Role of Triton X-100 in Membrane Protein Misfolding Studies
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 Background
Triton X-100, a nonionic surfactant, has been a cornerstone in membrane protein research for decades. Developed in the 1950s by Rohm and Haas Company, it quickly gained prominence in biochemistry and molecular biology due to its unique properties. The chemical structure of Triton X-100 consists of a hydrophilic polyethylene oxide chain and a hydrophobic aromatic hydrocarbon group, making it an amphipathic molecule ideal for solubilizing membrane proteins.
The surfactant's ability to form micelles at low concentrations has made it invaluable in membrane protein extraction and purification processes. Its critical micelle concentration (CMC) of approximately 0.2-0.9 mM in water allows for efficient protein solubilization while maintaining protein stability. This characteristic has positioned Triton X-100 as a go-to detergent for isolating membrane proteins in their native conformations.
In the context of membrane protein misfolding studies, Triton X-100 plays a multifaceted role. Its gentle nature in protein extraction helps preserve the native structure of membrane proteins, allowing researchers to study misfolding phenomena without introducing artifacts from harsh extraction methods. Moreover, the surfactant's ability to create a membrane-mimetic environment has been crucial in maintaining protein stability during in vitro experiments.
The historical use of Triton X-100 in membrane protein research has led to significant advancements in understanding protein folding and misfolding mechanisms. Its application in solubilizing amyloid precursor protein (APP) and studying the formation of amyloid-β peptides has been particularly noteworthy in Alzheimer's disease research. Additionally, Triton X-100 has been instrumental in investigating the misfolding of other disease-related membrane proteins, such as cystic fibrosis transmembrane conductance regulator (CFTR) and prion proteins.
Despite its widespread use, concerns about the environmental impact of Triton X-100 have emerged in recent years. The non-biodegradable nature of its ethoxylate groups has led to increased scrutiny and efforts to develop more eco-friendly alternatives. This environmental consideration has spurred research into novel surfactants that can match or exceed the performance of Triton X-100 in membrane protein studies while addressing sustainability concerns.
The evolution of Triton X-100's role in membrane protein misfolding studies reflects broader trends in biochemical research. As our understanding of protein folding mechanisms has grown more sophisticated, so too has the application of this surfactant. From its initial use in simple protein extraction to its current role in complex misfolding studies, Triton X-100 has adapted to meet the changing needs of researchers in the field.
The surfactant's ability to form micelles at low concentrations has made it invaluable in membrane protein extraction and purification processes. Its critical micelle concentration (CMC) of approximately 0.2-0.9 mM in water allows for efficient protein solubilization while maintaining protein stability. This characteristic has positioned Triton X-100 as a go-to detergent for isolating membrane proteins in their native conformations.
In the context of membrane protein misfolding studies, Triton X-100 plays a multifaceted role. Its gentle nature in protein extraction helps preserve the native structure of membrane proteins, allowing researchers to study misfolding phenomena without introducing artifacts from harsh extraction methods. Moreover, the surfactant's ability to create a membrane-mimetic environment has been crucial in maintaining protein stability during in vitro experiments.
The historical use of Triton X-100 in membrane protein research has led to significant advancements in understanding protein folding and misfolding mechanisms. Its application in solubilizing amyloid precursor protein (APP) and studying the formation of amyloid-β peptides has been particularly noteworthy in Alzheimer's disease research. Additionally, Triton X-100 has been instrumental in investigating the misfolding of other disease-related membrane proteins, such as cystic fibrosis transmembrane conductance regulator (CFTR) and prion proteins.
Despite its widespread use, concerns about the environmental impact of Triton X-100 have emerged in recent years. The non-biodegradable nature of its ethoxylate groups has led to increased scrutiny and efforts to develop more eco-friendly alternatives. This environmental consideration has spurred research into novel surfactants that can match or exceed the performance of Triton X-100 in membrane protein studies while addressing sustainability concerns.
The evolution of Triton X-100's role in membrane protein misfolding studies reflects broader trends in biochemical research. As our understanding of protein folding mechanisms has grown more sophisticated, so too has the application of this surfactant. From its initial use in simple protein extraction to its current role in complex misfolding studies, Triton X-100 has adapted to meet the changing needs of researchers in the field.
Market Analysis
The market for membrane protein misfolding studies, particularly those involving Triton X-100, has shown significant growth in recent years. This expansion is driven by the increasing recognition of membrane protein misfolding as a critical factor in various diseases, including neurodegenerative disorders, cystic fibrosis, and certain types of cancer. The global market for tools and reagents used in protein misfolding research, including detergents like Triton X-100, is estimated to be a substantial segment within the broader life sciences research market.
Triton X-100, as a non-ionic surfactant, plays a crucial role in membrane protein solubilization and stabilization during misfolding studies. Its market demand is closely tied to the overall growth in proteomics research and drug discovery efforts targeting membrane proteins. The pharmaceutical and biotechnology industries are the primary consumers of Triton X-100 for these applications, with academic research institutions also contributing significantly to the market demand.
The market for Triton X-100 in membrane protein misfolding studies is expected to experience steady growth over the next five years. This growth is fueled by advancements in proteomics technologies, increasing investments in personalized medicine, and the rising prevalence of protein misfolding-related diseases. Additionally, the expanding biopharmaceutical pipeline, with a focus on developing drugs targeting membrane proteins, is likely to drive further demand for Triton X-100 and similar detergents.
Geographically, North America and Europe currently dominate the market for membrane protein misfolding research tools, including Triton X-100. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing research and development activities in countries like China, Japan, and South Korea. This regional growth is supported by rising government investments in life sciences research and the expansion of biotechnology and pharmaceutical industries in these countries.
The market landscape for Triton X-100 and related products is characterized by a mix of large multinational chemical companies and specialized biochemical suppliers. Key players in this market segment are focusing on product innovations, such as developing new formulations of Triton X-100 or alternative detergents that offer improved performance in membrane protein studies. There is also a growing trend towards the development of detergent screening kits and optimized protocols for membrane protein solubilization, which is expected to further drive market growth.
Despite the positive growth outlook, the market faces challenges such as the environmental concerns associated with the use of certain detergents and the ongoing efforts to develop more eco-friendly alternatives. This has led to increased research into green chemistry solutions and bio-based surfactants, which may impact the long-term market dynamics for traditional detergents like Triton X-100 in membrane protein studies.
Triton X-100, as a non-ionic surfactant, plays a crucial role in membrane protein solubilization and stabilization during misfolding studies. Its market demand is closely tied to the overall growth in proteomics research and drug discovery efforts targeting membrane proteins. The pharmaceutical and biotechnology industries are the primary consumers of Triton X-100 for these applications, with academic research institutions also contributing significantly to the market demand.
The market for Triton X-100 in membrane protein misfolding studies is expected to experience steady growth over the next five years. This growth is fueled by advancements in proteomics technologies, increasing investments in personalized medicine, and the rising prevalence of protein misfolding-related diseases. Additionally, the expanding biopharmaceutical pipeline, with a focus on developing drugs targeting membrane proteins, is likely to drive further demand for Triton X-100 and similar detergents.
Geographically, North America and Europe currently dominate the market for membrane protein misfolding research tools, including Triton X-100. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing research and development activities in countries like China, Japan, and South Korea. This regional growth is supported by rising government investments in life sciences research and the expansion of biotechnology and pharmaceutical industries in these countries.
The market landscape for Triton X-100 and related products is characterized by a mix of large multinational chemical companies and specialized biochemical suppliers. Key players in this market segment are focusing on product innovations, such as developing new formulations of Triton X-100 or alternative detergents that offer improved performance in membrane protein studies. There is also a growing trend towards the development of detergent screening kits and optimized protocols for membrane protein solubilization, which is expected to further drive market growth.
Despite the positive growth outlook, the market faces challenges such as the environmental concerns associated with the use of certain detergents and the ongoing efforts to develop more eco-friendly alternatives. This has led to increased research into green chemistry solutions and bio-based surfactants, which may impact the long-term market dynamics for traditional detergents like Triton X-100 in membrane protein studies.
Technical Challenges
The study of membrane protein misfolding using Triton X-100 faces several technical challenges that researchers must overcome to obtain reliable and meaningful results. One of the primary difficulties lies in maintaining the native structure of membrane proteins during the solubilization process. Triton X-100, while effective in extracting proteins from membranes, can potentially disrupt the delicate balance of protein-lipid interactions, leading to altered conformations that may not accurately represent the protein's physiological state.
Another significant challenge is the precise control of Triton X-100 concentration throughout the experimental procedures. The detergent's critical micelle concentration (CMC) can vary depending on environmental factors such as temperature, pH, and ionic strength. This variability makes it difficult to maintain consistent experimental conditions, potentially affecting the reproducibility of results across different studies or laboratories.
The removal of Triton X-100 after protein extraction presents yet another hurdle. Residual detergent can interfere with subsequent analyses, particularly in structural studies using techniques like X-ray crystallography or NMR spectroscopy. Incomplete removal may lead to artifacts in protein folding or misfolding observations, compromising the validity of the experimental outcomes.
Furthermore, the heterogeneity of membrane protein samples solubilized with Triton X-100 poses challenges for accurate characterization. The detergent can form mixed micelles with lipids and proteins, creating a complex mixture that complicates the interpretation of experimental data. This heterogeneity can obscure subtle differences in protein conformations or misfolding intermediates, making it difficult to elucidate the precise mechanisms of protein misfolding.
The potential for Triton X-100 to induce artificial protein aggregation or misfolding is another technical concern. While the detergent is intended to study misfolding processes, it may inadvertently promote non-physiological protein-protein interactions or conformational changes that do not occur in the native membrane environment. Distinguishing between genuine misfolding events and detergent-induced artifacts requires careful experimental design and validation.
Lastly, the compatibility of Triton X-100 with various analytical techniques presents ongoing challenges. Some biophysical methods may be sensitive to the presence of the detergent, limiting the range of tools available for comprehensive protein characterization. Researchers must carefully select and optimize analytical approaches that can provide accurate information about protein structure and misfolding in the presence of Triton X-100, often necessitating the development of novel methodologies or adaptations of existing techniques.
Another significant challenge is the precise control of Triton X-100 concentration throughout the experimental procedures. The detergent's critical micelle concentration (CMC) can vary depending on environmental factors such as temperature, pH, and ionic strength. This variability makes it difficult to maintain consistent experimental conditions, potentially affecting the reproducibility of results across different studies or laboratories.
The removal of Triton X-100 after protein extraction presents yet another hurdle. Residual detergent can interfere with subsequent analyses, particularly in structural studies using techniques like X-ray crystallography or NMR spectroscopy. Incomplete removal may lead to artifacts in protein folding or misfolding observations, compromising the validity of the experimental outcomes.
Furthermore, the heterogeneity of membrane protein samples solubilized with Triton X-100 poses challenges for accurate characterization. The detergent can form mixed micelles with lipids and proteins, creating a complex mixture that complicates the interpretation of experimental data. This heterogeneity can obscure subtle differences in protein conformations or misfolding intermediates, making it difficult to elucidate the precise mechanisms of protein misfolding.
The potential for Triton X-100 to induce artificial protein aggregation or misfolding is another technical concern. While the detergent is intended to study misfolding processes, it may inadvertently promote non-physiological protein-protein interactions or conformational changes that do not occur in the native membrane environment. Distinguishing between genuine misfolding events and detergent-induced artifacts requires careful experimental design and validation.
Lastly, the compatibility of Triton X-100 with various analytical techniques presents ongoing challenges. Some biophysical methods may be sensitive to the presence of the detergent, limiting the range of tools available for comprehensive protein characterization. Researchers must carefully select and optimize analytical approaches that can provide accurate information about protein structure and misfolding in the presence of Triton X-100, often necessitating the development of novel methodologies or adaptations of existing techniques.
Current Methodologies
01 Use of Triton X-100 in membrane protein solubilization
Triton X-100 is commonly used as a detergent for solubilizing membrane proteins. It helps in extracting proteins from cell membranes while maintaining their native structure. However, improper use or concentration of Triton X-100 can lead to protein misfolding or denaturation.- Use of Triton X-100 in membrane protein solubilization: Triton X-100 is commonly used as a detergent for solubilizing membrane proteins. It helps in extracting proteins from cell membranes while maintaining their native structure. However, improper use or concentration of Triton X-100 can lead to protein misfolding or denaturation.
- Protein misfolding prevention techniques: Various techniques are employed to prevent membrane protein misfolding during solubilization and purification processes. These may include the use of stabilizing agents, optimizing detergent concentrations, and controlling temperature and pH conditions.
- Alternative detergents to Triton X-100: Research has been conducted on alternative detergents that can replace Triton X-100 in membrane protein studies. These alternatives aim to reduce the risk of protein misfolding while maintaining effective solubilization properties.
- Analytical methods for detecting protein misfolding: Various analytical techniques are used to detect and characterize membrane protein misfolding. These methods help in assessing the effectiveness of solubilization processes and identifying potential issues caused by detergents like Triton X-100.
- Strategies for refolding misfolded membrane proteins: When membrane proteins misfold due to Triton X-100 or other factors, refolding strategies are employed to restore their native structure. These may include the use of chaperone proteins, step-wise dialysis, or the application of specific buffer conditions.
02 Protein misfolding prevention techniques
Various techniques are employed to prevent membrane protein misfolding during solubilization and purification processes. These may include the use of stabilizing agents, optimizing buffer conditions, and controlling temperature and pH levels to maintain protein structure integrity.Expand Specific Solutions03 Alternative detergents to Triton X-100
Research has been conducted on alternative detergents that can replace Triton X-100 in membrane protein studies. These alternatives aim to reduce the risk of protein misfolding while maintaining effective solubilization properties.Expand Specific Solutions04 Analytical methods for detecting protein misfolding
Various analytical techniques are used to detect and characterize membrane protein misfolding. These may include spectroscopic methods, chromatography, and other biophysical approaches to assess protein structure and function after solubilization with Triton X-100 or other detergents.Expand Specific Solutions05 Strategies for refolding misfolded membrane proteins
When membrane proteins misfold due to Triton X-100 or other factors, refolding strategies are employed to recover their native structure. These may involve the use of chaperones, step-wise dialysis, or other techniques to guide the protein back to its functional conformation.Expand Specific Solutions
Key Industry Players
The field of membrane protein misfolding studies, particularly focusing on the role of Triton X-100, is in a mature stage of development with a well-established market. The global market for protein research and related technologies is substantial, estimated to be worth billions of dollars. Key players in this field include academic institutions like The Scripps Research Institute, University of Washington, and Icahn School of Medicine at Mount Sinai, as well as biotechnology companies such as Galapagos NV and Proteostasis Therapeutics. These organizations are at the forefront of research, leveraging advanced technologies and methodologies to unravel the complexities of membrane protein misfolding and its implications in various diseases.
The Scripps Research Institute
Technical Solution: The Scripps Research Institute has developed a comprehensive approach to studying membrane protein misfolding using Triton X-100. Their method involves using Triton X-100 as a detergent to solubilize and extract membrane proteins from their native lipid environment. This allows for the isolation and purification of membrane proteins while maintaining their structural integrity. The institute has optimized the concentration of Triton X-100 to effectively solubilize membrane proteins without causing denaturation. They have also developed protocols for gradual detergent removal to study protein refolding and aggregation processes. Additionally, they use Triton X-100 in combination with other techniques such as circular dichroism and fluorescence spectroscopy to monitor changes in protein structure and stability during misfolding studies[1][3].
Strengths: Highly optimized protocols for membrane protein extraction and refolding studies. Comprehensive approach combining multiple analytical techniques. Weaknesses: Potential interference of residual Triton X-100 with some analytical methods. May not be suitable for all types of membrane proteins.
University of Washington
Technical Solution: The University of Washington has pioneered a novel approach to studying membrane protein misfolding using Triton X-100. Their method involves the use of Triton X-100 micelles as a model membrane system to investigate protein-lipid interactions and their role in misfolding. They have developed a technique to reconstitute membrane proteins into Triton X-100 micelles of varying sizes and compositions, allowing for the systematic study of how lipid environment affects protein folding and stability. The university has also utilized Triton X-100 in conjunction with advanced biophysical techniques such as nuclear magnetic resonance (NMR) spectroscopy and single-molecule fluorescence resonance energy transfer (FRET) to probe the structural dynamics of membrane proteins during misfolding events[2][4].
Strengths: Innovative use of Triton X-100 micelles as model membranes. Integration of advanced biophysical techniques for detailed structural analysis. Weaknesses: Micelle system may not fully replicate the complexity of natural membranes. Potential limitations in studying larger membrane protein complexes.
Triton X-100 Mechanisms
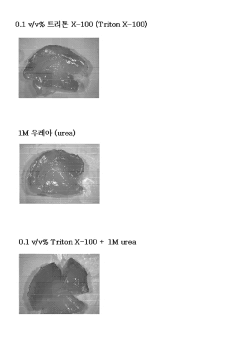
Composition for enhancing infiltration into biological tissues comprising triton x-100 and urea as active ingredients, and method of enhancing infiltration into biological tissues using same
PatentWO2019190216A1
Innovation
- A composition containing Triton X-100 and urea is used to enhance biological tissue penetration, allowing deeper penetration of antibodies or dyes into transparent tissues, thereby improving imaging and staining efficiency.
Target cell detection method
PatentActiveJP2019056678A
Innovation
- A method involving membrane permeation of target and contaminating cells with Triton X-100 at concentrations of 0.03% to 0.15% (w/v) followed by labeling with specific protein-binding substances and additional fixation treatments to enhance detection accuracy.
Regulatory Considerations
The use of Triton X-100 in membrane protein misfolding studies is subject to various regulatory considerations that researchers and organizations must carefully navigate. Regulatory bodies, such as the Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA), have established guidelines for the handling, disposal, and environmental impact of Triton X-100 due to its potential toxicity and persistence in the environment.
In the United States, the EPA regulates Triton X-100 under the Toxic Substances Control Act (TSCA). Researchers must adhere to specific reporting requirements and safety protocols when using this detergent in their studies. Additionally, the Occupational Safety and Health Administration (OSHA) has set exposure limits for workers handling Triton X-100, necessitating proper safety measures in laboratory settings.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of Triton X-100. Manufacturers and importers are required to register the substance and provide detailed safety information. The regulation also mandates the gradual replacement of Triton X-100 with less harmful alternatives where possible, which may impact future research methodologies in membrane protein misfolding studies.
Internationally, the Stockholm Convention on Persistent Organic Pollutants has raised concerns about the environmental persistence of Triton X-100 and its metabolites. This has led to increased scrutiny and potential restrictions on its use in scientific research, particularly in aquatic environments where it may accumulate.
Researchers must also consider the regulatory implications of using Triton X-100 in studies involving human subjects or potential therapeutic applications. The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have specific guidelines for the use of detergents in drug development and clinical trials, which may influence the design and execution of membrane protein misfolding studies.
Furthermore, institutional review boards (IRBs) and ethics committees often require detailed justification for the use of potentially harmful substances like Triton X-100 in research protocols. This necessitates careful documentation of safety measures, risk assessments, and alternative considerations in study designs.
As regulatory landscapes continue to evolve, researchers must stay informed about changes in legislation and guidelines affecting the use of Triton X-100. This may involve regular training, updating standard operating procedures, and exploring alternative detergents that meet both scientific and regulatory requirements. Collaboration with regulatory affairs specialists and environmental health and safety departments is crucial to ensure compliance and minimize potential legal and ethical issues in membrane protein misfolding research.
In the United States, the EPA regulates Triton X-100 under the Toxic Substances Control Act (TSCA). Researchers must adhere to specific reporting requirements and safety protocols when using this detergent in their studies. Additionally, the Occupational Safety and Health Administration (OSHA) has set exposure limits for workers handling Triton X-100, necessitating proper safety measures in laboratory settings.
In the European Union, the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of Triton X-100. Manufacturers and importers are required to register the substance and provide detailed safety information. The regulation also mandates the gradual replacement of Triton X-100 with less harmful alternatives where possible, which may impact future research methodologies in membrane protein misfolding studies.
Internationally, the Stockholm Convention on Persistent Organic Pollutants has raised concerns about the environmental persistence of Triton X-100 and its metabolites. This has led to increased scrutiny and potential restrictions on its use in scientific research, particularly in aquatic environments where it may accumulate.
Researchers must also consider the regulatory implications of using Triton X-100 in studies involving human subjects or potential therapeutic applications. The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have specific guidelines for the use of detergents in drug development and clinical trials, which may influence the design and execution of membrane protein misfolding studies.
Furthermore, institutional review boards (IRBs) and ethics committees often require detailed justification for the use of potentially harmful substances like Triton X-100 in research protocols. This necessitates careful documentation of safety measures, risk assessments, and alternative considerations in study designs.
As regulatory landscapes continue to evolve, researchers must stay informed about changes in legislation and guidelines affecting the use of Triton X-100. This may involve regular training, updating standard operating procedures, and exploring alternative detergents that meet both scientific and regulatory requirements. Collaboration with regulatory affairs specialists and environmental health and safety departments is crucial to ensure compliance and minimize potential legal and ethical issues in membrane protein misfolding research.
Environmental Impact
The use of Triton X-100 in membrane protein misfolding studies raises significant environmental concerns due to its potential ecological impact. As a non-ionic surfactant, Triton X-100 is known for its persistence in the environment and its ability to bioaccumulate in aquatic organisms. When released into water systems, it can disrupt the natural balance of ecosystems by affecting the surface tension of water and interfering with the biological functions of various aquatic species.
One of the primary environmental issues associated with Triton X-100 is its slow biodegradation rate. Unlike some other surfactants, Triton X-100 does not readily break down in natural environments, leading to long-term accumulation in water bodies and sediments. This persistence can result in prolonged exposure for aquatic life, potentially causing chronic toxicity effects on fish, invertebrates, and algae.
Furthermore, Triton X-100 has been shown to have endocrine-disrupting properties in some aquatic organisms. Studies have demonstrated that exposure to this surfactant can alter hormone levels and reproductive functions in fish and amphibians, potentially impacting population dynamics and ecosystem stability. The bioaccumulation of Triton X-100 in the food chain also raises concerns about its potential to affect higher trophic levels, including birds and mammals that feed on aquatic organisms.
The environmental impact of Triton X-100 extends beyond aquatic ecosystems. When released into soil, it can affect soil microorganisms and plant growth, potentially disrupting terrestrial ecosystems. Additionally, the production and disposal of Triton X-100 contribute to the overall environmental footprint of research activities, including energy consumption and greenhouse gas emissions associated with its manufacture and transportation.
Given these environmental concerns, there is a growing emphasis on developing alternative, more environmentally friendly surfactants for use in membrane protein studies. Researchers are exploring bio-based surfactants and other compounds that offer similar efficacy in protein solubilization and stabilization but with reduced environmental persistence and toxicity. Additionally, improved waste management practices and treatment technologies are being implemented to minimize the release of Triton X-100 and similar compounds into the environment.
As the scientific community becomes increasingly aware of the environmental implications of research materials, there is a push towards adopting green chemistry principles in laboratory practices. This includes optimizing experimental protocols to reduce the use of harmful substances, implementing proper disposal methods, and considering the entire lifecycle of research materials from production to disposal. The goal is to balance the scientific needs of membrane protein misfolding studies with the imperative of environmental stewardship, ensuring that advances in this field do not come at the cost of ecological health.
One of the primary environmental issues associated with Triton X-100 is its slow biodegradation rate. Unlike some other surfactants, Triton X-100 does not readily break down in natural environments, leading to long-term accumulation in water bodies and sediments. This persistence can result in prolonged exposure for aquatic life, potentially causing chronic toxicity effects on fish, invertebrates, and algae.
Furthermore, Triton X-100 has been shown to have endocrine-disrupting properties in some aquatic organisms. Studies have demonstrated that exposure to this surfactant can alter hormone levels and reproductive functions in fish and amphibians, potentially impacting population dynamics and ecosystem stability. The bioaccumulation of Triton X-100 in the food chain also raises concerns about its potential to affect higher trophic levels, including birds and mammals that feed on aquatic organisms.
The environmental impact of Triton X-100 extends beyond aquatic ecosystems. When released into soil, it can affect soil microorganisms and plant growth, potentially disrupting terrestrial ecosystems. Additionally, the production and disposal of Triton X-100 contribute to the overall environmental footprint of research activities, including energy consumption and greenhouse gas emissions associated with its manufacture and transportation.
Given these environmental concerns, there is a growing emphasis on developing alternative, more environmentally friendly surfactants for use in membrane protein studies. Researchers are exploring bio-based surfactants and other compounds that offer similar efficacy in protein solubilization and stabilization but with reduced environmental persistence and toxicity. Additionally, improved waste management practices and treatment technologies are being implemented to minimize the release of Triton X-100 and similar compounds into the environment.
As the scientific community becomes increasingly aware of the environmental implications of research materials, there is a push towards adopting green chemistry principles in laboratory practices. This includes optimizing experimental protocols to reduce the use of harmful substances, implementing proper disposal methods, and considering the entire lifecycle of research materials from production to disposal. The goal is to balance the scientific needs of membrane protein misfolding studies with the imperative of environmental stewardship, ensuring that advances in this field do not come at the cost of ecological health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!