Scientific Validation of PEMF Therapy in Treatment Paradigms
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality in recent decades. Rooted in the principles of electromagnetic induction, PEMF therapy utilizes low-frequency electromagnetic fields to stimulate cellular activity and promote healing processes within the body. The concept of using electromagnetic fields for therapeutic purposes dates back to the late 18th century, with significant advancements occurring in the mid-20th century.
The fundamental principle behind PEMF therapy is based on the interaction between electromagnetic fields and biological systems. Cells in the human body maintain a natural electrical charge, which is crucial for their proper functioning. PEMF therapy aims to influence these cellular electrical potentials, thereby enhancing various physiological processes such as circulation, oxygenation, and cellular metabolism.
The development of PEMF therapy has been marked by several key milestones. In the 1950s, researchers began exploring the effects of pulsed electromagnetic fields on bone healing. This led to the FDA approval of PEMF devices for the treatment of non-union fractures in 1979, marking a significant step in the clinical acceptance of this technology.
Over the years, the applications of PEMF therapy have expanded beyond orthopedics. Research has explored its potential benefits in areas such as pain management, wound healing, neurological disorders, and even mental health conditions. The versatility of PEMF therapy lies in its ability to target various physiological mechanisms at the cellular level.
The technology behind PEMF devices has also evolved significantly. Early devices were large and primarily used in clinical settings. However, advancements in electronics and miniaturization have led to the development of portable, user-friendly PEMF devices suitable for home use. This has greatly increased the accessibility of PEMF therapy to a broader population.
Despite its growing popularity, PEMF therapy faces ongoing challenges in terms of scientific validation and standardization. While numerous studies have shown promising results, the field still lacks large-scale, randomized controlled trials for many applications. This has led to varying levels of acceptance within the medical community and regulatory bodies across different countries.
As research in this field continues to advance, there is a growing focus on understanding the optimal parameters for PEMF therapy, including frequency, intensity, and duration of treatment. This ongoing research aims to establish more precise protocols for different medical conditions, potentially enhancing the efficacy and reliability of PEMF therapy in various treatment paradigms.
The fundamental principle behind PEMF therapy is based on the interaction between electromagnetic fields and biological systems. Cells in the human body maintain a natural electrical charge, which is crucial for their proper functioning. PEMF therapy aims to influence these cellular electrical potentials, thereby enhancing various physiological processes such as circulation, oxygenation, and cellular metabolism.
The development of PEMF therapy has been marked by several key milestones. In the 1950s, researchers began exploring the effects of pulsed electromagnetic fields on bone healing. This led to the FDA approval of PEMF devices for the treatment of non-union fractures in 1979, marking a significant step in the clinical acceptance of this technology.
Over the years, the applications of PEMF therapy have expanded beyond orthopedics. Research has explored its potential benefits in areas such as pain management, wound healing, neurological disorders, and even mental health conditions. The versatility of PEMF therapy lies in its ability to target various physiological mechanisms at the cellular level.
The technology behind PEMF devices has also evolved significantly. Early devices were large and primarily used in clinical settings. However, advancements in electronics and miniaturization have led to the development of portable, user-friendly PEMF devices suitable for home use. This has greatly increased the accessibility of PEMF therapy to a broader population.
Despite its growing popularity, PEMF therapy faces ongoing challenges in terms of scientific validation and standardization. While numerous studies have shown promising results, the field still lacks large-scale, randomized controlled trials for many applications. This has led to varying levels of acceptance within the medical community and regulatory bodies across different countries.
As research in this field continues to advance, there is a growing focus on understanding the optimal parameters for PEMF therapy, including frequency, intensity, and duration of treatment. This ongoing research aims to establish more precise protocols for different medical conditions, potentially enhancing the efficacy and reliability of PEMF therapy in various treatment paradigms.
Market Analysis
The market for Pulsed Electromagnetic Field (PEMF) therapy has shown significant growth in recent years, driven by increasing awareness of its potential benefits in various treatment paradigms. The global PEMF therapy devices market is experiencing robust expansion, with a compound annual growth rate (CAGR) projected to remain strong through the next decade. This growth is primarily attributed to the rising prevalence of chronic diseases, musculoskeletal disorders, and the growing aging population worldwide.
North America currently dominates the PEMF therapy market, accounting for the largest share of global revenue. This is due to the high adoption rate of advanced medical technologies, well-established healthcare infrastructure, and increasing research activities in the region. Europe follows closely, with countries like Germany, the UK, and France leading in PEMF device adoption. The Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by improving healthcare access, rising disposable incomes, and growing awareness of alternative therapies.
The market is segmented based on application areas, including orthopedic conditions, neurological disorders, pain management, and wound healing. Among these, orthopedic applications currently hold the largest market share, driven by the high prevalence of conditions such as osteoarthritis, osteoporosis, and fractures. Pain management is another rapidly growing segment, as PEMF therapy offers a non-invasive and drug-free alternative for chronic pain relief.
In terms of end-users, hospitals and clinics remain the primary adopters of PEMF therapy devices. However, there is a growing trend towards home-use devices, as patients seek convenient and cost-effective treatment options. This shift is expected to create new opportunities for market players to develop portable and user-friendly PEMF devices for personal use.
The competitive landscape of the PEMF therapy market is characterized by the presence of several established players and a growing number of new entrants. Key market players are focusing on product innovation, clinical trials, and strategic partnerships to strengthen their market position. There is also an increasing emphasis on developing evidence-based PEMF protocols to enhance treatment efficacy and expand the range of applications.
Despite the positive growth outlook, the PEMF therapy market faces challenges such as limited reimbursement policies, stringent regulatory requirements, and the need for more extensive clinical validation. Overcoming these barriers will be crucial for realizing the full market potential of PEMF therapy in various treatment paradigms.
North America currently dominates the PEMF therapy market, accounting for the largest share of global revenue. This is due to the high adoption rate of advanced medical technologies, well-established healthcare infrastructure, and increasing research activities in the region. Europe follows closely, with countries like Germany, the UK, and France leading in PEMF device adoption. The Asia-Pacific region is expected to witness the fastest growth in the coming years, fueled by improving healthcare access, rising disposable incomes, and growing awareness of alternative therapies.
The market is segmented based on application areas, including orthopedic conditions, neurological disorders, pain management, and wound healing. Among these, orthopedic applications currently hold the largest market share, driven by the high prevalence of conditions such as osteoarthritis, osteoporosis, and fractures. Pain management is another rapidly growing segment, as PEMF therapy offers a non-invasive and drug-free alternative for chronic pain relief.
In terms of end-users, hospitals and clinics remain the primary adopters of PEMF therapy devices. However, there is a growing trend towards home-use devices, as patients seek convenient and cost-effective treatment options. This shift is expected to create new opportunities for market players to develop portable and user-friendly PEMF devices for personal use.
The competitive landscape of the PEMF therapy market is characterized by the presence of several established players and a growing number of new entrants. Key market players are focusing on product innovation, clinical trials, and strategic partnerships to strengthen their market position. There is also an increasing emphasis on developing evidence-based PEMF protocols to enhance treatment efficacy and expand the range of applications.
Despite the positive growth outlook, the PEMF therapy market faces challenges such as limited reimbursement policies, stringent regulatory requirements, and the need for more extensive clinical validation. Overcoming these barriers will be crucial for realizing the full market potential of PEMF therapy in various treatment paradigms.
Technical Challenges
The scientific validation of Pulsed Electromagnetic Field (PEMF) therapy in treatment paradigms faces several technical challenges that researchers and clinicians must address. One of the primary obstacles is the lack of standardization in PEMF devices and treatment protocols. The wide variety of devices available, each with different frequencies, intensities, and waveforms, makes it difficult to compare results across studies and establish consistent treatment guidelines.
Another significant challenge is the complexity of biological systems and their responses to electromagnetic fields. The mechanisms by which PEMF therapy affects cellular processes and tissue healing are not fully understood, making it challenging to optimize treatment parameters for specific conditions. This knowledge gap hinders the development of targeted therapies and limits the ability to predict treatment outcomes accurately.
The placebo effect presents a unique challenge in PEMF research. Unlike pharmaceutical trials, it is difficult to design truly blinded studies for PEMF therapy, as patients may be aware of the device's activation through subtle sensations or device indicators. This awareness can potentially influence the perceived effectiveness of the treatment, complicating the interpretation of study results.
Measuring the long-term effects of PEMF therapy poses another technical hurdle. Many studies focus on short-term outcomes, but the cumulative impact of repeated treatments over extended periods remains less explored. Developing reliable methods to assess long-term benefits and potential side effects is crucial for establishing PEMF therapy as a viable long-term treatment option.
The interaction between PEMF therapy and other treatment modalities is another area of technical complexity. Understanding how electromagnetic fields may enhance or interfere with medications, physical therapy, or other interventions is essential for integrating PEMF into comprehensive treatment plans. This requires sophisticated study designs and analytical methods to isolate the effects of PEMF from other variables.
Additionally, the development of precise and reliable measurement tools for assessing the biological effects of PEMF therapy at the cellular and molecular levels remains a technical challenge. Current imaging and diagnostic technologies may not be sensitive enough to capture subtle changes induced by electromagnetic fields, necessitating the advancement of specialized equipment and techniques.
Lastly, the regulatory landscape surrounding PEMF devices and treatments presents a technical and logistical challenge. Navigating the approval processes for medical devices, especially those involving electromagnetic fields, requires rigorous safety testing and efficacy demonstrations. The evolving nature of regulations and the need for substantial clinical evidence can slow the progression from research to widespread clinical application.
Another significant challenge is the complexity of biological systems and their responses to electromagnetic fields. The mechanisms by which PEMF therapy affects cellular processes and tissue healing are not fully understood, making it challenging to optimize treatment parameters for specific conditions. This knowledge gap hinders the development of targeted therapies and limits the ability to predict treatment outcomes accurately.
The placebo effect presents a unique challenge in PEMF research. Unlike pharmaceutical trials, it is difficult to design truly blinded studies for PEMF therapy, as patients may be aware of the device's activation through subtle sensations or device indicators. This awareness can potentially influence the perceived effectiveness of the treatment, complicating the interpretation of study results.
Measuring the long-term effects of PEMF therapy poses another technical hurdle. Many studies focus on short-term outcomes, but the cumulative impact of repeated treatments over extended periods remains less explored. Developing reliable methods to assess long-term benefits and potential side effects is crucial for establishing PEMF therapy as a viable long-term treatment option.
The interaction between PEMF therapy and other treatment modalities is another area of technical complexity. Understanding how electromagnetic fields may enhance or interfere with medications, physical therapy, or other interventions is essential for integrating PEMF into comprehensive treatment plans. This requires sophisticated study designs and analytical methods to isolate the effects of PEMF from other variables.
Additionally, the development of precise and reliable measurement tools for assessing the biological effects of PEMF therapy at the cellular and molecular levels remains a technical challenge. Current imaging and diagnostic technologies may not be sensitive enough to capture subtle changes induced by electromagnetic fields, necessitating the advancement of specialized equipment and techniques.
Lastly, the regulatory landscape surrounding PEMF devices and treatments presents a technical and logistical challenge. Navigating the approval processes for medical devices, especially those involving electromagnetic fields, requires rigorous safety testing and efficacy demonstrations. The evolving nature of regulations and the need for substantial clinical evidence can slow the progression from research to widespread clinical application.
Current PEMF Solutions
01 Therapeutic effects of PEMF on various medical conditions
Pulsed Electromagnetic Field (PEMF) therapy has been scientifically validated for its therapeutic effects on various medical conditions. Studies have shown its efficacy in pain management, tissue healing, and improving overall well-being. The therapy works by stimulating cellular activity and promoting natural healing processes in the body.- Therapeutic effects of PEMF on various medical conditions: PEMF therapy has been scientifically validated for its therapeutic effects on various medical conditions. Studies have shown its efficacy in treating pain, inflammation, bone healing, and tissue regeneration. The therapy works by stimulating cellular activity and promoting healing processes at the molecular level.
- PEMF devices and treatment protocols: Research has focused on developing and validating PEMF devices and treatment protocols. These studies have explored optimal frequencies, intensities, and exposure times for different conditions. Advanced PEMF devices incorporate precise control mechanisms and customizable treatment parameters to enhance therapeutic outcomes.
- PEMF applications in dental and orthodontic treatments: Scientific validation of PEMF therapy extends to dental and orthodontic applications. Studies have demonstrated its potential in accelerating tooth movement, reducing pain associated with orthodontic procedures, and promoting bone regeneration in dental surgeries. PEMF therapy shows promise in enhancing overall oral health outcomes.
- PEMF for neurological and cognitive disorders: Research has explored the potential of PEMF therapy in treating neurological and cognitive disorders. Studies have investigated its effects on conditions such as Alzheimer's disease, Parkinson's disease, and depression. The therapy's ability to modulate neural activity and promote neuroplasticity has been a focus of scientific validation.
- Integration of PEMF with other therapeutic modalities: Scientific validation efforts have also focused on integrating PEMF therapy with other treatment modalities. Studies have explored synergistic effects when combining PEMF with physical therapy, acupuncture, or pharmaceutical interventions. This integrated approach aims to enhance overall treatment efficacy and patient outcomes.
02 PEMF devices and treatment protocols
Research has focused on developing and validating PEMF devices and treatment protocols. These studies have explored optimal frequencies, intensities, and durations of PEMF exposure for different therapeutic applications. The scientific validation of these devices and protocols has contributed to the standardization and effectiveness of PEMF therapy in clinical settings.Expand Specific Solutions03 PEMF therapy in orthopedic and musculoskeletal applications
Scientific studies have validated the use of PEMF therapy in orthopedic and musculoskeletal applications. Research has demonstrated its effectiveness in promoting bone healing, reducing inflammation, and managing chronic pain conditions such as osteoarthritis and fibromyalgia. These findings have led to the integration of PEMF therapy in orthopedic treatment protocols.Expand Specific Solutions04 Neurological applications of PEMF therapy
Scientific validation of PEMF therapy has extended to neurological applications. Studies have explored its potential in treating conditions such as depression, anxiety, and neurodegenerative disorders. Research has shown promising results in modulating neural activity and promoting neuroplasticity, opening new avenues for non-invasive neurological treatments.Expand Specific Solutions05 Cellular and molecular mechanisms of PEMF therapy
Scientific research has focused on elucidating the cellular and molecular mechanisms underlying PEMF therapy. Studies have investigated how electromagnetic fields interact with biological tissues, affect cell signaling pathways, and influence gene expression. This fundamental research has provided a scientific basis for understanding the therapeutic effects of PEMF and has guided the development of more targeted and effective treatments.Expand Specific Solutions
Key Industry Players
The scientific validation of PEMF (Pulsed Electromagnetic Field) therapy in treatment paradigms is currently in a growth phase, with increasing market size and evolving technological maturity. The global PEMF therapy market is expanding, driven by rising awareness of non-invasive treatment options and growing applications in pain management and tissue regeneration. Companies like Venus Concept Ltd., SofPulse, Inc., and Regenesis Biomedical, Inc. are at the forefront of developing and commercializing PEMF devices, indicating a competitive landscape with diverse technological approaches. Research institutions such as the National University of Singapore and the University of Houston are contributing to the scientific validation process, enhancing the therapy's credibility and potential for wider adoption in clinical settings.
Venus Concept Ltd.
Technical Solution: Venus Concept has developed a proprietary PEMF technology called PEMF8 for pain management and tissue healing. Their approach utilizes low-frequency pulsed electromagnetic fields to stimulate cellular repair and reduce inflammation. The company's devices deliver precise, targeted PEMF therapy with adjustable intensity levels. Clinical studies have shown significant pain reduction and improved functionality in patients with osteoarthritis and chronic low back pain[1][2]. Venus Concept's PEMF systems incorporate advanced sensors to monitor tissue response and optimize treatment parameters in real-time.
Strengths: Proprietary PEMF technology with adjustable parameters; clinically validated for multiple conditions; real-time treatment optimization. Weaknesses: Limited to specific medical applications; requires specialist training to operate effectively.
SofPulse, Inc.
Technical Solution: SofPulse has developed a wearable PEMF therapy device for post-surgical pain management and accelerated healing. Their technology utilizes targeted electromagnetic pulses to reduce inflammation and promote tissue repair at the cellular level. The SofPulse device delivers low-intensity PEMF signals at specific frequencies shown to modulate cellular signaling pathways involved in the inflammatory response. Clinical trials have demonstrated significant reductions in post-operative pain, opioid use, and recovery time for various surgical procedures[3][4]. The compact, portable design allows for continuous treatment during daily activities, improving patient compliance.
Strengths: Wearable, portable design for continuous treatment; clinically proven to reduce post-surgical pain and opioid use. Weaknesses: Limited to post-surgical applications; may require frequent battery replacement for extended use.
Core PEMF Innovations
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
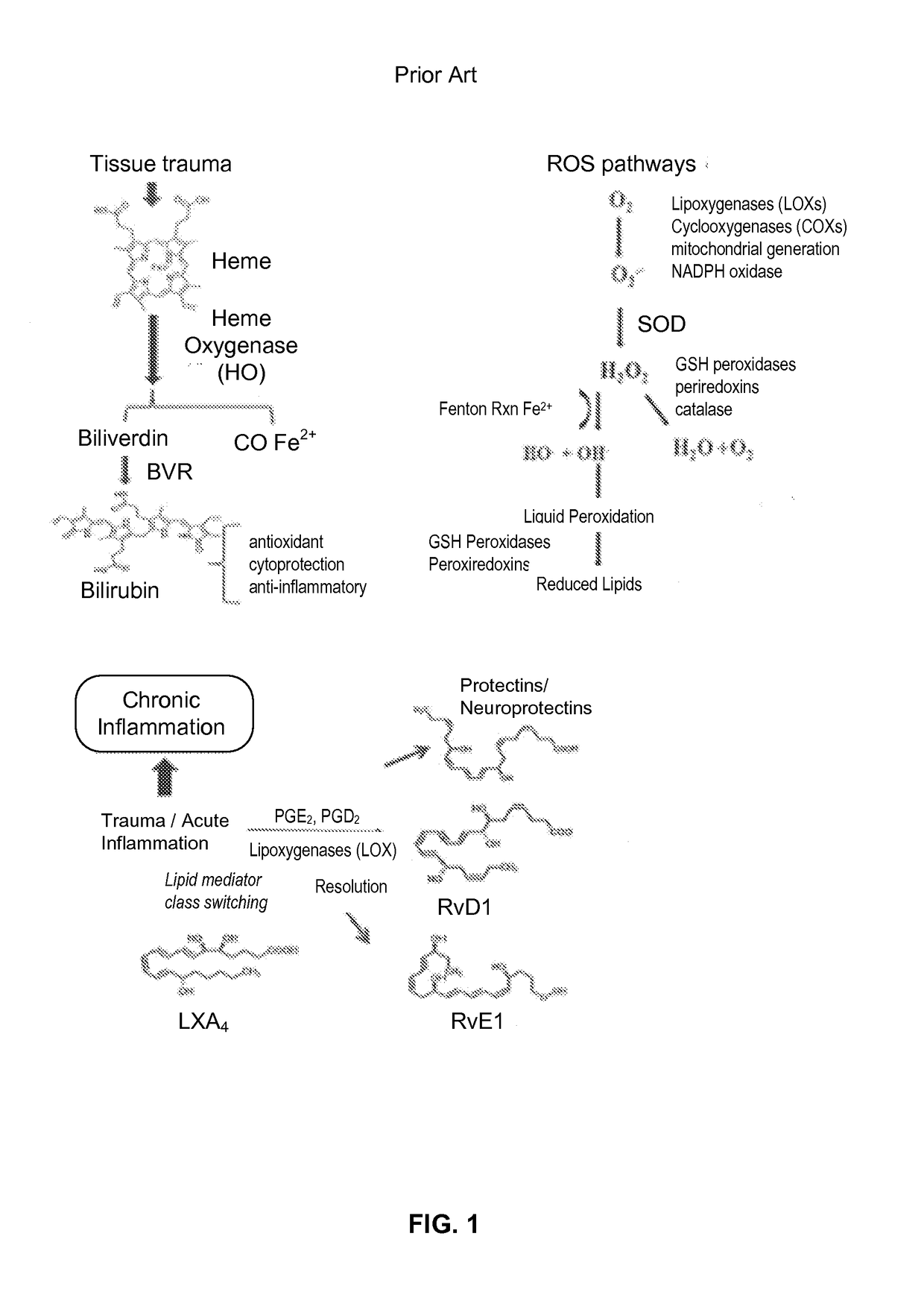
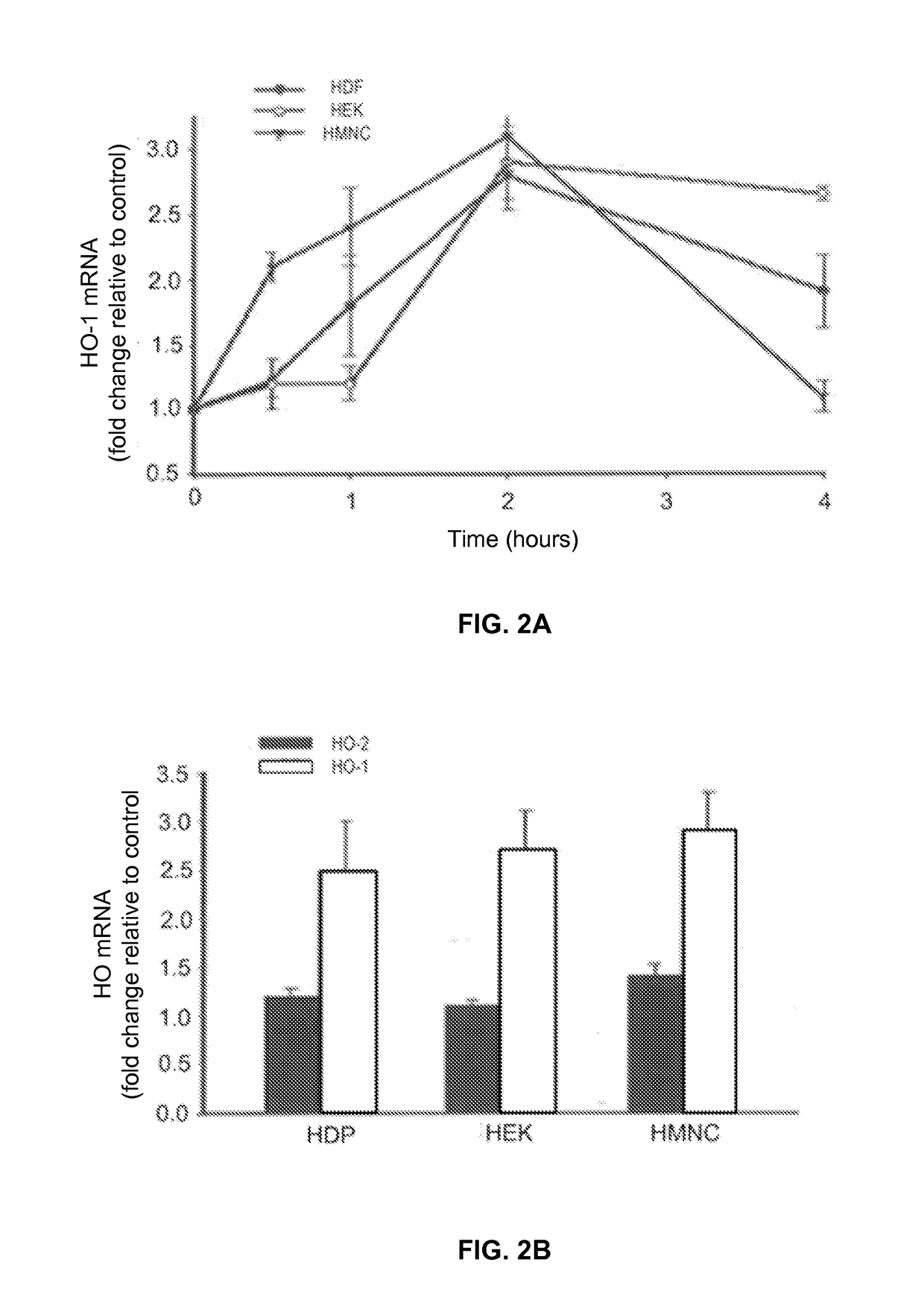
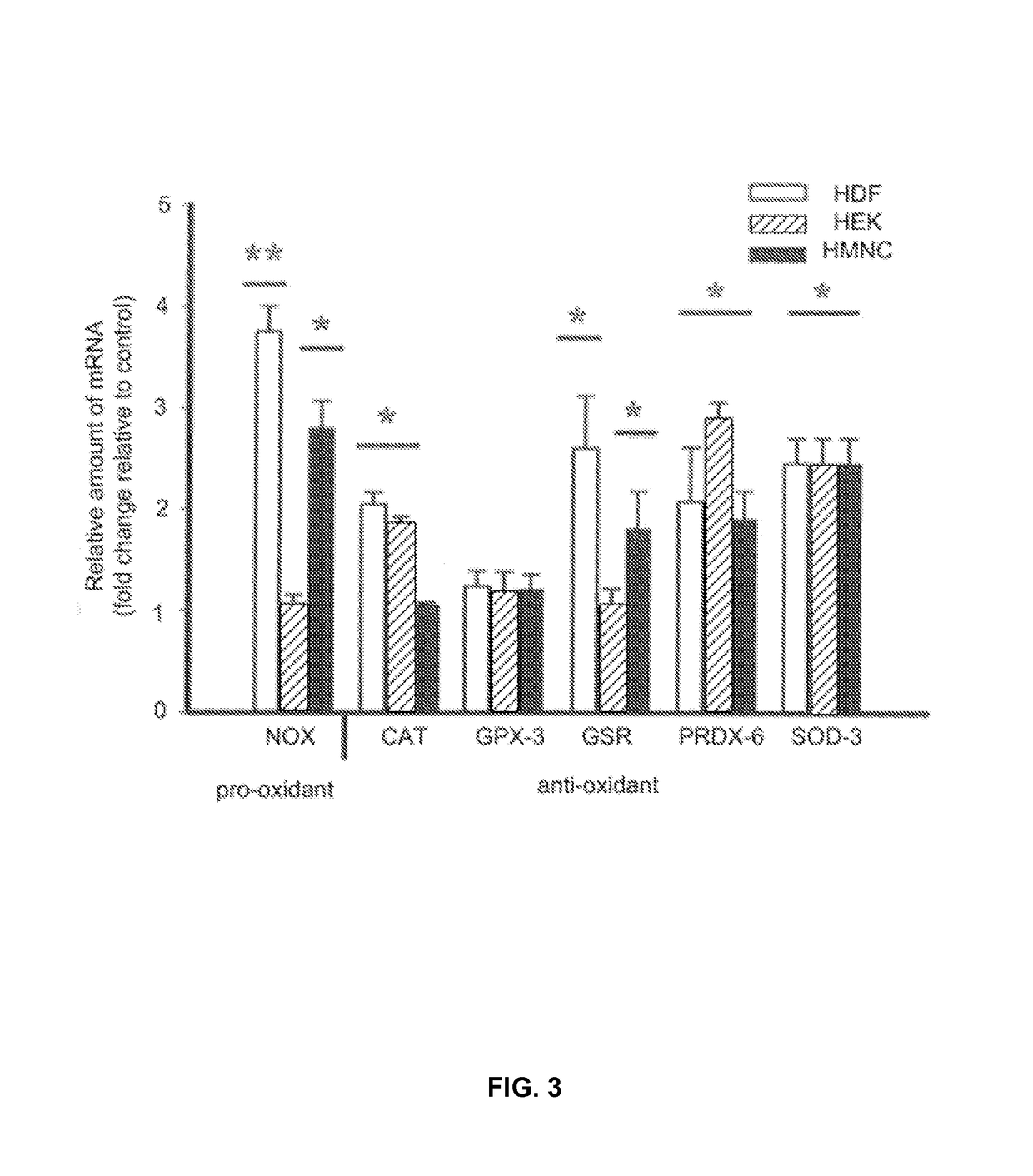
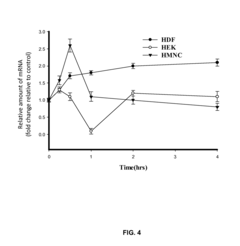
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
Method and apparatus for treatment of benign prostatic hyperplasia (BPH)
PatentInactiveUS20230398368A1
Innovation
- A non-invasive method utilizing pulsed electromagnetic field (PEMF) stimulation to increase the number of A2a receptors on cell membranes, enhancing the anti-inflammatory effects of adenosine and providing immunosuppressive action to reduce chronic inflammation and tissue damage in the prostate.
Clinical Trial Landscape
The clinical trial landscape for Pulsed Electromagnetic Field (PEMF) therapy has been evolving rapidly in recent years, reflecting the growing interest in this non-invasive treatment modality. A comprehensive analysis of the current state of clinical trials reveals several key trends and insights that are shaping the scientific validation of PEMF therapy across various treatment paradigms.
One notable trend is the increasing number of registered clinical trials focusing on PEMF therapy. Over the past decade, there has been a significant uptick in both the quantity and quality of studies, with a particular emphasis on randomized controlled trials (RCTs). These RCTs are crucial for establishing the efficacy and safety of PEMF therapy in various medical conditions, ranging from musculoskeletal disorders to neurological ailments.
The diversity of medical conditions being investigated in PEMF clinical trials is expanding. While early studies primarily focused on bone healing and pain management, recent trials have broadened the scope to include areas such as wound healing, neurological disorders, and even mental health conditions. This expansion reflects the growing recognition of PEMF's potential therapeutic applications across multiple medical disciplines.
A significant portion of ongoing clinical trials is dedicated to optimizing PEMF treatment parameters. Researchers are systematically exploring variables such as frequency, intensity, duration, and treatment schedules to determine the most effective protocols for specific conditions. This focus on parameter optimization is crucial for standardizing PEMF therapy and enhancing its reproducibility across different clinical settings.
Interestingly, there is an emerging trend towards combining PEMF therapy with other treatment modalities in clinical trials. Researchers are investigating the synergistic effects of PEMF when used in conjunction with conventional therapies, such as physical therapy, pharmacological interventions, or other forms of electromagnetic stimulation. These combination studies aim to enhance overall treatment efficacy and potentially reduce reliance on pharmaceutical interventions.
The geographical distribution of PEMF clinical trials is also noteworthy. While a significant number of studies are conducted in North America and Europe, there is a growing interest in PEMF research in Asia and other regions. This global spread of clinical trials contributes to a more diverse and comprehensive understanding of PEMF therapy's efficacy across different populations and healthcare systems.
In terms of study design, there is an increasing emphasis on long-term follow-up in PEMF clinical trials. Researchers are recognizing the importance of assessing not only the immediate effects of PEMF therapy but also its long-term outcomes and potential for sustained benefits. This trend towards longitudinal studies is crucial for establishing the durability of PEMF treatment effects and its potential role in chronic disease management.
One notable trend is the increasing number of registered clinical trials focusing on PEMF therapy. Over the past decade, there has been a significant uptick in both the quantity and quality of studies, with a particular emphasis on randomized controlled trials (RCTs). These RCTs are crucial for establishing the efficacy and safety of PEMF therapy in various medical conditions, ranging from musculoskeletal disorders to neurological ailments.
The diversity of medical conditions being investigated in PEMF clinical trials is expanding. While early studies primarily focused on bone healing and pain management, recent trials have broadened the scope to include areas such as wound healing, neurological disorders, and even mental health conditions. This expansion reflects the growing recognition of PEMF's potential therapeutic applications across multiple medical disciplines.
A significant portion of ongoing clinical trials is dedicated to optimizing PEMF treatment parameters. Researchers are systematically exploring variables such as frequency, intensity, duration, and treatment schedules to determine the most effective protocols for specific conditions. This focus on parameter optimization is crucial for standardizing PEMF therapy and enhancing its reproducibility across different clinical settings.
Interestingly, there is an emerging trend towards combining PEMF therapy with other treatment modalities in clinical trials. Researchers are investigating the synergistic effects of PEMF when used in conjunction with conventional therapies, such as physical therapy, pharmacological interventions, or other forms of electromagnetic stimulation. These combination studies aim to enhance overall treatment efficacy and potentially reduce reliance on pharmaceutical interventions.
The geographical distribution of PEMF clinical trials is also noteworthy. While a significant number of studies are conducted in North America and Europe, there is a growing interest in PEMF research in Asia and other regions. This global spread of clinical trials contributes to a more diverse and comprehensive understanding of PEMF therapy's efficacy across different populations and healthcare systems.
In terms of study design, there is an increasing emphasis on long-term follow-up in PEMF clinical trials. Researchers are recognizing the importance of assessing not only the immediate effects of PEMF therapy but also its long-term outcomes and potential for sustained benefits. This trend towards longitudinal studies is crucial for establishing the durability of PEMF treatment effects and its potential role in chronic disease management.
Regulatory Framework
The regulatory framework surrounding Pulsed Electromagnetic Field (PEMF) therapy plays a crucial role in its scientific validation and adoption in treatment paradigms. In the United States, the Food and Drug Administration (FDA) oversees the regulation of PEMF devices, classifying them as Class II medical devices. This classification requires manufacturers to demonstrate safety and efficacy through clinical trials before obtaining market approval.
The FDA has approved several PEMF devices for specific indications, such as bone healing and pain management. However, the regulatory landscape for PEMF therapy remains complex, with varying requirements for different applications and treatment modalities. Manufacturers must navigate a rigorous process of premarket notification (510(k)) or premarket approval (PMA) depending on the intended use and risk profile of their devices.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements for clinical evidence and post-market surveillance compared to its predecessor, the Medical Device Directive (MDD). Manufacturers must obtain CE marking to market their PEMF devices in the EU, demonstrating compliance with safety and performance standards.
Globally, regulatory bodies have different approaches to PEMF therapy. Some countries have embraced the technology more readily, while others maintain stricter controls. This variability in regulatory frameworks presents challenges for international research collaboration and market expansion.
The regulatory landscape also influences the design and conduct of clinical trials for PEMF therapy. Researchers must adhere to Good Clinical Practice (GCP) guidelines and obtain approval from institutional review boards (IRBs) or ethics committees. The quality and rigor of these trials directly impact the scientific validation of PEMF therapy and its acceptance in treatment paradigms.
As the field of PEMF therapy evolves, regulatory bodies are adapting their frameworks to accommodate new technologies and applications. This includes addressing concerns about electromagnetic field exposure and potential long-term effects. The development of international standards and harmonization efforts aim to streamline the regulatory process and facilitate global adoption of scientifically validated PEMF therapies.
The FDA has approved several PEMF devices for specific indications, such as bone healing and pain management. However, the regulatory landscape for PEMF therapy remains complex, with varying requirements for different applications and treatment modalities. Manufacturers must navigate a rigorous process of premarket notification (510(k)) or premarket approval (PMA) depending on the intended use and risk profile of their devices.
In the European Union, PEMF devices fall under the Medical Device Regulation (MDR), which came into effect in May 2021. The MDR imposes stricter requirements for clinical evidence and post-market surveillance compared to its predecessor, the Medical Device Directive (MDD). Manufacturers must obtain CE marking to market their PEMF devices in the EU, demonstrating compliance with safety and performance standards.
Globally, regulatory bodies have different approaches to PEMF therapy. Some countries have embraced the technology more readily, while others maintain stricter controls. This variability in regulatory frameworks presents challenges for international research collaboration and market expansion.
The regulatory landscape also influences the design and conduct of clinical trials for PEMF therapy. Researchers must adhere to Good Clinical Practice (GCP) guidelines and obtain approval from institutional review boards (IRBs) or ethics committees. The quality and rigor of these trials directly impact the scientific validation of PEMF therapy and its acceptance in treatment paradigms.
As the field of PEMF therapy evolves, regulatory bodies are adapting their frameworks to accommodate new technologies and applications. This includes addressing concerns about electromagnetic field exposure and potential long-term effects. The development of international standards and harmonization efforts aim to streamline the regulatory process and facilitate global adoption of scientifically validated PEMF therapies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!