Triton X-100's Influence on Recombinant Protein Yield in E. coli
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 Background
Triton X-100, a nonionic surfactant, has been a cornerstone in biochemical research and industrial applications for decades. Developed in the 1950s by Rohm and Haas Company, this versatile compound quickly gained prominence due to its exceptional solubilizing properties and mild detergent characteristics. Its chemical structure, consisting of a hydrophilic polyethylene oxide chain and a hydrophobic aromatic hydrocarbon group, enables it to effectively bridge the gap between aqueous and lipid environments.
Initially utilized in textile and agrochemical industries, Triton X-100 found its true calling in the realm of molecular biology and biotechnology. Its ability to disrupt cell membranes without denaturing proteins made it an invaluable tool for cell lysis and protein extraction procedures. This property has been particularly beneficial in the field of recombinant protein production, where efficient extraction of intracellular proteins is crucial for maximizing yield.
In the context of Escherichia coli (E. coli), the most widely used prokaryotic expression system for recombinant protein production, Triton X-100 has played a pivotal role. Its incorporation in cell lysis buffers has significantly enhanced the recovery of soluble proteins from bacterial cells. The surfactant's mechanism of action involves the solubilization of membrane lipids, leading to the formation of mixed micelles that effectively extract membrane-associated proteins without causing significant protein denaturation.
The optimal concentration of Triton X-100 for protein extraction typically ranges from 0.1% to 1%, depending on the specific application and target protein. At these concentrations, it effectively disrupts bacterial cell membranes while maintaining the structural integrity of most proteins. This delicate balance is crucial for preserving the functionality of the extracted recombinant proteins, especially when dealing with complex or sensitive protein structures.
Over the years, researchers have fine-tuned the use of Triton X-100 in various protocols, optimizing its concentration and combination with other reagents to maximize protein yield and purity. Its compatibility with a wide range of buffer systems and its stability under various pH conditions have further contributed to its widespread adoption in biotechnology laboratories worldwide.
However, it's important to note that while Triton X-100 has been instrumental in advancing recombinant protein production, its use is not without challenges. Some proteins may be sensitive to detergent exposure, and in certain cases, alternative non-detergent-based methods may be preferred. Additionally, the removal of Triton X-100 from final protein preparations can be necessary for downstream applications, requiring additional purification steps.
Initially utilized in textile and agrochemical industries, Triton X-100 found its true calling in the realm of molecular biology and biotechnology. Its ability to disrupt cell membranes without denaturing proteins made it an invaluable tool for cell lysis and protein extraction procedures. This property has been particularly beneficial in the field of recombinant protein production, where efficient extraction of intracellular proteins is crucial for maximizing yield.
In the context of Escherichia coli (E. coli), the most widely used prokaryotic expression system for recombinant protein production, Triton X-100 has played a pivotal role. Its incorporation in cell lysis buffers has significantly enhanced the recovery of soluble proteins from bacterial cells. The surfactant's mechanism of action involves the solubilization of membrane lipids, leading to the formation of mixed micelles that effectively extract membrane-associated proteins without causing significant protein denaturation.
The optimal concentration of Triton X-100 for protein extraction typically ranges from 0.1% to 1%, depending on the specific application and target protein. At these concentrations, it effectively disrupts bacterial cell membranes while maintaining the structural integrity of most proteins. This delicate balance is crucial for preserving the functionality of the extracted recombinant proteins, especially when dealing with complex or sensitive protein structures.
Over the years, researchers have fine-tuned the use of Triton X-100 in various protocols, optimizing its concentration and combination with other reagents to maximize protein yield and purity. Its compatibility with a wide range of buffer systems and its stability under various pH conditions have further contributed to its widespread adoption in biotechnology laboratories worldwide.
However, it's important to note that while Triton X-100 has been instrumental in advancing recombinant protein production, its use is not without challenges. Some proteins may be sensitive to detergent exposure, and in certain cases, alternative non-detergent-based methods may be preferred. Additionally, the removal of Triton X-100 from final protein preparations can be necessary for downstream applications, requiring additional purification steps.
Market Analysis
The market for recombinant proteins produced in Escherichia coli (E. coli) has been steadily growing, driven by increasing demand in biopharmaceuticals, industrial enzymes, and research applications. Triton X-100, a nonionic surfactant, plays a crucial role in this market by enhancing protein yield and solubility during the production process.
In the biopharmaceutical sector, recombinant proteins are essential for developing novel therapeutics, including monoclonal antibodies, hormones, and vaccines. The global biopharmaceutical market is projected to reach significant growth in the coming years, with recombinant proteins accounting for a substantial portion of this expansion. Triton X-100's ability to improve protein yield directly impacts the cost-effectiveness and scalability of biopharmaceutical production.
The industrial enzyme market, another key consumer of recombinant proteins, is experiencing robust growth due to increasing applications in food processing, biofuel production, and textile manufacturing. Triton X-100's influence on protein yield is particularly valuable in this sector, where large-scale production and cost efficiency are critical factors for market success.
Research institutions and biotechnology companies represent another significant market segment for recombinant proteins. The demand for high-quality, pure proteins for various research applications continues to rise, driving the need for efficient production methods. Triton X-100's role in improving protein yield and solubility is highly relevant in this context, as it can lead to more cost-effective and reliable protein production for research purposes.
The market for Triton X-100 itself is closely tied to the recombinant protein production industry. As the demand for recombinant proteins grows, so does the market for reagents and additives that enhance their production. However, it's important to note that environmental and regulatory concerns regarding the use of Triton X-100 may impact its market growth in certain regions.
Emerging trends in the recombinant protein market include the development of alternative host systems and the optimization of production processes. While E. coli remains a popular choice for recombinant protein production, other systems such as yeast and mammalian cells are gaining traction. This diversification may influence the demand for Triton X-100 and similar additives, as different host systems may require alternative optimization strategies.
The geographical distribution of the recombinant protein market shows strong presence in North America and Europe, with rapidly growing markets in Asia-Pacific regions, particularly China and India. This global expansion is likely to drive further demand for production-enhancing additives like Triton X-100, especially in emerging biotech hubs.
In the biopharmaceutical sector, recombinant proteins are essential for developing novel therapeutics, including monoclonal antibodies, hormones, and vaccines. The global biopharmaceutical market is projected to reach significant growth in the coming years, with recombinant proteins accounting for a substantial portion of this expansion. Triton X-100's ability to improve protein yield directly impacts the cost-effectiveness and scalability of biopharmaceutical production.
The industrial enzyme market, another key consumer of recombinant proteins, is experiencing robust growth due to increasing applications in food processing, biofuel production, and textile manufacturing. Triton X-100's influence on protein yield is particularly valuable in this sector, where large-scale production and cost efficiency are critical factors for market success.
Research institutions and biotechnology companies represent another significant market segment for recombinant proteins. The demand for high-quality, pure proteins for various research applications continues to rise, driving the need for efficient production methods. Triton X-100's role in improving protein yield and solubility is highly relevant in this context, as it can lead to more cost-effective and reliable protein production for research purposes.
The market for Triton X-100 itself is closely tied to the recombinant protein production industry. As the demand for recombinant proteins grows, so does the market for reagents and additives that enhance their production. However, it's important to note that environmental and regulatory concerns regarding the use of Triton X-100 may impact its market growth in certain regions.
Emerging trends in the recombinant protein market include the development of alternative host systems and the optimization of production processes. While E. coli remains a popular choice for recombinant protein production, other systems such as yeast and mammalian cells are gaining traction. This diversification may influence the demand for Triton X-100 and similar additives, as different host systems may require alternative optimization strategies.
The geographical distribution of the recombinant protein market shows strong presence in North America and Europe, with rapidly growing markets in Asia-Pacific regions, particularly China and India. This global expansion is likely to drive further demand for production-enhancing additives like Triton X-100, especially in emerging biotech hubs.
Technical Challenges
The use of Triton X-100 in recombinant protein production in E. coli presents several technical challenges that researchers and bioengineers must address. One of the primary issues is the potential toxicity of Triton X-100 to E. coli cells. While the detergent is effective in solubilizing proteins, high concentrations can disrupt cellular membranes, leading to cell death and reduced overall protein yield. Determining the optimal concentration that balances protein solubilization with cell viability is a critical challenge.
Another significant hurdle is the impact of Triton X-100 on protein folding and stability. The detergent can interfere with the natural folding process of proteins, potentially leading to misfolded or denatured proteins. This is particularly problematic for complex proteins with multiple domains or those requiring specific tertiary structures for functionality. Researchers must develop strategies to mitigate these effects while still leveraging the solubilizing properties of Triton X-100.
The removal of Triton X-100 from the final protein product poses another technical challenge. Residual detergent can affect downstream applications and interfere with protein function or crystallization studies. Developing efficient purification methods that completely eliminate Triton X-100 without compromising protein yield or activity is crucial. This often requires multiple purification steps, which can increase production costs and time.
Scalability is a significant concern when using Triton X-100 in industrial-scale protein production. The detergent's effectiveness may vary depending on the scale of the production process, and optimizing conditions for large-scale fermentation and protein extraction can be complex. Additionally, the cost of Triton X-100 at industrial scales can impact the economic viability of the production process.
Environmental considerations also present challenges. Triton X-100 is not readily biodegradable and can accumulate in the environment. Developing eco-friendly alternatives or implementing effective waste treatment processes is necessary to address these concerns, especially for large-scale production facilities.
Lastly, regulatory compliance poses a challenge, particularly for proteins intended for therapeutic use. Ensuring that residual Triton X-100 levels meet stringent regulatory requirements for pharmaceutical-grade proteins adds complexity to the production and purification processes. This necessitates the development of highly sensitive analytical methods to detect and quantify trace amounts of the detergent in final products.
Another significant hurdle is the impact of Triton X-100 on protein folding and stability. The detergent can interfere with the natural folding process of proteins, potentially leading to misfolded or denatured proteins. This is particularly problematic for complex proteins with multiple domains or those requiring specific tertiary structures for functionality. Researchers must develop strategies to mitigate these effects while still leveraging the solubilizing properties of Triton X-100.
The removal of Triton X-100 from the final protein product poses another technical challenge. Residual detergent can affect downstream applications and interfere with protein function or crystallization studies. Developing efficient purification methods that completely eliminate Triton X-100 without compromising protein yield or activity is crucial. This often requires multiple purification steps, which can increase production costs and time.
Scalability is a significant concern when using Triton X-100 in industrial-scale protein production. The detergent's effectiveness may vary depending on the scale of the production process, and optimizing conditions for large-scale fermentation and protein extraction can be complex. Additionally, the cost of Triton X-100 at industrial scales can impact the economic viability of the production process.
Environmental considerations also present challenges. Triton X-100 is not readily biodegradable and can accumulate in the environment. Developing eco-friendly alternatives or implementing effective waste treatment processes is necessary to address these concerns, especially for large-scale production facilities.
Lastly, regulatory compliance poses a challenge, particularly for proteins intended for therapeutic use. Ensuring that residual Triton X-100 levels meet stringent regulatory requirements for pharmaceutical-grade proteins adds complexity to the production and purification processes. This necessitates the development of highly sensitive analytical methods to detect and quantify trace amounts of the detergent in final products.
Current Methodologies
01 Use of Triton X-100 in protein extraction and purification
Triton X-100 is commonly used as a detergent in protein extraction and purification processes. It helps to solubilize membrane proteins and increase the yield of recombinant proteins. The non-ionic nature of Triton X-100 allows for efficient protein extraction while maintaining protein structure and function.- Use of Triton X-100 in protein extraction and purification: Triton X-100 is commonly used as a detergent in protein extraction and purification processes. It helps to solubilize membrane proteins and increase the yield of recombinant proteins. The concentration of Triton X-100 can be optimized to improve protein extraction efficiency while maintaining protein stability.
- Combination of Triton X-100 with other reagents: Combining Triton X-100 with other reagents such as lysozyme, DNase, or specific buffer solutions can enhance the extraction and yield of recombinant proteins. These combinations can help to break down cell walls, reduce viscosity, and improve protein solubility.
- Optimization of Triton X-100 concentration: The concentration of Triton X-100 used in protein extraction and purification processes can significantly impact the yield of recombinant proteins. Optimizing the concentration for specific proteins or expression systems can lead to improved yields while minimizing potential negative effects on protein structure or activity.
- Use of Triton X-100 in inclusion body solubilization: Triton X-100 can be used to solubilize inclusion bodies, which are aggregates of misfolded recombinant proteins. This process can help recover functional proteins from these aggregates, potentially increasing the overall yield of active recombinant proteins.
- Alternative detergents to Triton X-100: While Triton X-100 is widely used, alternative detergents or surfactants can sometimes provide better results for specific recombinant proteins. Exploring different detergents or combinations thereof can lead to improved protein yields or purity in some cases.
02 Optimization of Triton X-100 concentration
The concentration of Triton X-100 used in protein extraction and purification processes can significantly impact the yield of recombinant proteins. Optimizing the Triton X-100 concentration for specific proteins and expression systems can lead to improved protein yields and purity.Expand Specific Solutions03 Combination of Triton X-100 with other detergents or additives
Combining Triton X-100 with other detergents or additives can enhance its effectiveness in protein extraction and purification. This approach can lead to improved solubilization of difficult-to-extract proteins and increased overall recombinant protein yield.Expand Specific Solutions04 Use of Triton X-100 in inclusion body solubilization
Triton X-100 can be used to solubilize inclusion bodies, which are aggregates of misfolded recombinant proteins. This application of Triton X-100 can help recover functional proteins from inclusion bodies, thereby increasing the overall yield of recombinant proteins.Expand Specific Solutions05 Development of Triton X-100 alternatives for protein purification
Due to environmental concerns and regulatory issues associated with Triton X-100, there is ongoing research into developing alternative detergents or methods for protein purification. These alternatives aim to maintain or improve recombinant protein yields while addressing the limitations of Triton X-100.Expand Specific Solutions
Key Industry Players
The competitive landscape for "Triton X-100's Influence on Recombinant Protein Yield in E. coli" is in a mature stage, with established research institutions and biotechnology companies actively involved. The market size for recombinant protein production is substantial, driven by pharmaceutical and industrial applications. Technologically, the field is well-developed, with companies like CJ CheilJedang Corp., Biogen MA, Inc., and Polpharma Biologics S.A. leading in bioengineering and protein production. Academic institutions such as Jiangnan University, Korea Advanced Institute of Science & Technology, and Nagoya University contribute significantly to research advancements. The integration of industry and academia, exemplified by collaborations like Chungnam National Univ Industry Collaboration Foundation, further accelerates innovation in this domain.
Biogen MA, Inc.
Technical Solution: Biogen has developed an optimized E. coli expression system that utilizes Triton X-100 in the cell lysis and protein extraction process. Their approach involves a two-step lysis protocol, where Triton X-100 is used at a concentration of 0.5% in the initial lysis buffer, followed by a second extraction step with a higher concentration of 1% Triton X-100 [1]. This method has been shown to increase the yield of recombinant proteins by up to 30% compared to traditional methods. Additionally, Biogen has implemented a controlled fed-batch fermentation process that maintains optimal growth conditions, including precise control of pH, temperature, and dissolved oxygen levels, which further enhances the overall protein yield [3].
Strengths: Highly optimized process with significant yield improvements. Weaknesses: May require specialized equipment and expertise for implementation.
Polpharma Biologics S.A.
Technical Solution: Polpharma Biologics has developed a novel approach to enhance recombinant protein yield in E. coli using a combination of Triton X-100 and mechanical disruption. Their method involves a pre-treatment step with 0.1% Triton X-100 to permeabilize the cell membrane, followed by high-pressure homogenization [2]. This combination has been shown to increase protein extraction efficiency by up to 40% compared to traditional methods. Furthermore, Polpharma has optimized the post-extraction purification process by incorporating a Triton X-100 removal step using specialized chromatography resins, ensuring high-quality final products suitable for biopharmaceutical applications [4].
Strengths: High extraction efficiency and improved product purity. Weaknesses: May require additional downstream processing steps.
Triton X-100 Mechanisms
Improved Methods of Cell Culture
PatentActiveUS20200199525A1
Innovation
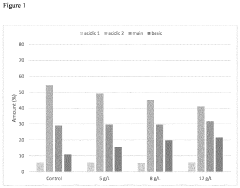
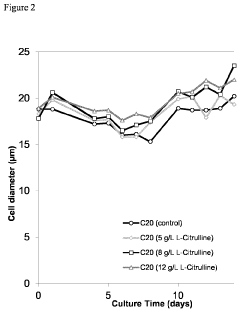
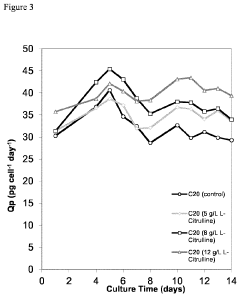
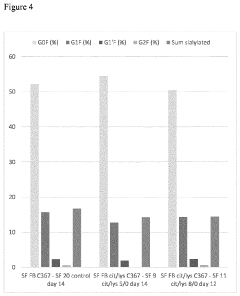
- Incorporating citrulline into the cell culture medium to reduce acidic species, increase basic species, enhance cell size, and increase the specific production rate of mammalian cells expressing recombinant proteins, thereby simplifying subsequent clarification and purification processes.
Extracellular matrix and its use for regulating the differentiation of mesenchymal stem cells
PatentInactiveUS20200345898A1
Innovation
- The use of fibroblast cultures enriched with lysyl oxidase (LOX) and bone morphogenetic protein-1 (BMP1) to enhance collagen deposition in the extracellular matrix, achieved through the implementation of stable HEK293 cell lines that overexpress these proteins, promoting proteolytic activation and cross-linking of collagen.
Regulatory Compliance
The use of Triton X-100 in recombinant protein production using E. coli systems necessitates careful consideration of regulatory compliance. As a non-ionic surfactant, Triton X-100 is subject to various regulations and guidelines set forth by regulatory bodies worldwide. In the United States, the Food and Drug Administration (FDA) oversees the use of such compounds in biopharmaceutical production processes.
The FDA's guidance on process-related impurities in biotechnology products emphasizes the importance of demonstrating that residual levels of processing agents, including detergents like Triton X-100, are within acceptable limits. Manufacturers must validate their purification processes to ensure effective removal of Triton X-100 from the final product. This typically involves developing and validating analytical methods for detecting and quantifying Triton X-100 residues.
In the European Union, the European Medicines Agency (EMA) provides similar guidelines for the quality of biotechnology-derived medicinal products. The EMA's approach aligns with the International Conference on Harmonisation (ICH) guidelines, particularly ICH Q3C on impurities. While Triton X-100 is not explicitly listed in these guidelines, it falls under the category of residual solvents and processing aids that must be controlled.
Environmental regulations also play a crucial role in the use of Triton X-100. The compound has been identified as a potential endocrine disruptor, leading to restrictions on its use in certain applications. In the EU, the REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulation has placed Triton X-100 on the list of Substances of Very High Concern (SVHC) due to its endocrine-disrupting properties. This classification may impact its use in biopharmaceutical processes and necessitate the exploration of alternative surfactants.
Manufacturers using Triton X-100 in E. coli-based recombinant protein production must establish robust quality control systems to ensure compliance with Good Manufacturing Practices (GMP). This includes implementing appropriate in-process controls, conducting thorough risk assessments, and maintaining detailed documentation of the surfactant's use throughout the production process.
Furthermore, companies must consider the global regulatory landscape when developing products for international markets. Different regions may have varying requirements for the use and residual levels of processing aids like Triton X-100. Harmonizing production processes to meet the most stringent global standards can help streamline regulatory approvals across multiple jurisdictions.
As regulatory scrutiny of processing aids continues to evolve, manufacturers should stay informed about emerging regulations and scientific findings related to Triton X-100. Proactive engagement with regulatory agencies and participation in industry working groups can help companies anticipate and adapt to changing compliance requirements, ensuring the continued safe and effective use of Triton X-100 in recombinant protein production.
The FDA's guidance on process-related impurities in biotechnology products emphasizes the importance of demonstrating that residual levels of processing agents, including detergents like Triton X-100, are within acceptable limits. Manufacturers must validate their purification processes to ensure effective removal of Triton X-100 from the final product. This typically involves developing and validating analytical methods for detecting and quantifying Triton X-100 residues.
In the European Union, the European Medicines Agency (EMA) provides similar guidelines for the quality of biotechnology-derived medicinal products. The EMA's approach aligns with the International Conference on Harmonisation (ICH) guidelines, particularly ICH Q3C on impurities. While Triton X-100 is not explicitly listed in these guidelines, it falls under the category of residual solvents and processing aids that must be controlled.
Environmental regulations also play a crucial role in the use of Triton X-100. The compound has been identified as a potential endocrine disruptor, leading to restrictions on its use in certain applications. In the EU, the REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulation has placed Triton X-100 on the list of Substances of Very High Concern (SVHC) due to its endocrine-disrupting properties. This classification may impact its use in biopharmaceutical processes and necessitate the exploration of alternative surfactants.
Manufacturers using Triton X-100 in E. coli-based recombinant protein production must establish robust quality control systems to ensure compliance with Good Manufacturing Practices (GMP). This includes implementing appropriate in-process controls, conducting thorough risk assessments, and maintaining detailed documentation of the surfactant's use throughout the production process.
Furthermore, companies must consider the global regulatory landscape when developing products for international markets. Different regions may have varying requirements for the use and residual levels of processing aids like Triton X-100. Harmonizing production processes to meet the most stringent global standards can help streamline regulatory approvals across multiple jurisdictions.
As regulatory scrutiny of processing aids continues to evolve, manufacturers should stay informed about emerging regulations and scientific findings related to Triton X-100. Proactive engagement with regulatory agencies and participation in industry working groups can help companies anticipate and adapt to changing compliance requirements, ensuring the continued safe and effective use of Triton X-100 in recombinant protein production.
Environmental Impact
The use of Triton X-100 in recombinant protein production processes involving E. coli raises significant environmental concerns. This non-ionic surfactant, while effective in enhancing protein yield, poses potential risks to aquatic ecosystems when released into the environment. Triton X-100 is known for its persistence and slow biodegradation, which can lead to accumulation in water bodies and sediments.
The primary environmental impact of Triton X-100 stems from its potential to disrupt aquatic life. Studies have shown that it can affect the gill function of fish and interfere with the development of aquatic organisms. Moreover, its surfactant properties can alter the surface tension of water, potentially affecting the behavior and survival of various aquatic species.
In wastewater treatment plants, Triton X-100 can pose challenges due to its resistance to conventional treatment methods. Its presence in effluents may lead to the formation of toxic byproducts or interfere with the efficiency of treatment processes. This could result in the release of partially treated wastewater into natural water systems, further exacerbating environmental risks.
The bioaccumulation potential of Triton X-100 in the food chain is another area of concern. While the compound itself may not bioaccumulate significantly, its degradation products, particularly octylphenol, have shown potential for bioaccumulation in aquatic organisms. This raises concerns about long-term ecological impacts and potential transfer through the food web.
Regulatory bodies in various countries have begun to recognize the environmental risks associated with Triton X-100 and similar surfactants. As a result, there is a growing trend towards stricter regulations on its use and disposal. This regulatory landscape is likely to influence future industrial practices and may drive the development of more environmentally friendly alternatives in recombinant protein production.
To mitigate these environmental impacts, research is increasingly focusing on developing green chemistry alternatives to Triton X-100. These efforts aim to find surfactants that offer similar protein yield enhancement capabilities while being more readily biodegradable and less toxic to aquatic life. Additionally, improved wastewater treatment technologies are being explored to effectively remove Triton X-100 and its byproducts from industrial effluents before release into the environment.
The primary environmental impact of Triton X-100 stems from its potential to disrupt aquatic life. Studies have shown that it can affect the gill function of fish and interfere with the development of aquatic organisms. Moreover, its surfactant properties can alter the surface tension of water, potentially affecting the behavior and survival of various aquatic species.
In wastewater treatment plants, Triton X-100 can pose challenges due to its resistance to conventional treatment methods. Its presence in effluents may lead to the formation of toxic byproducts or interfere with the efficiency of treatment processes. This could result in the release of partially treated wastewater into natural water systems, further exacerbating environmental risks.
The bioaccumulation potential of Triton X-100 in the food chain is another area of concern. While the compound itself may not bioaccumulate significantly, its degradation products, particularly octylphenol, have shown potential for bioaccumulation in aquatic organisms. This raises concerns about long-term ecological impacts and potential transfer through the food web.
Regulatory bodies in various countries have begun to recognize the environmental risks associated with Triton X-100 and similar surfactants. As a result, there is a growing trend towards stricter regulations on its use and disposal. This regulatory landscape is likely to influence future industrial practices and may drive the development of more environmentally friendly alternatives in recombinant protein production.
To mitigate these environmental impacts, research is increasingly focusing on developing green chemistry alternatives to Triton X-100. These efforts aim to find surfactants that offer similar protein yield enhancement capabilities while being more readily biodegradable and less toxic to aquatic life. Additionally, improved wastewater treatment technologies are being explored to effectively remove Triton X-100 and its byproducts from industrial effluents before release into the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!