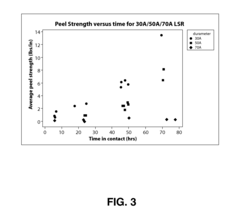
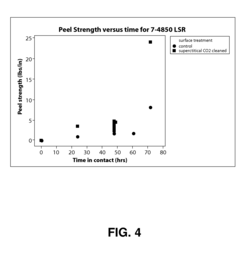
Silicone Rubber Applications in Medical Devices
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicone Rubber in Medical Devices: Overview and Objectives
Silicone rubber has emerged as a pivotal material in the medical device industry, revolutionizing the design and functionality of numerous healthcare products. This versatile elastomer offers a unique combination of properties that make it exceptionally suitable for medical applications. Its biocompatibility, chemical inertness, and flexibility have positioned silicone rubber as a preferred choice for manufacturers and healthcare professionals alike.
The evolution of silicone rubber in medical devices can be traced back to the mid-20th century, with significant advancements occurring in recent decades. Initially used in simple applications such as tubing and seals, silicone rubber has since found its way into complex medical devices, including implants, prosthetics, and drug delivery systems. This progression highlights the material's adaptability and the continuous innovation in its formulation and processing techniques.
The primary objective of utilizing silicone rubber in medical devices is to enhance patient care and improve treatment outcomes. By leveraging its unique properties, manufacturers aim to develop devices that are safer, more comfortable, and more effective than their predecessors. Silicone rubber's ability to be molded into intricate shapes, its resistance to bacterial growth, and its durability under various environmental conditions contribute significantly to achieving these goals.
Another crucial objective is to meet the stringent regulatory requirements imposed on medical devices. Silicone rubber's well-documented safety profile and its ability to maintain its properties over extended periods make it an attractive option for devices that require long-term implantation or repeated sterilization. This aspect is particularly important as the medical industry faces increasing scrutiny regarding the long-term safety and efficacy of materials used in healthcare.
The future trajectory of silicone rubber in medical devices is focused on further expanding its capabilities and applications. Research efforts are directed towards enhancing its mechanical properties, improving its integration with electronic components for smart medical devices, and exploring its potential in emerging fields such as 3D-printed medical implants. These advancements aim to address unmet medical needs and push the boundaries of what is possible in healthcare technology.
As we delve deeper into the applications of silicone rubber in medical devices, it becomes evident that this material plays a crucial role in driving innovation and improving patient outcomes across various medical specialties. From cardiovascular devices to orthopedic implants, silicone rubber continues to demonstrate its versatility and indispensability in modern healthcare solutions.
The evolution of silicone rubber in medical devices can be traced back to the mid-20th century, with significant advancements occurring in recent decades. Initially used in simple applications such as tubing and seals, silicone rubber has since found its way into complex medical devices, including implants, prosthetics, and drug delivery systems. This progression highlights the material's adaptability and the continuous innovation in its formulation and processing techniques.
The primary objective of utilizing silicone rubber in medical devices is to enhance patient care and improve treatment outcomes. By leveraging its unique properties, manufacturers aim to develop devices that are safer, more comfortable, and more effective than their predecessors. Silicone rubber's ability to be molded into intricate shapes, its resistance to bacterial growth, and its durability under various environmental conditions contribute significantly to achieving these goals.
Another crucial objective is to meet the stringent regulatory requirements imposed on medical devices. Silicone rubber's well-documented safety profile and its ability to maintain its properties over extended periods make it an attractive option for devices that require long-term implantation or repeated sterilization. This aspect is particularly important as the medical industry faces increasing scrutiny regarding the long-term safety and efficacy of materials used in healthcare.
The future trajectory of silicone rubber in medical devices is focused on further expanding its capabilities and applications. Research efforts are directed towards enhancing its mechanical properties, improving its integration with electronic components for smart medical devices, and exploring its potential in emerging fields such as 3D-printed medical implants. These advancements aim to address unmet medical needs and push the boundaries of what is possible in healthcare technology.
As we delve deeper into the applications of silicone rubber in medical devices, it becomes evident that this material plays a crucial role in driving innovation and improving patient outcomes across various medical specialties. From cardiovascular devices to orthopedic implants, silicone rubber continues to demonstrate its versatility and indispensability in modern healthcare solutions.
Market Analysis for Silicone-Based Medical Products
The global market for silicone-based medical products has experienced significant growth in recent years, driven by the increasing demand for advanced medical devices and the unique properties of silicone rubber. This market segment is expected to continue its upward trajectory, with projections indicating robust growth over the next decade.
Silicone rubber's biocompatibility, durability, and versatility make it an ideal material for a wide range of medical applications. The market for silicone-based medical products spans various sectors, including implantable devices, wound care, drug delivery systems, and medical equipment components. Each of these sectors contributes to the overall market growth, with implantable devices and wound care products showing particularly strong demand.
The healthcare industry's shift towards minimally invasive procedures and the growing aging population are key factors driving the demand for silicone-based medical products. Silicone's ability to be molded into complex shapes and its resistance to bacterial growth make it particularly suitable for these applications. Additionally, the increasing prevalence of chronic diseases and the need for long-term medical care solutions further boost market demand.
Geographically, North America and Europe currently dominate the market for silicone-based medical products, owing to their advanced healthcare infrastructure and high healthcare expenditure. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by improving healthcare access, rising disposable incomes, and increasing awareness of advanced medical technologies.
The market is characterized by intense competition among key players, including major silicone manufacturers and specialized medical device companies. These companies are investing heavily in research and development to innovate new products and improve existing ones, further driving market growth. Collaborations between silicone manufacturers and medical device companies are becoming increasingly common, leading to the development of more sophisticated and effective medical solutions.
Regulatory factors play a crucial role in shaping the market landscape for silicone-based medical products. Stringent approval processes and quality standards set by regulatory bodies such as the FDA and EMA ensure product safety and efficacy but can also impact market entry and product development timelines. Companies that can navigate these regulatory challenges effectively are likely to gain a competitive edge in the market.
Looking ahead, emerging technologies such as 3D printing and nanotechnology are expected to open up new opportunities for silicone-based medical products. These advancements could lead to more personalized medical solutions and expand the application scope of silicone in the medical field, potentially revolutionizing patient care and treatment outcomes.
Silicone rubber's biocompatibility, durability, and versatility make it an ideal material for a wide range of medical applications. The market for silicone-based medical products spans various sectors, including implantable devices, wound care, drug delivery systems, and medical equipment components. Each of these sectors contributes to the overall market growth, with implantable devices and wound care products showing particularly strong demand.
The healthcare industry's shift towards minimally invasive procedures and the growing aging population are key factors driving the demand for silicone-based medical products. Silicone's ability to be molded into complex shapes and its resistance to bacterial growth make it particularly suitable for these applications. Additionally, the increasing prevalence of chronic diseases and the need for long-term medical care solutions further boost market demand.
Geographically, North America and Europe currently dominate the market for silicone-based medical products, owing to their advanced healthcare infrastructure and high healthcare expenditure. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by improving healthcare access, rising disposable incomes, and increasing awareness of advanced medical technologies.
The market is characterized by intense competition among key players, including major silicone manufacturers and specialized medical device companies. These companies are investing heavily in research and development to innovate new products and improve existing ones, further driving market growth. Collaborations between silicone manufacturers and medical device companies are becoming increasingly common, leading to the development of more sophisticated and effective medical solutions.
Regulatory factors play a crucial role in shaping the market landscape for silicone-based medical products. Stringent approval processes and quality standards set by regulatory bodies such as the FDA and EMA ensure product safety and efficacy but can also impact market entry and product development timelines. Companies that can navigate these regulatory challenges effectively are likely to gain a competitive edge in the market.
Looking ahead, emerging technologies such as 3D printing and nanotechnology are expected to open up new opportunities for silicone-based medical products. These advancements could lead to more personalized medical solutions and expand the application scope of silicone in the medical field, potentially revolutionizing patient care and treatment outcomes.
Current Challenges in Silicone Rubber Medical Applications
Despite the widespread use of silicone rubber in medical devices, several challenges persist in its application. One of the primary concerns is the potential for material degradation over time, which can lead to compromised device performance and safety issues. Silicone rubber, when exposed to certain environmental factors or chemical agents, may undergo changes in its physical and mechanical properties, affecting its durability and functionality.
Another significant challenge is the biocompatibility of silicone rubber in long-term implantable devices. While generally considered biocompatible, there are instances where patients develop adverse reactions or sensitivities to silicone-based implants. This has led to ongoing research into improving the biocompatibility of silicone rubber and developing alternative materials for sensitive applications.
The sterilization of silicone rubber medical devices presents another hurdle. Traditional sterilization methods, such as high-temperature autoclaving or radiation, can potentially alter the material's properties or accelerate its degradation. This necessitates the development of specialized sterilization techniques that maintain the integrity of silicone rubber components without compromising their performance or longevity.
Manufacturing consistency and quality control pose additional challenges in silicone rubber medical applications. Ensuring uniform material properties across different production batches is crucial for maintaining device reliability and regulatory compliance. Variations in raw material quality, processing conditions, and curing methods can all impact the final product's characteristics, requiring stringent quality assurance protocols.
The integration of silicone rubber with other materials in complex medical devices also presents technical difficulties. Achieving strong, durable bonds between silicone and dissimilar materials, such as metals or other polymers, often requires specialized surface treatments or adhesive systems. These bonding challenges can affect device assembly, performance, and long-term reliability.
Furthermore, the development of advanced functionalities in silicone rubber, such as drug-eluting capabilities or smart, responsive properties, is an ongoing challenge. Incorporating active pharmaceutical ingredients or sensors into silicone rubber matrices while maintaining material integrity and controlled release profiles requires sophisticated engineering and formulation techniques.
Lastly, regulatory compliance and documentation for silicone rubber medical devices continue to evolve, presenting ongoing challenges for manufacturers. Stringent requirements for material characterization, biocompatibility testing, and long-term performance data necessitate extensive research and development efforts to bring new silicone rubber-based medical devices to market.
Another significant challenge is the biocompatibility of silicone rubber in long-term implantable devices. While generally considered biocompatible, there are instances where patients develop adverse reactions or sensitivities to silicone-based implants. This has led to ongoing research into improving the biocompatibility of silicone rubber and developing alternative materials for sensitive applications.
The sterilization of silicone rubber medical devices presents another hurdle. Traditional sterilization methods, such as high-temperature autoclaving or radiation, can potentially alter the material's properties or accelerate its degradation. This necessitates the development of specialized sterilization techniques that maintain the integrity of silicone rubber components without compromising their performance or longevity.
Manufacturing consistency and quality control pose additional challenges in silicone rubber medical applications. Ensuring uniform material properties across different production batches is crucial for maintaining device reliability and regulatory compliance. Variations in raw material quality, processing conditions, and curing methods can all impact the final product's characteristics, requiring stringent quality assurance protocols.
The integration of silicone rubber with other materials in complex medical devices also presents technical difficulties. Achieving strong, durable bonds between silicone and dissimilar materials, such as metals or other polymers, often requires specialized surface treatments or adhesive systems. These bonding challenges can affect device assembly, performance, and long-term reliability.
Furthermore, the development of advanced functionalities in silicone rubber, such as drug-eluting capabilities or smart, responsive properties, is an ongoing challenge. Incorporating active pharmaceutical ingredients or sensors into silicone rubber matrices while maintaining material integrity and controlled release profiles requires sophisticated engineering and formulation techniques.
Lastly, regulatory compliance and documentation for silicone rubber medical devices continue to evolve, presenting ongoing challenges for manufacturers. Stringent requirements for material characterization, biocompatibility testing, and long-term performance data necessitate extensive research and development efforts to bring new silicone rubber-based medical devices to market.
Existing Silicone Rubber Solutions for Medical Devices
01 Composition and preparation of silicone rubber
Silicone rubber is typically composed of silicone polymers, fillers, and curing agents. The preparation process often involves mixing these components, shaping the mixture, and then curing it to form the final rubber product. Various additives can be incorporated to enhance specific properties such as strength, heat resistance, or electrical insulation.- Composition and preparation of silicone rubber: Silicone rubber is typically composed of silicone polymers, fillers, and curing agents. The preparation process often involves mixing these components, shaping the mixture, and then curing it to form the final rubber product. Various additives can be incorporated to modify properties such as strength, flexibility, and heat resistance.
- Modification of silicone rubber properties: The properties of silicone rubber can be modified through the addition of specific compounds or by altering the polymer structure. This can include improving thermal stability, enhancing electrical properties, or increasing chemical resistance. Techniques may involve blending with other polymers or incorporating functional groups into the silicone backbone.
- Applications of silicone rubber: Silicone rubber finds wide applications across various industries due to its unique properties. It is used in medical devices, automotive parts, electrical insulation, cookware, and construction materials. Its biocompatibility, heat resistance, and flexibility make it suitable for diverse applications ranging from prosthetics to gaskets and seals.
- Processing and manufacturing techniques: Various processing techniques are employed in the manufacturing of silicone rubber products. These may include extrusion, injection molding, compression molding, and liquid injection molding. Each method is suited to different product types and production volumes, and may require specific formulations or equipment.
- Innovations in silicone rubber technology: Recent innovations in silicone rubber technology focus on enhancing performance characteristics and expanding application areas. This includes developing self-healing silicone rubbers, improving adhesion properties, creating conductive silicone composites, and formulating silicone rubbers with extreme temperature resistance or unique optical properties.
02 Modification of silicone rubber properties
The properties of silicone rubber can be modified through the addition of specific compounds or by altering the molecular structure. This can include improving heat resistance, increasing tensile strength, enhancing electrical properties, or modifying the surface characteristics. Such modifications allow for the customization of silicone rubber for various applications.Expand Specific Solutions03 Silicone rubber in medical and healthcare applications
Silicone rubber is widely used in medical and healthcare applications due to its biocompatibility, flexibility, and durability. It is used in various medical devices, implants, and prosthetics. The material can be formulated to meet specific requirements such as sterilization resistance and long-term stability in the human body.Expand Specific Solutions04 Silicone rubber in electronic and electrical applications
Silicone rubber finds extensive use in electronic and electrical applications due to its excellent insulating properties and temperature resistance. It is used in cable insulation, connectors, gaskets, and seals for electronic devices. The material can be formulated to provide specific dielectric properties or to dissipate heat effectively.Expand Specific Solutions05 Environmental and sustainability aspects of silicone rubber
Research is ongoing to improve the environmental profile of silicone rubber, including developing bio-based alternatives, improving recycling methods, and reducing the environmental impact of production processes. This includes efforts to create more sustainable curing methods and to incorporate renewable resources into silicone rubber formulations.Expand Specific Solutions
Key Players in Medical-Grade Silicone Industry
The silicone rubber applications in medical devices market is in a growth phase, driven by increasing demand for advanced medical equipment and an aging population. The global market size is projected to reach several billion dollars by 2025, with a compound annual growth rate of around 6-8%. Technologically, silicone rubber for medical applications is relatively mature, but ongoing innovations are enhancing its properties and expanding its uses. Key players like Shin-Etsu Chemical, Momentive Performance Materials, and Sumitomo Bakelite are leading in product development and market share. Major medical device manufacturers such as Medtronic and St. Jude Medical are also significant stakeholders, integrating silicone rubber components into their products. The competitive landscape is characterized by a mix of large chemical companies and specialized medical material suppliers, with increasing focus on biocompatibility and customization for specific medical applications.
Shin-Etsu Chemical Co., Ltd.
Technical Solution: Shin-Etsu Chemical Co., Ltd. has developed advanced silicone rubber compounds specifically tailored for medical device applications. Their SILPOT™ series of liquid silicone rubbers (LSRs) offers excellent biocompatibility, high purity, and customizable mechanical properties[1]. These LSRs are used in various medical devices, including catheters, respiratory masks, and implantable components. Shin-Etsu's silicone rubbers undergo rigorous testing to meet FDA and ISO 10993 standards for biocompatibility[2]. The company has also introduced self-adhesive LSRs that can bond directly to thermoplastics during injection molding, streamlining the production of complex medical devices[3]. Additionally, Shin-Etsu has developed antimicrobial silicone rubbers incorporating silver-based additives, which provide long-lasting protection against bacterial growth on medical device surfaces[4].
Strengths: Extensive experience in silicone technology, wide range of medical-grade silicone products, strong focus on innovation and customization. Weaknesses: Potential higher costs compared to some competitors, reliance on global supply chains for raw materials.
Momentive Performance Materials, Inc.
Technical Solution: Momentive Performance Materials has developed a comprehensive range of silicone elastomers for medical applications under their Silopren™ brand. Their liquid silicone rubber (LSR) technology offers excellent processability and consistency for high-volume production of medical devices[1]. Momentive's LSRs feature adjustable durometers ranging from ultra-soft to rigid, allowing for precise tuning of mechanical properties for specific medical applications[2]. The company has also introduced self-lubricating silicone rubbers that reduce friction in medical devices like catheters and syringes, improving patient comfort and device performance[3]. Momentive's silicone elastomers are engineered to meet stringent biocompatibility requirements and can withstand various sterilization methods, including gamma radiation and ethylene oxide[4]. Additionally, they have developed specialty silicone formulations with enhanced tear strength and elongation properties for applications in prosthetics and wearable medical devices[5].
Strengths: Broad portfolio of medical-grade silicone products, strong R&D capabilities, global manufacturing presence. Weaknesses: Potential vulnerability to market fluctuations, competition from other major silicone manufacturers.
Innovative Silicone Rubber Formulations for Healthcare
Medical devices with plasma-treated surface and methods
PatentInactiveUS20120035544A1
Innovation
- A medical device with a sealing apparatus featuring plasma-treated surfaces to immobilize mobile species, reducing adhesion and self-healing by creating an oxygen-rich surface that pins low molecular weight silicone oligomers, thereby preventing bond interchange and stress relaxation.
Silicone rubber-based curable composition, method of producing silicone rubber, silicone rubber, molded body and medical tube
PatentInactiveUS20140242312A1
Innovation
- A silicone rubber-based curable composition comprising a combination of vinyl group-containing organopolysiloxanes, organohydrogen polysiloxanes, silica particles, and a silane coupling agent, with specific molecular structures and ratios to enhance crosslink density and mechanical properties, such as the use of both linear and branch organopolysiloxanes and a silane coupling agent with hydrolysable and vinyl groups.
Regulatory Framework for Silicone in Medical Devices
The regulatory framework for silicone in medical devices is a complex and evolving landscape that manufacturers must navigate to ensure compliance and patient safety. In the United States, the Food and Drug Administration (FDA) plays a central role in regulating medical devices containing silicone materials. The FDA classifies medical devices into three categories based on their risk level, with Class III devices being the most stringently regulated.
For silicone-based medical devices, manufacturers must adhere to the premarket approval (PMA) process for high-risk devices or the 510(k) clearance pathway for lower-risk devices. The PMA process requires extensive clinical data to demonstrate safety and efficacy, while the 510(k) pathway focuses on demonstrating substantial equivalence to a predicate device already on the market.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of silicone in medical devices. These regulations emphasize a life-cycle approach to device safety and performance, requiring manufacturers to implement robust post-market surveillance systems and maintain up-to-date technical documentation.
The International Organization for Standardization (ISO) provides several standards relevant to silicone in medical devices, including ISO 10993 for biocompatibility testing and ISO 14971 for risk management. These standards are often recognized by regulatory bodies and serve as important guidelines for manufacturers in developing and testing silicone-based medical devices.
Regulatory requirements also extend to the manufacturing processes of silicone medical devices. Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) are essential components of regulatory compliance. Manufacturers must implement and maintain these systems to ensure consistent product quality and traceability throughout the production process.
As the use of silicone in medical devices continues to expand, regulatory bodies are increasingly focusing on long-term safety data and potential risks associated with silicone implants. This has led to more stringent post-market surveillance requirements and the need for manufacturers to conduct long-term clinical studies to monitor device performance and patient outcomes.
Emerging technologies and novel applications of silicone in medical devices may require adaptations to existing regulatory frameworks. Regulatory bodies are working to develop guidance documents and standards that address the unique challenges posed by innovative silicone-based medical technologies, such as combination products or devices incorporating nanotechnology.
For silicone-based medical devices, manufacturers must adhere to the premarket approval (PMA) process for high-risk devices or the 510(k) clearance pathway for lower-risk devices. The PMA process requires extensive clinical data to demonstrate safety and efficacy, while the 510(k) pathway focuses on demonstrating substantial equivalence to a predicate device already on the market.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the use of silicone in medical devices. These regulations emphasize a life-cycle approach to device safety and performance, requiring manufacturers to implement robust post-market surveillance systems and maintain up-to-date technical documentation.
The International Organization for Standardization (ISO) provides several standards relevant to silicone in medical devices, including ISO 10993 for biocompatibility testing and ISO 14971 for risk management. These standards are often recognized by regulatory bodies and serve as important guidelines for manufacturers in developing and testing silicone-based medical devices.
Regulatory requirements also extend to the manufacturing processes of silicone medical devices. Good Manufacturing Practices (GMP) and Quality Management Systems (QMS) are essential components of regulatory compliance. Manufacturers must implement and maintain these systems to ensure consistent product quality and traceability throughout the production process.
As the use of silicone in medical devices continues to expand, regulatory bodies are increasingly focusing on long-term safety data and potential risks associated with silicone implants. This has led to more stringent post-market surveillance requirements and the need for manufacturers to conduct long-term clinical studies to monitor device performance and patient outcomes.
Emerging technologies and novel applications of silicone in medical devices may require adaptations to existing regulatory frameworks. Regulatory bodies are working to develop guidance documents and standards that address the unique challenges posed by innovative silicone-based medical technologies, such as combination products or devices incorporating nanotechnology.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the application of silicone rubber in medical devices. The material's interaction with biological systems must be thoroughly evaluated to ensure patient safety and regulatory compliance.
Silicone rubber's biocompatibility is attributed to its chemical inertness, low toxicity, and resistance to degradation in biological environments. These properties make it suitable for both short-term and long-term implantable devices. However, the specific formulation of silicone rubber used in medical applications must undergo rigorous testing to confirm its safety profile.
The evaluation of biocompatibility typically involves a series of standardized tests outlined in ISO 10993, which covers various aspects of biological safety. These tests assess cytotoxicity, sensitization, irritation, systemic toxicity, and hemocompatibility, among others. The selection of specific tests depends on the intended use and duration of contact with the body.
One critical safety consideration is the potential for leaching of low molecular weight siloxanes or other additives from the silicone rubber. While silicone rubber is generally considered stable, certain conditions may lead to the release of these compounds. Manufacturers must carefully control the curing process and select appropriate additives to minimize this risk.
The sterilization compatibility of silicone rubber is another crucial factor. Common sterilization methods such as ethylene oxide, gamma irradiation, and steam autoclaving can affect the material's properties. Manufacturers must validate that the chosen sterilization method does not compromise the silicone rubber's integrity or introduce harmful byproducts.
Long-term implantable devices made from silicone rubber require additional scrutiny. Factors such as biodurability, resistance to calcification, and potential for capsular contracture in breast implants must be thoroughly investigated. The material's ability to maintain its mechanical and chemical properties over extended periods in vivo is essential for ensuring device performance and patient safety.
Regulatory bodies, including the FDA and EMA, have established stringent guidelines for the use of silicone rubber in medical devices. Manufacturers must provide comprehensive data on biocompatibility, toxicological assessments, and clinical performance to obtain regulatory approval. This process often involves extensive pre-clinical and clinical studies to demonstrate the safety and efficacy of silicone rubber-based medical devices.
In recent years, there has been increased focus on the potential health effects of silicone implants, particularly in the context of breast implants. While the overall safety profile of silicone rubber remains favorable, ongoing research continues to investigate rare complications and long-term effects. This underscores the importance of post-market surveillance and continuous evaluation of biocompatibility and safety data.
Silicone rubber's biocompatibility is attributed to its chemical inertness, low toxicity, and resistance to degradation in biological environments. These properties make it suitable for both short-term and long-term implantable devices. However, the specific formulation of silicone rubber used in medical applications must undergo rigorous testing to confirm its safety profile.
The evaluation of biocompatibility typically involves a series of standardized tests outlined in ISO 10993, which covers various aspects of biological safety. These tests assess cytotoxicity, sensitization, irritation, systemic toxicity, and hemocompatibility, among others. The selection of specific tests depends on the intended use and duration of contact with the body.
One critical safety consideration is the potential for leaching of low molecular weight siloxanes or other additives from the silicone rubber. While silicone rubber is generally considered stable, certain conditions may lead to the release of these compounds. Manufacturers must carefully control the curing process and select appropriate additives to minimize this risk.
The sterilization compatibility of silicone rubber is another crucial factor. Common sterilization methods such as ethylene oxide, gamma irradiation, and steam autoclaving can affect the material's properties. Manufacturers must validate that the chosen sterilization method does not compromise the silicone rubber's integrity or introduce harmful byproducts.
Long-term implantable devices made from silicone rubber require additional scrutiny. Factors such as biodurability, resistance to calcification, and potential for capsular contracture in breast implants must be thoroughly investigated. The material's ability to maintain its mechanical and chemical properties over extended periods in vivo is essential for ensuring device performance and patient safety.
Regulatory bodies, including the FDA and EMA, have established stringent guidelines for the use of silicone rubber in medical devices. Manufacturers must provide comprehensive data on biocompatibility, toxicological assessments, and clinical performance to obtain regulatory approval. This process often involves extensive pre-clinical and clinical studies to demonstrate the safety and efficacy of silicone rubber-based medical devices.
In recent years, there has been increased focus on the potential health effects of silicone implants, particularly in the context of breast implants. While the overall safety profile of silicone rubber remains favorable, ongoing research continues to investigate rare complications and long-term effects. This underscores the importance of post-market surveillance and continuous evaluation of biocompatibility and safety data.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!