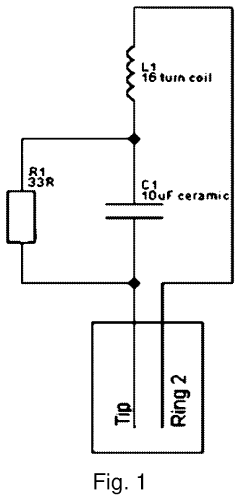
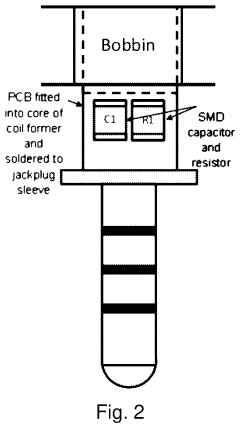
Understanding PEMF Therapy's Role in Pain Perception Modulation
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMF Therapy Background and Objectives
Pulsed Electromagnetic Field (PEMF) therapy has emerged as a promising non-invasive treatment modality for pain management. The technology harnesses the power of electromagnetic fields to stimulate cellular activity and promote healing. PEMF therapy's roots can be traced back to the mid-20th century, with initial applications in bone healing. Over the decades, its potential in pain modulation has garnered increasing attention from researchers and clinicians alike.
The evolution of PEMF therapy has been marked by significant advancements in device design, treatment protocols, and understanding of its biological mechanisms. Early devices were bulky and limited in their applications, while modern PEMF systems offer greater precision, portability, and versatility. This progression has expanded the therapy's potential across various medical fields, including orthopedics, neurology, and rehabilitation medicine.
Current research in PEMF therapy focuses on elucidating its role in pain perception modulation. The primary objective is to comprehend the neurophysiological pathways through which PEMF influences pain signaling and processing. This understanding is crucial for optimizing treatment parameters and expanding the therapy's applications in chronic pain conditions.
One of the key goals in PEMF research is to establish standardized protocols for different pain conditions. This involves determining optimal frequency ranges, field intensities, and treatment durations for specific types of pain, such as neuropathic, inflammatory, or musculoskeletal pain. Researchers aim to develop evidence-based guidelines that can enhance the efficacy and reliability of PEMF therapy in clinical settings.
Another important objective is to investigate the long-term effects of PEMF therapy on pain perception and overall quality of life. This includes assessing its potential for reducing reliance on pharmacological interventions, particularly in the context of the ongoing opioid crisis. Researchers are exploring whether PEMF therapy can offer a safer, non-addictive alternative for long-term pain management.
The integration of PEMF therapy with other treatment modalities is also a significant area of interest. Scientists are examining how PEMF can complement or enhance the effects of physical therapy, acupuncture, or cognitive behavioral therapies in comprehensive pain management strategies. This holistic approach aims to address the multifaceted nature of chronic pain and improve overall patient outcomes.
As the field progresses, there is a growing emphasis on personalized PEMF therapy. Researchers are working on developing adaptive PEMF systems that can adjust treatment parameters based on individual patient responses and real-time physiological data. This tailored approach holds promise for maximizing therapeutic benefits while minimizing potential side effects.
The evolution of PEMF therapy has been marked by significant advancements in device design, treatment protocols, and understanding of its biological mechanisms. Early devices were bulky and limited in their applications, while modern PEMF systems offer greater precision, portability, and versatility. This progression has expanded the therapy's potential across various medical fields, including orthopedics, neurology, and rehabilitation medicine.
Current research in PEMF therapy focuses on elucidating its role in pain perception modulation. The primary objective is to comprehend the neurophysiological pathways through which PEMF influences pain signaling and processing. This understanding is crucial for optimizing treatment parameters and expanding the therapy's applications in chronic pain conditions.
One of the key goals in PEMF research is to establish standardized protocols for different pain conditions. This involves determining optimal frequency ranges, field intensities, and treatment durations for specific types of pain, such as neuropathic, inflammatory, or musculoskeletal pain. Researchers aim to develop evidence-based guidelines that can enhance the efficacy and reliability of PEMF therapy in clinical settings.
Another important objective is to investigate the long-term effects of PEMF therapy on pain perception and overall quality of life. This includes assessing its potential for reducing reliance on pharmacological interventions, particularly in the context of the ongoing opioid crisis. Researchers are exploring whether PEMF therapy can offer a safer, non-addictive alternative for long-term pain management.
The integration of PEMF therapy with other treatment modalities is also a significant area of interest. Scientists are examining how PEMF can complement or enhance the effects of physical therapy, acupuncture, or cognitive behavioral therapies in comprehensive pain management strategies. This holistic approach aims to address the multifaceted nature of chronic pain and improve overall patient outcomes.
As the field progresses, there is a growing emphasis on personalized PEMF therapy. Researchers are working on developing adaptive PEMF systems that can adjust treatment parameters based on individual patient responses and real-time physiological data. This tailored approach holds promise for maximizing therapeutic benefits while minimizing potential side effects.
Market Analysis for PEMF Pain Management
The PEMF (Pulsed Electromagnetic Field) therapy market for pain management is experiencing significant growth, driven by increasing awareness of non-invasive treatment options and the rising prevalence of chronic pain conditions. The global PEMF therapy devices market was valued at approximately $500 million in 2020 and is projected to reach $1.2 billion by 2027, growing at a CAGR of around 13% during the forecast period.
The demand for PEMF therapy in pain management is primarily fueled by the growing aging population, rising incidence of musculoskeletal disorders, and the need for alternative pain management solutions amid the opioid crisis. North America currently holds the largest market share, accounting for about 40% of the global market, followed by Europe and Asia-Pacific regions.
Key market segments include home-use devices, clinical-grade equipment, and portable PEMF systems. Home-use devices are gaining traction due to their convenience and cost-effectiveness, while clinical-grade equipment remains essential for professional healthcare settings. The portable PEMF systems segment is expected to witness the highest growth rate, driven by increasing demand for on-the-go pain management solutions.
The market landscape is characterized by a mix of established medical device companies and innovative startups. Major players include OMI PLC, Orthofix Medical Inc., and Bemer Group, which collectively hold about 30% of the market share. These companies are investing heavily in research and development to enhance the efficacy and user-friendliness of PEMF devices.
Emerging trends in the PEMF therapy market for pain management include the integration of smart technologies, such as mobile apps for treatment tracking and personalization, and the development of wearable PEMF devices. These innovations are expected to drive market growth and improve patient outcomes.
Challenges facing the market include the need for more extensive clinical evidence to support PEMF therapy's efficacy in various pain conditions and regulatory hurdles in some regions. However, ongoing research and clinical trials are addressing these concerns, potentially expanding the market's reach in the coming years.
In conclusion, the PEMF therapy market for pain management presents significant opportunities for growth and innovation. As the technology continues to evolve and gain acceptance among healthcare professionals and patients, it is poised to play an increasingly important role in the broader pain management landscape.
The demand for PEMF therapy in pain management is primarily fueled by the growing aging population, rising incidence of musculoskeletal disorders, and the need for alternative pain management solutions amid the opioid crisis. North America currently holds the largest market share, accounting for about 40% of the global market, followed by Europe and Asia-Pacific regions.
Key market segments include home-use devices, clinical-grade equipment, and portable PEMF systems. Home-use devices are gaining traction due to their convenience and cost-effectiveness, while clinical-grade equipment remains essential for professional healthcare settings. The portable PEMF systems segment is expected to witness the highest growth rate, driven by increasing demand for on-the-go pain management solutions.
The market landscape is characterized by a mix of established medical device companies and innovative startups. Major players include OMI PLC, Orthofix Medical Inc., and Bemer Group, which collectively hold about 30% of the market share. These companies are investing heavily in research and development to enhance the efficacy and user-friendliness of PEMF devices.
Emerging trends in the PEMF therapy market for pain management include the integration of smart technologies, such as mobile apps for treatment tracking and personalization, and the development of wearable PEMF devices. These innovations are expected to drive market growth and improve patient outcomes.
Challenges facing the market include the need for more extensive clinical evidence to support PEMF therapy's efficacy in various pain conditions and regulatory hurdles in some regions. However, ongoing research and clinical trials are addressing these concerns, potentially expanding the market's reach in the coming years.
In conclusion, the PEMF therapy market for pain management presents significant opportunities for growth and innovation. As the technology continues to evolve and gain acceptance among healthcare professionals and patients, it is poised to play an increasingly important role in the broader pain management landscape.
Current PEMF Technology and Challenges
Pulsed Electromagnetic Field (PEMF) therapy has gained significant attention in recent years as a non-invasive approach to pain management. The current state of PEMF technology demonstrates promising advancements, yet it also faces several challenges in its widespread adoption and efficacy optimization.
PEMF devices have evolved from bulky, stationary units to portable, user-friendly devices suitable for home use. Modern PEMF systems utilize a range of frequencies and intensities, typically between 1 Hz to 100 Hz and 0.1 mT to 20 mT, respectively. These parameters can be adjusted to target specific conditions or pain types, enhancing the therapy's versatility.
One of the primary technological advancements in PEMF therapy is the development of precise waveform generation. Current devices can produce various waveforms, including sine, square, and sawtooth waves, each potentially offering different therapeutic benefits. This allows for more targeted treatment protocols based on the specific pain condition being addressed.
Despite these advancements, PEMF technology faces several challenges. One significant hurdle is the lack of standardization in treatment protocols. The optimal frequency, intensity, and duration of PEMF therapy for different pain conditions remain subjects of ongoing research and debate. This variability in treatment parameters makes it difficult to establish universally accepted guidelines for PEMF therapy in pain management.
Another challenge lies in the mechanism of action of PEMF therapy on pain perception. While studies have shown that PEMF can modulate pain signaling pathways, the exact neurophysiological processes involved are not fully understood. This knowledge gap hinders the development of more targeted and effective PEMF treatments.
The miniaturization of PEMF devices, while beneficial for portability, presents technical challenges in maintaining consistent field strength and penetration depth. Ensuring that the electromagnetic field reaches the target tissues at therapeutic levels, especially in deeper structures, remains a significant technological hurdle.
Moreover, the long-term effects of PEMF therapy on human physiology are not yet comprehensively documented. This lack of long-term safety data poses a challenge in gaining widespread acceptance among healthcare professionals and regulatory bodies.
Lastly, the integration of PEMF technology with other pain management modalities and its incorporation into comprehensive pain treatment plans presents both opportunities and challenges. Developing systems that can work synergistically with other therapies, such as pharmacological interventions or physical therapy, is an area that requires further technological innovation and clinical validation.
PEMF devices have evolved from bulky, stationary units to portable, user-friendly devices suitable for home use. Modern PEMF systems utilize a range of frequencies and intensities, typically between 1 Hz to 100 Hz and 0.1 mT to 20 mT, respectively. These parameters can be adjusted to target specific conditions or pain types, enhancing the therapy's versatility.
One of the primary technological advancements in PEMF therapy is the development of precise waveform generation. Current devices can produce various waveforms, including sine, square, and sawtooth waves, each potentially offering different therapeutic benefits. This allows for more targeted treatment protocols based on the specific pain condition being addressed.
Despite these advancements, PEMF technology faces several challenges. One significant hurdle is the lack of standardization in treatment protocols. The optimal frequency, intensity, and duration of PEMF therapy for different pain conditions remain subjects of ongoing research and debate. This variability in treatment parameters makes it difficult to establish universally accepted guidelines for PEMF therapy in pain management.
Another challenge lies in the mechanism of action of PEMF therapy on pain perception. While studies have shown that PEMF can modulate pain signaling pathways, the exact neurophysiological processes involved are not fully understood. This knowledge gap hinders the development of more targeted and effective PEMF treatments.
The miniaturization of PEMF devices, while beneficial for portability, presents technical challenges in maintaining consistent field strength and penetration depth. Ensuring that the electromagnetic field reaches the target tissues at therapeutic levels, especially in deeper structures, remains a significant technological hurdle.
Moreover, the long-term effects of PEMF therapy on human physiology are not yet comprehensively documented. This lack of long-term safety data poses a challenge in gaining widespread acceptance among healthcare professionals and regulatory bodies.
Lastly, the integration of PEMF technology with other pain management modalities and its incorporation into comprehensive pain treatment plans presents both opportunities and challenges. Developing systems that can work synergistically with other therapies, such as pharmacological interventions or physical therapy, is an area that requires further technological innovation and clinical validation.
Existing PEMF Pain Modulation Techniques
01 PEMF therapy for pain reduction
Pulsed electromagnetic field (PEMF) therapy is used to reduce pain perception by applying electromagnetic fields to affected areas. This non-invasive treatment stimulates cellular repair and reduces inflammation, leading to pain relief in various conditions.- PEMF therapy for pain reduction: Pulsed Electromagnetic Field (PEMF) therapy is used to reduce pain perception by applying electromagnetic fields to affected areas. This non-invasive treatment stimulates cellular repair and reduces inflammation, leading to pain relief in various conditions.
- Customizable PEMF treatment protocols: Advanced PEMF devices allow for customizable treatment protocols, adjusting frequency, intensity, and duration based on the specific pain condition and patient needs. This personalization enhances the effectiveness of pain management.
- Combination of PEMF with other therapies: PEMF therapy can be combined with other pain management techniques, such as medication, physical therapy, or cognitive behavioral therapy, to create a comprehensive approach to pain reduction and improve overall treatment outcomes.
- PEMF devices for specific body areas: Specialized PEMF devices are designed to target specific body areas, such as joints, muscles, or the spine. These devices are optimized to deliver electromagnetic fields to the precise location of pain, enhancing the therapy's effectiveness.
- Monitoring and feedback systems in PEMF therapy: Advanced PEMF devices incorporate monitoring and feedback systems to track treatment progress and adjust therapy parameters in real-time. This allows for more precise pain management and helps healthcare providers optimize treatment plans.
02 Customizable PEMF treatment protocols
Advanced PEMF devices allow for customizable treatment protocols, including adjustable frequency, intensity, and duration of electromagnetic pulses. This personalization enables targeted therapy for different types of pain and individual patient needs.Expand Specific Solutions03 Combination of PEMF with other therapies
PEMF therapy can be combined with other pain management techniques, such as medication, physical therapy, or cognitive behavioral therapy, to enhance overall pain relief and improve patient outcomes.Expand Specific Solutions04 PEMF devices for specific body areas
Specialized PEMF devices are designed to target specific body areas, such as joints, muscles, or the spine. These devices are shaped to conform to the anatomy of the treatment area, ensuring optimal electromagnetic field delivery and pain relief.Expand Specific Solutions05 Monitoring and feedback systems in PEMF therapy
Advanced PEMF devices incorporate monitoring and feedback systems to track treatment progress and adjust therapy parameters in real-time. This allows for more effective pain management and helps healthcare providers optimize treatment plans based on patient responses.Expand Specific Solutions
Key PEMF Device Manufacturers
The field of PEMF (Pulsed Electromagnetic Field) therapy for pain perception modulation is in a growth phase, with increasing market size and technological advancements. The global PEMF therapy market is expanding due to rising chronic pain prevalence and growing awareness of non-invasive treatment options. Companies like Regenesis Biomedical, Medtronic, and Boston Scientific Neuromodulation are at the forefront, developing sophisticated PEMF devices. Emerging players such as SPR Therapeutics and Venus Concept are also contributing to innovation. The technology's maturity is progressing, with established firms like Orthofix and LG Chem investing in research and development to enhance efficacy and expand applications, indicating a competitive and evolving landscape in pain management solutions.
Regenesis Biomedical, Inc.
Technical Solution: Regenesis Biomedical has developed the Provant® Therapy System, a non-invasive PEMF device specifically designed for post-operative pain management and chronic wound healing. The system utilizes a proprietary pulsed radio frequency energy (PRFE) technology that operates at 27.12 MHz, a frequency shown to be effective in modulating cellular activity and reducing inflammation[10]. The Provant system delivers short treatment sessions of 30 minutes, making it convenient for outpatient use. Regenesis's approach focuses on promoting the body's natural healing processes, potentially reducing the need for pharmacological interventions in pain management[11].
Strengths: Non-invasive, short treatment sessions, dual application for pain and wound healing. Weaknesses: May have limited efficacy for deep-tissue pain, requires consistent use for optimal results.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced PEMF therapy devices for pain management, utilizing their proprietary AdaptiveStim technology. This system automatically adjusts stimulation based on the patient's body position and activity level, ensuring consistent pain relief[1]. Their devices employ multiple waveforms and frequencies to target different pain types, with some models reaching frequencies up to 10,000 Hz[2]. Medtronic's PEMF therapy integrates with their neurostimulation platforms, allowing for comprehensive pain management solutions that can be tailored to individual patient needs[3].
Strengths: Cutting-edge adaptive technology, wide range of frequencies, integration with existing neurostimulation platforms. Weaknesses: Higher cost compared to simpler PEMF devices, may require more complex patient training.
Core PEMF Pain Perception Research
Treatment of conditions susceptible to pulsed electromagnetic field therapy
PatentActiveUS20170354830A1
Innovation
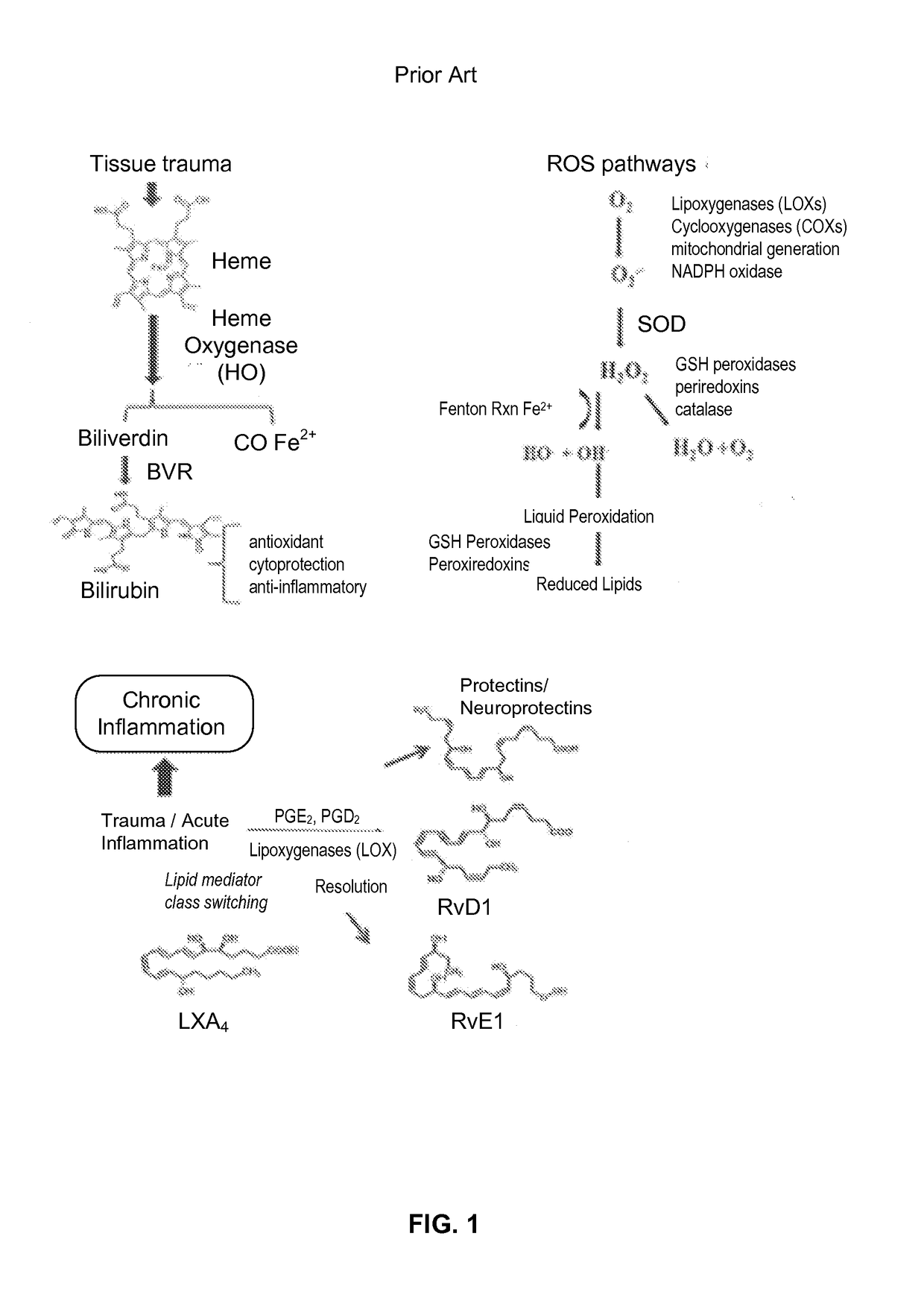
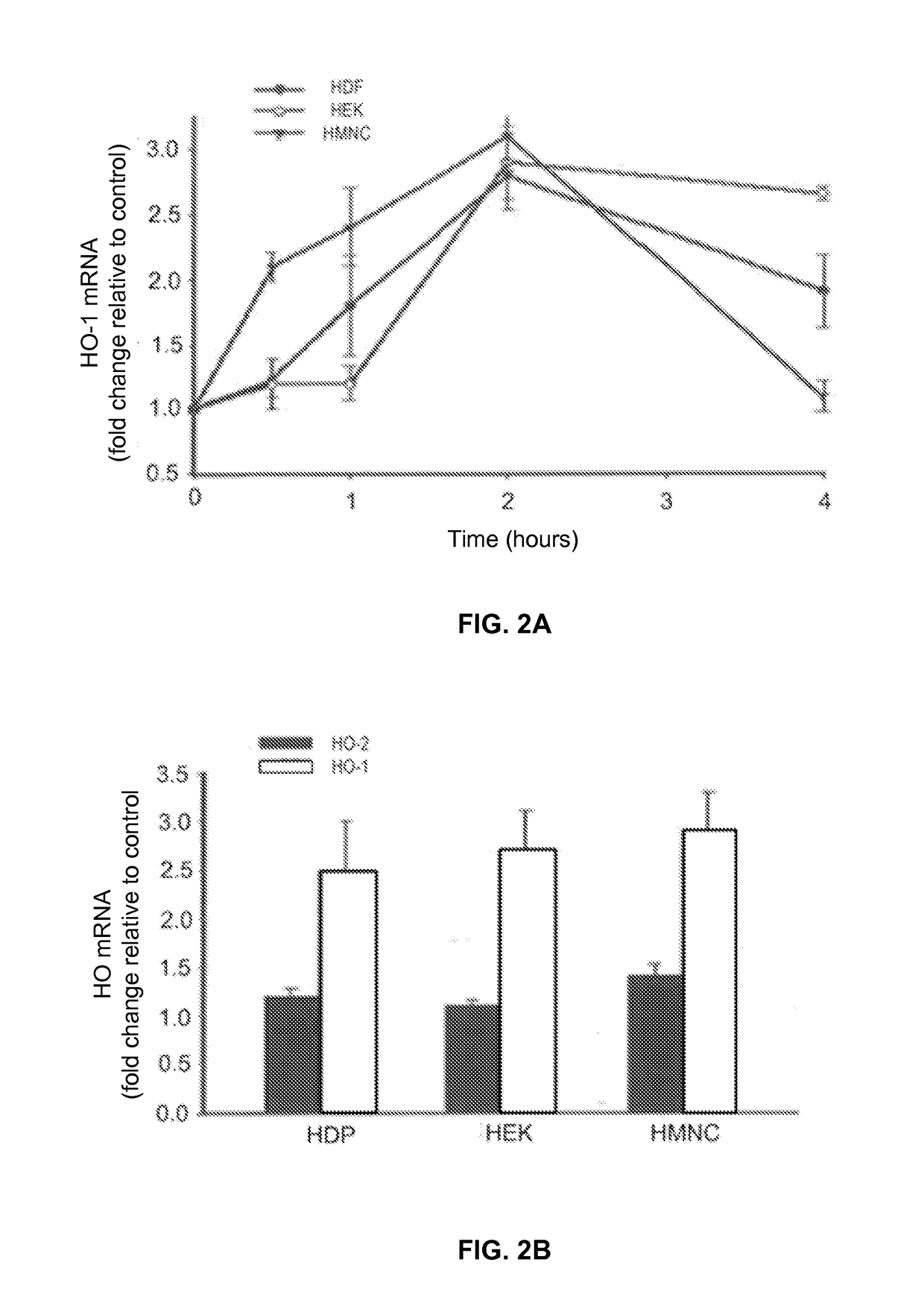
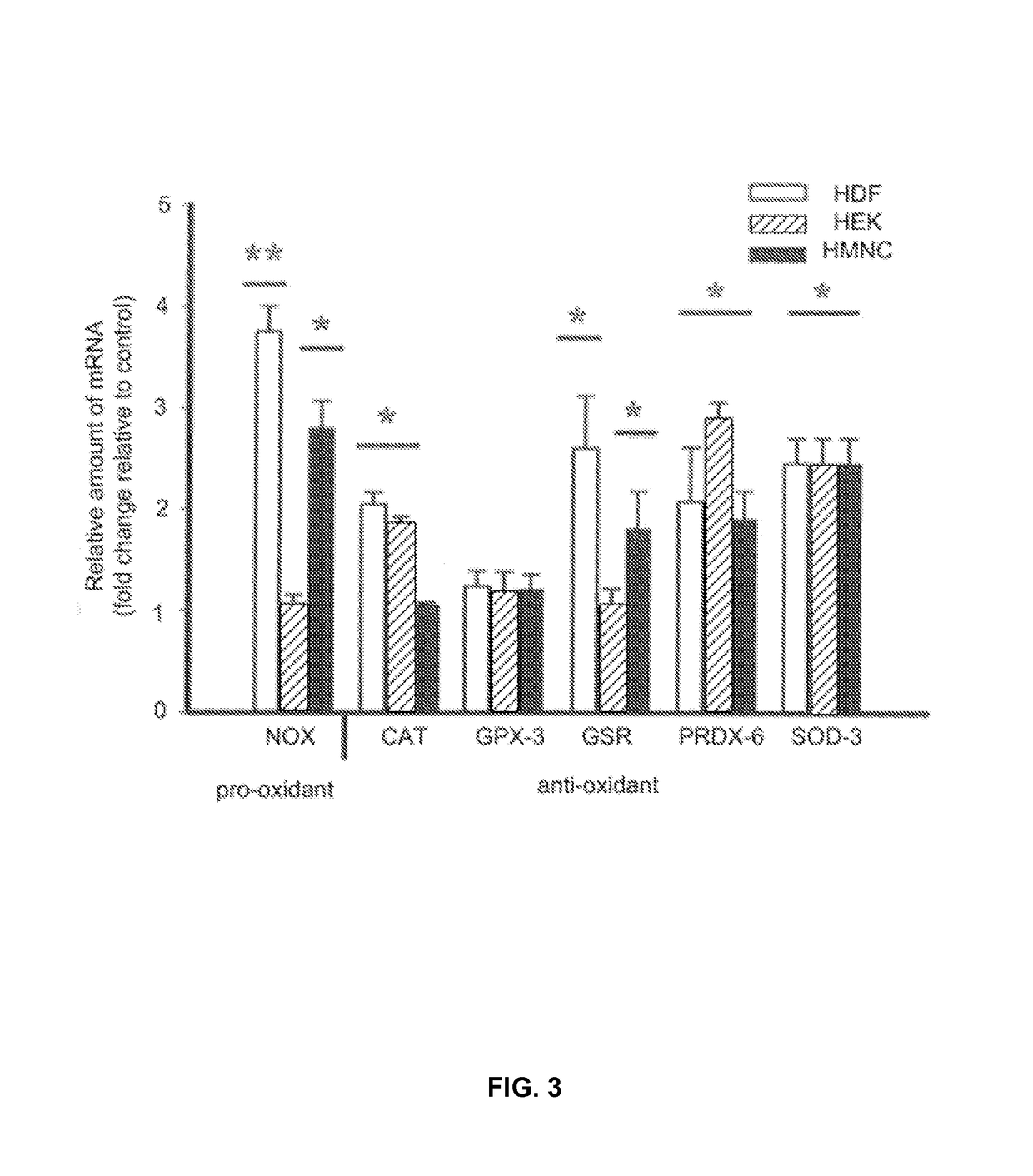
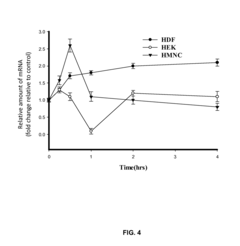
- PEMF therapy is administered to modulate gene expression associated with inflammation pathways, including heme oxygenase, antioxidant enzymes, lipid mediator biosynthesis, and cytokines, using specific parameters such as electric field strength, pulse rate, and duration to produce measurable clinical effects on pain, nerve function, and wound healing.
System and method for applying a low frequency magnetic field to biological tissues
PatentActiveUS20240091548A1
Innovation
- The use of pulsed electromagnetic fields (PEMF) with specific frequency ranges (5-50 kHz) and intensities (millitesla range) is proposed to target cellular mechanisms, including ion movement and voltage-gated channels, to enhance healing and reduce inflammation.
Clinical Trials and Efficacy Data
Clinical trials and efficacy data play a crucial role in understanding the effectiveness of Pulsed Electromagnetic Field (PEMF) therapy in pain perception modulation. Numerous studies have been conducted to evaluate the impact of PEMF on various types of pain conditions, providing valuable insights into its potential as a non-invasive treatment option.
A systematic review of randomized controlled trials published in the Journal of Pain Research in 2019 analyzed the efficacy of PEMF therapy for chronic musculoskeletal pain. The review included 12 studies with a total of 576 participants. Results showed that PEMF therapy significantly reduced pain intensity compared to placebo treatments, with a moderate effect size. The most substantial improvements were observed in patients with osteoarthritis and chronic low back pain.
Another notable clinical trial, published in the European Journal of Pain in 2020, focused on the use of PEMF therapy for fibromyalgia-related pain. This double-blind, placebo-controlled study involved 108 participants over a 12-week treatment period. The PEMF group reported a significant reduction in pain scores (measured using the Visual Analog Scale) compared to the placebo group, with a mean difference of 1.8 points on a 10-point scale.
A meta-analysis of PEMF therapy for knee osteoarthritis, published in Rheumatology International in 2018, included 10 randomized controlled trials with a total of 626 participants. The analysis revealed that PEMF therapy significantly improved pain and physical function in patients with knee osteoarthritis, with effect sizes ranging from small to moderate.
Efficacy data from these clinical trials suggest that PEMF therapy may modulate pain perception through various mechanisms. One proposed mechanism is the stimulation of endogenous opioid production, as evidenced by a study published in Pain Medicine in 2017. This placebo-controlled trial demonstrated increased levels of endorphins in patients receiving PEMF therapy for chronic low back pain.
While the majority of clinical trials show promising results, it is important to note that some studies have reported mixed or inconclusive findings. A Cochrane review on PEMF for osteoarthritis, updated in 2021, concluded that while PEMF may provide small to moderate benefits for pain relief, the quality of evidence was low to moderate, highlighting the need for further high-quality research.
The duration and frequency of PEMF therapy sessions vary across studies, ranging from daily treatments of 20-30 minutes to weekly sessions over several months. This variability in treatment protocols presents a challenge in standardizing PEMF therapy for pain management and underscores the importance of conducting dose-response studies to optimize treatment parameters.
A systematic review of randomized controlled trials published in the Journal of Pain Research in 2019 analyzed the efficacy of PEMF therapy for chronic musculoskeletal pain. The review included 12 studies with a total of 576 participants. Results showed that PEMF therapy significantly reduced pain intensity compared to placebo treatments, with a moderate effect size. The most substantial improvements were observed in patients with osteoarthritis and chronic low back pain.
Another notable clinical trial, published in the European Journal of Pain in 2020, focused on the use of PEMF therapy for fibromyalgia-related pain. This double-blind, placebo-controlled study involved 108 participants over a 12-week treatment period. The PEMF group reported a significant reduction in pain scores (measured using the Visual Analog Scale) compared to the placebo group, with a mean difference of 1.8 points on a 10-point scale.
A meta-analysis of PEMF therapy for knee osteoarthritis, published in Rheumatology International in 2018, included 10 randomized controlled trials with a total of 626 participants. The analysis revealed that PEMF therapy significantly improved pain and physical function in patients with knee osteoarthritis, with effect sizes ranging from small to moderate.
Efficacy data from these clinical trials suggest that PEMF therapy may modulate pain perception through various mechanisms. One proposed mechanism is the stimulation of endogenous opioid production, as evidenced by a study published in Pain Medicine in 2017. This placebo-controlled trial demonstrated increased levels of endorphins in patients receiving PEMF therapy for chronic low back pain.
While the majority of clinical trials show promising results, it is important to note that some studies have reported mixed or inconclusive findings. A Cochrane review on PEMF for osteoarthritis, updated in 2021, concluded that while PEMF may provide small to moderate benefits for pain relief, the quality of evidence was low to moderate, highlighting the need for further high-quality research.
The duration and frequency of PEMF therapy sessions vary across studies, ranging from daily treatments of 20-30 minutes to weekly sessions over several months. This variability in treatment protocols presents a challenge in standardizing PEMF therapy for pain management and underscores the importance of conducting dose-response studies to optimize treatment parameters.
Regulatory Framework for PEMF Devices
The regulatory framework for PEMF (Pulsed Electromagnetic Field) devices plays a crucial role in ensuring the safety and efficacy of these therapeutic tools in pain perception modulation. In the United States, the Food and Drug Administration (FDA) oversees the regulation of PEMF devices, classifying them as Class II medical devices. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
The FDA's regulatory approach for PEMF devices focuses on several key aspects. Firstly, manufacturers must provide evidence of the device's safety profile, including data on potential adverse effects and contraindications. This involves conducting clinical trials and safety studies to assess the device's impact on various patient populations. Secondly, the efficacy of the device in pain modulation must be demonstrated through well-designed clinical studies, with clear endpoints and measurable outcomes.
In addition to safety and efficacy, the FDA requires PEMF device manufacturers to adhere to strict quality control standards in their production processes. This includes implementing and maintaining a quality management system that complies with the FDA's Quality System Regulation (QSR). The QSR ensures that devices are consistently manufactured to meet the intended specifications and performance criteria.
Electromagnetic compatibility (EMC) is another critical aspect of PEMF device regulation. Manufacturers must demonstrate that their devices do not interfere with other electronic medical equipment and are not susceptible to electromagnetic interference from external sources. This involves compliance with specific EMC standards and testing protocols.
Internationally, regulatory frameworks for PEMF devices vary, but many countries follow guidelines similar to those of the FDA. The European Union, for instance, regulates PEMF devices under the Medical Device Regulation (MDR), which requires CE marking for market access. This process involves a conformity assessment to ensure compliance with safety and performance requirements.
As the field of PEMF therapy continues to evolve, regulatory bodies are adapting their frameworks to address emerging technologies and applications. This includes considering the integration of PEMF devices with digital health platforms and the use of artificial intelligence in treatment planning and monitoring. Regulatory agencies are also increasingly focusing on post-market surveillance to gather real-world data on the long-term safety and effectiveness of PEMF devices in pain management.
The FDA's regulatory approach for PEMF devices focuses on several key aspects. Firstly, manufacturers must provide evidence of the device's safety profile, including data on potential adverse effects and contraindications. This involves conducting clinical trials and safety studies to assess the device's impact on various patient populations. Secondly, the efficacy of the device in pain modulation must be demonstrated through well-designed clinical studies, with clear endpoints and measurable outcomes.
In addition to safety and efficacy, the FDA requires PEMF device manufacturers to adhere to strict quality control standards in their production processes. This includes implementing and maintaining a quality management system that complies with the FDA's Quality System Regulation (QSR). The QSR ensures that devices are consistently manufactured to meet the intended specifications and performance criteria.
Electromagnetic compatibility (EMC) is another critical aspect of PEMF device regulation. Manufacturers must demonstrate that their devices do not interfere with other electronic medical equipment and are not susceptible to electromagnetic interference from external sources. This involves compliance with specific EMC standards and testing protocols.
Internationally, regulatory frameworks for PEMF devices vary, but many countries follow guidelines similar to those of the FDA. The European Union, for instance, regulates PEMF devices under the Medical Device Regulation (MDR), which requires CE marking for market access. This process involves a conformity assessment to ensure compliance with safety and performance requirements.
As the field of PEMF therapy continues to evolve, regulatory bodies are adapting their frameworks to address emerging technologies and applications. This includes considering the integration of PEMF devices with digital health platforms and the use of artificial intelligence in treatment planning and monitoring. Regulatory agencies are also increasingly focusing on post-market surveillance to gather real-world data on the long-term safety and effectiveness of PEMF devices in pain management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!