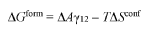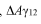Triton X-100 in Studies of Electrostatic and Steric Stabilization Mechanisms
JUL 31, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 Background and Research Objectives
Triton X-100, a nonionic surfactant, has been a subject of extensive research in the field of colloidal science and nanotechnology for several decades. This versatile compound, with its unique molecular structure consisting of a hydrophilic polyethylene oxide chain and a hydrophobic aromatic hydrocarbon group, has found widespread applications in various industries, including pharmaceuticals, biotechnology, and materials science.
The evolution of Triton X-100 research can be traced back to the mid-20th century when scientists began exploring its surface-active properties. Initially, it was primarily used as a detergent and emulsifier in laboratory settings. However, as the understanding of colloidal systems advanced, researchers recognized the potential of Triton X-100 in stabilizing dispersions and controlling interfacial phenomena.
In recent years, the focus of Triton X-100 research has shifted towards its role in electrostatic and steric stabilization mechanisms. These mechanisms are crucial for maintaining the stability of colloidal systems, preventing aggregation, and controlling particle interactions. The ability of Triton X-100 to adsorb onto particle surfaces and create a protective layer has made it an invaluable tool in the development of advanced materials and formulations.
The primary objective of current research on Triton X-100 is to elucidate its precise mechanisms of action in electrostatic and steric stabilization. This involves investigating how Triton X-100 molecules interact with different types of particles, the influence of concentration on stabilization efficiency, and the interplay between electrostatic repulsion and steric hindrance in various environmental conditions.
Furthermore, researchers aim to optimize the use of Triton X-100 in specific applications, such as drug delivery systems, nanoparticle synthesis, and emulsion stabilization. This requires a comprehensive understanding of how Triton X-100 behaves in complex multi-component systems and its compatibility with other additives and solvents.
Another important research goal is to explore the environmental impact and biodegradability of Triton X-100. As sustainability becomes increasingly important in industrial processes, there is a growing need to develop eco-friendly alternatives or modify existing surfactants to reduce their environmental footprint.
The study of Triton X-100 in electrostatic and steric stabilization mechanisms also has broader implications for the field of soft matter physics and colloid science. By unraveling the fundamental principles governing these stabilization processes, researchers hope to develop predictive models and design guidelines for creating stable colloidal systems with tailored properties.
The evolution of Triton X-100 research can be traced back to the mid-20th century when scientists began exploring its surface-active properties. Initially, it was primarily used as a detergent and emulsifier in laboratory settings. However, as the understanding of colloidal systems advanced, researchers recognized the potential of Triton X-100 in stabilizing dispersions and controlling interfacial phenomena.
In recent years, the focus of Triton X-100 research has shifted towards its role in electrostatic and steric stabilization mechanisms. These mechanisms are crucial for maintaining the stability of colloidal systems, preventing aggregation, and controlling particle interactions. The ability of Triton X-100 to adsorb onto particle surfaces and create a protective layer has made it an invaluable tool in the development of advanced materials and formulations.
The primary objective of current research on Triton X-100 is to elucidate its precise mechanisms of action in electrostatic and steric stabilization. This involves investigating how Triton X-100 molecules interact with different types of particles, the influence of concentration on stabilization efficiency, and the interplay between electrostatic repulsion and steric hindrance in various environmental conditions.
Furthermore, researchers aim to optimize the use of Triton X-100 in specific applications, such as drug delivery systems, nanoparticle synthesis, and emulsion stabilization. This requires a comprehensive understanding of how Triton X-100 behaves in complex multi-component systems and its compatibility with other additives and solvents.
Another important research goal is to explore the environmental impact and biodegradability of Triton X-100. As sustainability becomes increasingly important in industrial processes, there is a growing need to develop eco-friendly alternatives or modify existing surfactants to reduce their environmental footprint.
The study of Triton X-100 in electrostatic and steric stabilization mechanisms also has broader implications for the field of soft matter physics and colloid science. By unraveling the fundamental principles governing these stabilization processes, researchers hope to develop predictive models and design guidelines for creating stable colloidal systems with tailored properties.
Market Analysis for Triton X-100 Applications
The market for Triton X-100 applications has shown significant growth in recent years, driven by its versatile properties as a non-ionic surfactant. This compound finds extensive use across various industries, including biotechnology, pharmaceuticals, and industrial cleaning. In the biotechnology sector, Triton X-100 plays a crucial role in cell lysis and protein extraction processes, contributing to the expanding field of proteomics and genomics research.
The pharmaceutical industry represents another major market for Triton X-100, where it is utilized in drug formulation and delivery systems. Its ability to enhance the solubility and stability of certain drugs has made it an invaluable component in pharmaceutical development. The growing emphasis on targeted drug delivery and personalized medicine is expected to further boost the demand for Triton X-100 in this sector.
In the industrial cleaning market, Triton X-100 is widely used as an effective detergent and emulsifier. Its excellent wetting and dispersing properties make it ideal for various cleaning applications, from household products to industrial degreasers. The increasing focus on environmentally friendly cleaning solutions has led to the development of Triton X-100-based formulations that meet stringent ecological standards.
The global market for Triton X-100 is closely tied to the overall growth of the surfactants industry. As emerging economies continue to industrialize and urbanize, the demand for surfactants, including Triton X-100, is projected to rise. This growth is particularly evident in Asia-Pacific regions, where rapid industrial development and increasing consumer awareness of hygiene products are driving market expansion.
However, the market for Triton X-100 also faces challenges. Environmental concerns regarding its biodegradability and potential ecological impact have led to increased scrutiny and regulatory pressure in some regions. This has spurred research into more environmentally friendly alternatives, which could potentially impact the long-term market dynamics for Triton X-100.
Despite these challenges, the market for Triton X-100 applications remains robust. The compound's unique properties in electrostatic and steric stabilization mechanisms continue to make it a preferred choice in many applications. As research in nanotechnology and advanced materials progresses, new applications for Triton X-100 are likely to emerge, potentially opening up new market segments and opportunities for growth.
The pharmaceutical industry represents another major market for Triton X-100, where it is utilized in drug formulation and delivery systems. Its ability to enhance the solubility and stability of certain drugs has made it an invaluable component in pharmaceutical development. The growing emphasis on targeted drug delivery and personalized medicine is expected to further boost the demand for Triton X-100 in this sector.
In the industrial cleaning market, Triton X-100 is widely used as an effective detergent and emulsifier. Its excellent wetting and dispersing properties make it ideal for various cleaning applications, from household products to industrial degreasers. The increasing focus on environmentally friendly cleaning solutions has led to the development of Triton X-100-based formulations that meet stringent ecological standards.
The global market for Triton X-100 is closely tied to the overall growth of the surfactants industry. As emerging economies continue to industrialize and urbanize, the demand for surfactants, including Triton X-100, is projected to rise. This growth is particularly evident in Asia-Pacific regions, where rapid industrial development and increasing consumer awareness of hygiene products are driving market expansion.
However, the market for Triton X-100 also faces challenges. Environmental concerns regarding its biodegradability and potential ecological impact have led to increased scrutiny and regulatory pressure in some regions. This has spurred research into more environmentally friendly alternatives, which could potentially impact the long-term market dynamics for Triton X-100.
Despite these challenges, the market for Triton X-100 applications remains robust. The compound's unique properties in electrostatic and steric stabilization mechanisms continue to make it a preferred choice in many applications. As research in nanotechnology and advanced materials progresses, new applications for Triton X-100 are likely to emerge, potentially opening up new market segments and opportunities for growth.
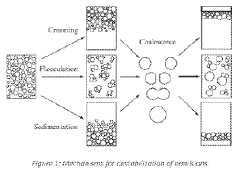
Current Challenges in Stabilization Mechanisms
The current challenges in stabilization mechanisms for Triton X-100 in electrostatic and steric stabilization are multifaceted and complex. One of the primary issues is the difficulty in achieving a delicate balance between electrostatic and steric stabilization effects. While Triton X-100 is known for its ability to provide both types of stabilization, optimizing their synergistic effects remains a significant challenge.
Electrostatic stabilization, which relies on the repulsion between charged particles, faces limitations in high ionic strength environments. As the concentration of ions in the solution increases, the electrostatic double layer around particles becomes compressed, reducing the effectiveness of this stabilization mechanism. This poses a particular challenge in applications where Triton X-100 is used in physiological or high-salt conditions.
Steric stabilization, on the other hand, encounters issues related to the conformation and density of the Triton X-100 molecules on particle surfaces. Achieving uniform coverage and maintaining the extended conformation of the surfactant molecules are crucial for effective steric stabilization. However, controlling these factors across different particle sizes and surface chemistries remains challenging.
Another significant hurdle is the temperature sensitivity of Triton X-100-based stabilization systems. The cloud point of Triton X-100, above which the surfactant becomes less soluble and may precipitate, can limit its effectiveness in certain temperature ranges. This temperature-dependent behavior complicates the use of Triton X-100 in applications requiring thermal stability or those involving temperature cycling.
The long-term stability of Triton X-100 stabilized systems is also a concern. Over time, surfactant molecules may desorb from particle surfaces or undergo chemical degradation, potentially leading to system instability. This is particularly problematic in applications requiring extended shelf life or prolonged functionality.
Furthermore, the interaction of Triton X-100 with other components in complex formulations presents additional challenges. The surfactant may compete for adsorption sites with other molecules or alter the properties of other stabilizing agents, leading to unexpected changes in overall system stability.
Lastly, there are growing environmental and regulatory concerns surrounding the use of Triton X-100. Its potential persistence in the environment and toxicity to aquatic organisms have led to increased scrutiny and restrictions in certain regions. This necessitates the development of more environmentally friendly alternatives that can match or exceed the stabilization performance of Triton X-100, adding another layer of complexity to the field.
Electrostatic stabilization, which relies on the repulsion between charged particles, faces limitations in high ionic strength environments. As the concentration of ions in the solution increases, the electrostatic double layer around particles becomes compressed, reducing the effectiveness of this stabilization mechanism. This poses a particular challenge in applications where Triton X-100 is used in physiological or high-salt conditions.
Steric stabilization, on the other hand, encounters issues related to the conformation and density of the Triton X-100 molecules on particle surfaces. Achieving uniform coverage and maintaining the extended conformation of the surfactant molecules are crucial for effective steric stabilization. However, controlling these factors across different particle sizes and surface chemistries remains challenging.
Another significant hurdle is the temperature sensitivity of Triton X-100-based stabilization systems. The cloud point of Triton X-100, above which the surfactant becomes less soluble and may precipitate, can limit its effectiveness in certain temperature ranges. This temperature-dependent behavior complicates the use of Triton X-100 in applications requiring thermal stability or those involving temperature cycling.
The long-term stability of Triton X-100 stabilized systems is also a concern. Over time, surfactant molecules may desorb from particle surfaces or undergo chemical degradation, potentially leading to system instability. This is particularly problematic in applications requiring extended shelf life or prolonged functionality.
Furthermore, the interaction of Triton X-100 with other components in complex formulations presents additional challenges. The surfactant may compete for adsorption sites with other molecules or alter the properties of other stabilizing agents, leading to unexpected changes in overall system stability.
Lastly, there are growing environmental and regulatory concerns surrounding the use of Triton X-100. Its potential persistence in the environment and toxicity to aquatic organisms have led to increased scrutiny and restrictions in certain regions. This necessitates the development of more environmentally friendly alternatives that can match or exceed the stabilization performance of Triton X-100, adding another layer of complexity to the field.
Existing Stabilization Solutions
01 Use of Triton X-100 in stabilizing biological samples
Triton X-100 is utilized to stabilize various biological samples, including proteins, enzymes, and cellular components. It helps maintain the structural integrity and activity of these biomolecules during storage, analysis, or experimental procedures. The surfactant properties of Triton X-100 contribute to its stabilizing effects by preventing aggregation and denaturation.- Use of Triton X-100 in stabilizing biological samples: Triton X-100 is utilized to stabilize various biological samples, including proteins, enzymes, and cellular components. It helps maintain the structural integrity and activity of these biomolecules during storage and analysis. The surfactant properties of Triton X-100 contribute to its stabilizing effects by preventing aggregation and denaturation.
- Triton X-100 in formulation of stable emulsions and suspensions: Triton X-100 is employed as a stabilizer in the preparation of emulsions and suspensions. It helps to reduce surface tension and prevent coalescence of dispersed particles or droplets. This surfactant is particularly useful in creating stable formulations for pharmaceutical, cosmetic, and industrial applications.
- Stabilization of nanoparticles and nanomaterials using Triton X-100: Triton X-100 is effective in stabilizing nanoparticles and nanomaterials in various applications. It helps prevent agglomeration and sedimentation of nanoparticles in suspensions, improving their stability and dispersibility. This is particularly useful in the development of nanomedicine, nanocomposites, and other nanotechnology applications.
- Triton X-100 in membrane protein solubilization and stabilization: Triton X-100 is widely used in the solubilization and stabilization of membrane proteins. It helps extract and maintain the native structure of membrane proteins, which is crucial for studying their functions and interactions. This application is particularly important in biochemistry and structural biology research.
- Triton X-100 as a stabilizer in analytical and diagnostic methods: Triton X-100 is employed as a stabilizing agent in various analytical and diagnostic techniques. It helps improve the stability and reproducibility of assays, including immunoassays, PCR, and other molecular biology methods. The surfactant properties of Triton X-100 contribute to reducing non-specific binding and improving signal-to-noise ratios in these applications.
02 Triton X-100 in formulation of stable emulsions and suspensions
Triton X-100 is employed as a stabilizer in the preparation of emulsions and suspensions. It helps to reduce surface tension and prevent coalescence of dispersed particles or droplets, resulting in improved stability of the formulation. This is particularly useful in pharmaceutical, cosmetic, and industrial applications where long-term stability is crucial.Expand Specific Solutions03 Stabilization of nanoparticles and nanomaterials using Triton X-100
Triton X-100 is used to stabilize nanoparticles and nanomaterials in various applications. It helps prevent agglomeration and sedimentation of nanoparticles in suspension, maintaining their unique properties and ensuring uniform dispersion. This is particularly important in the development of nanomedicine, nanocomposites, and other nanotechnology-based products.Expand Specific Solutions04 Triton X-100 in membrane protein stabilization
Triton X-100 is utilized to stabilize membrane proteins during extraction, purification, and characterization processes. It helps maintain the native conformation of membrane proteins by mimicking the lipid bilayer environment. This is crucial for studying the structure and function of membrane proteins, which are important targets for drug discovery and biotechnology applications.Expand Specific Solutions05 Triton X-100 in stabilizing analytical and diagnostic reagents
Triton X-100 is employed to stabilize various analytical and diagnostic reagents, including enzymes, antibodies, and other biomolecules used in assays and tests. It helps maintain the activity and specificity of these reagents during storage and use, ensuring reliable and reproducible results in analytical and diagnostic applications.Expand Specific Solutions
Key Players in Surfactant Industry
The competitive landscape for studies on Triton X-100 in electrostatic and steric stabilization mechanisms is characterized by a diverse mix of academic institutions and industrial players. The field is in a mature stage of development, with established research methodologies and applications across various sectors. The market size is moderate, driven by the widespread use of Triton X-100 in industrial and research settings. Key players include universities like Xi'an Jiaotong University and Beihang University, which contribute significant academic research, alongside industrial entities such as NTN Corp. and Mitsubishi Electric Corp., which likely focus on practical applications. The technology's maturity is evident from the involvement of both specialized research institutions and large corporations, indicating a well-developed understanding of Triton X-100's stabilization mechanisms.
Xi'an Jiaotong University
Technical Solution: Xi'an Jiaotong University has conducted extensive research on the use of Triton X-100 in electrostatic and steric stabilization mechanisms. Their approach involves utilizing Triton X-100 as a non-ionic surfactant to enhance the stability of colloidal systems. The research team has developed a novel method combining electrostatic repulsion and steric hindrance to achieve superior dispersion stability. They have successfully applied this technique to various nanoparticle systems, demonstrating improved stability in both aqueous and non-aqueous media [1][3]. The university's work has also focused on optimizing the concentration of Triton X-100 to achieve the best balance between electrostatic and steric effects, leading to enhanced long-term stability of suspensions [2].
Strengths: Comprehensive understanding of Triton X-100's role in stabilization mechanisms; innovative combination of electrostatic and steric effects. Weaknesses: Potential limitations in scaling up the technology for industrial applications; possible environmental concerns due to the use of surfactants.
Beihang University
Technical Solution: Beihang University has made significant advancements in the study of Triton X-100 for electrostatic and steric stabilization. Their research focuses on the development of a multi-functional stabilization system incorporating Triton X-100. The university's approach involves creating a synergistic effect between the surfactant and other stabilizing agents to enhance both electrostatic repulsion and steric hindrance. They have successfully applied this technology to improve the stability of nanomaterials in various industrial processes, including aerospace coatings and advanced materials manufacturing [4]. The research team has also explored the use of Triton X-100 in combination with polymeric stabilizers to create hybrid stabilization systems with enhanced performance in extreme conditions [5].
Strengths: Innovative multi-functional stabilization approach; practical applications in advanced industries. Weaknesses: Complexity of the hybrid system may increase production costs; potential challenges in maintaining consistent performance across different material systems.
Core Innovations in Triton X-100 Research
Processing of personal care emulsions using phase inversion temperature methods
PatentWO2019195361A1
Innovation
- Applying phase inversion temperature (PIT) methods to personal care formulations containing ethoxylated nonionic surfactants, which reduces energy needs and enhances stability by processing at the temperature where the surfactant's hydrophobicity changes, minimizing surface tension and allowing for finer droplet dispersion.
Environmental Impact of Triton X-100
The environmental impact of Triton X-100 is a significant concern due to its widespread use in various industrial and consumer applications. This non-ionic surfactant, while effective in its intended purposes, has been found to have detrimental effects on aquatic ecosystems and potentially on human health.
In aquatic environments, Triton X-100 has been shown to disrupt the endocrine systems of marine organisms. Studies have demonstrated that even at low concentrations, it can interfere with the hormonal balance of fish and amphibians, leading to reproductive abnormalities and developmental issues. The surfactant's ability to persist in water bodies for extended periods exacerbates its potential for long-term ecological damage.
Biodegradation of Triton X-100 in natural environments is relatively slow, contributing to its accumulation in water and soil. This persistence allows it to enter food chains, potentially biomagnifying up trophic levels. Research has indicated that the compound can be detected in various aquatic species, raising concerns about its impact on biodiversity and ecosystem health.
The use of Triton X-100 in household and personal care products has led to its presence in wastewater streams. Conventional wastewater treatment processes are not always effective in completely removing this surfactant, resulting in its release into natural water bodies. This has prompted investigations into advanced treatment technologies to mitigate its environmental release.
Soil contamination is another area of concern. When Triton X-100 enters soil ecosystems, it can alter soil properties and affect microbial communities. This may have cascading effects on soil fertility and plant growth, potentially impacting agricultural productivity and natural vegetation.
Recent regulatory actions in various countries have begun to address the environmental risks posed by Triton X-100. Some jurisdictions have implemented restrictions on its use in certain products, particularly those with high potential for environmental release. These regulatory measures aim to reduce the compound's environmental footprint and protect sensitive ecosystems.
Research into alternative, more environmentally friendly surfactants has gained momentum in response to these concerns. Scientists are exploring bio-based surfactants and other compounds that offer similar performance characteristics to Triton X-100 but with reduced environmental persistence and toxicity. This shift towards greener alternatives represents a growing trend in the chemical industry towards more sustainable practices.
In aquatic environments, Triton X-100 has been shown to disrupt the endocrine systems of marine organisms. Studies have demonstrated that even at low concentrations, it can interfere with the hormonal balance of fish and amphibians, leading to reproductive abnormalities and developmental issues. The surfactant's ability to persist in water bodies for extended periods exacerbates its potential for long-term ecological damage.
Biodegradation of Triton X-100 in natural environments is relatively slow, contributing to its accumulation in water and soil. This persistence allows it to enter food chains, potentially biomagnifying up trophic levels. Research has indicated that the compound can be detected in various aquatic species, raising concerns about its impact on biodiversity and ecosystem health.
The use of Triton X-100 in household and personal care products has led to its presence in wastewater streams. Conventional wastewater treatment processes are not always effective in completely removing this surfactant, resulting in its release into natural water bodies. This has prompted investigations into advanced treatment technologies to mitigate its environmental release.
Soil contamination is another area of concern. When Triton X-100 enters soil ecosystems, it can alter soil properties and affect microbial communities. This may have cascading effects on soil fertility and plant growth, potentially impacting agricultural productivity and natural vegetation.
Recent regulatory actions in various countries have begun to address the environmental risks posed by Triton X-100. Some jurisdictions have implemented restrictions on its use in certain products, particularly those with high potential for environmental release. These regulatory measures aim to reduce the compound's environmental footprint and protect sensitive ecosystems.
Research into alternative, more environmentally friendly surfactants has gained momentum in response to these concerns. Scientists are exploring bio-based surfactants and other compounds that offer similar performance characteristics to Triton X-100 but with reduced environmental persistence and toxicity. This shift towards greener alternatives represents a growing trend in the chemical industry towards more sustainable practices.
Regulatory Framework for Surfactants
The regulatory framework for surfactants, including Triton X-100, is complex and multifaceted, involving various international, regional, and national regulations. These regulations aim to ensure the safe use of surfactants while minimizing their potential environmental and health impacts.
At the international level, the United Nations Environment Programme (UNEP) has established guidelines for the production and use of surfactants, focusing on their biodegradability and environmental impact. The Stockholm Convention on Persistent Organic Pollutants (POPs) also plays a crucial role in regulating certain surfactants that may have long-lasting effects on the environment.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation is a key piece of legislation governing surfactants. REACH requires manufacturers and importers to register chemicals, including surfactants, and provide safety data. The Detergents Regulation (EC) No 648/2004 specifically addresses the biodegradability of surfactants used in detergents.
The United States Environmental Protection Agency (EPA) regulates surfactants under various acts, including the Toxic Substances Control Act (TSCA) and the Clean Water Act. The EPA's Design for the Environment (DfE) program also provides guidance on safer surfactant alternatives.
Specific to Triton X-100, its use is regulated due to its potential environmental persistence and toxicity to aquatic organisms. In the EU, Triton X-100 is classified as a substance of very high concern (SVHC) under REACH due to its endocrine-disrupting properties. This classification imposes strict requirements on its use and may lead to future restrictions or phase-outs.
Many countries have implemented their own regulatory frameworks for surfactants. For example, Japan's Chemical Substances Control Law (CSCL) and China's Measures for Environmental Management of New Chemical Substances both include provisions for surfactant regulation.
The regulatory landscape for surfactants is continually evolving, with increasing focus on environmental sustainability and human health. Recent trends include the promotion of bio-based surfactants and stricter controls on per- and polyfluoroalkyl substances (PFAS), which are used in some surfactant applications.
As research on the electrostatic and steric stabilization mechanisms of surfactants like Triton X-100 progresses, regulatory bodies may update their frameworks to reflect new scientific understanding. This could lead to more targeted regulations that consider not only the chemical properties of surfactants but also their specific mechanisms of action in various applications.
At the international level, the United Nations Environment Programme (UNEP) has established guidelines for the production and use of surfactants, focusing on their biodegradability and environmental impact. The Stockholm Convention on Persistent Organic Pollutants (POPs) also plays a crucial role in regulating certain surfactants that may have long-lasting effects on the environment.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation is a key piece of legislation governing surfactants. REACH requires manufacturers and importers to register chemicals, including surfactants, and provide safety data. The Detergents Regulation (EC) No 648/2004 specifically addresses the biodegradability of surfactants used in detergents.
The United States Environmental Protection Agency (EPA) regulates surfactants under various acts, including the Toxic Substances Control Act (TSCA) and the Clean Water Act. The EPA's Design for the Environment (DfE) program also provides guidance on safer surfactant alternatives.
Specific to Triton X-100, its use is regulated due to its potential environmental persistence and toxicity to aquatic organisms. In the EU, Triton X-100 is classified as a substance of very high concern (SVHC) under REACH due to its endocrine-disrupting properties. This classification imposes strict requirements on its use and may lead to future restrictions or phase-outs.
Many countries have implemented their own regulatory frameworks for surfactants. For example, Japan's Chemical Substances Control Law (CSCL) and China's Measures for Environmental Management of New Chemical Substances both include provisions for surfactant regulation.
The regulatory landscape for surfactants is continually evolving, with increasing focus on environmental sustainability and human health. Recent trends include the promotion of bio-based surfactants and stricter controls on per- and polyfluoroalkyl substances (PFAS), which are used in some surfactant applications.
As research on the electrostatic and steric stabilization mechanisms of surfactants like Triton X-100 progresses, regulatory bodies may update their frameworks to reflect new scientific understanding. This could lead to more targeted regulations that consider not only the chemical properties of surfactants but also their specific mechanisms of action in various applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!