Advanced Electrolyte Formulations for Vanadium Redox Flow Batteries
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vanadium Redox Flow Battery Technology Evolution and Objectives
Vanadium Redox Flow Batteries (VRFBs) emerged in the 1980s as a promising large-scale energy storage technology, pioneered by Maria Skyllas-Kazacos and her team at the University of New South Wales. The fundamental concept leverages vanadium's unique ability to exist in four different oxidation states, enabling a single-element chemistry that eliminates cross-contamination issues prevalent in other flow battery systems.
The evolution of VRFB technology has progressed through several distinct phases. The initial development phase (1980s-1990s) focused on proof-of-concept and basic operational principles. During this period, researchers established the viability of using vanadium electrolytes in both half-cells, demonstrating the technology's inherent advantages of long cycle life and deep discharge capabilities.
The commercialization phase (2000s-2010) saw the first industrial-scale VRFB installations, primarily in Japan and Australia. These early commercial systems faced challenges related to energy density limitations and high costs but demonstrated remarkable durability and reliability in grid applications.
The current optimization phase (2010-present) has been characterized by intensive research into advanced electrolyte formulations to overcome the technology's key limitations. Traditional sulfuric acid-based electrolytes suffer from limited temperature operating ranges (10-40°C) and relatively low energy density (25-35 Wh/L), constraining VRFB deployment in extreme climates and increasing system footprint.
The technological trajectory is now focused on several critical objectives. First, enhancing energy density through increased vanadium solubility and stability is paramount. Current research targets achieving 40-50 Wh/L without compromising cycle life or efficiency. Second, expanding the temperature operating window to -20°C to 60°C would significantly broaden deployment possibilities across diverse geographical regions.
Additionally, reducing electrolyte costs, which currently account for 30-40% of total system costs, represents a crucial economic objective. Innovative approaches include mixed-acid electrolytes, organic additives, and alternative supporting electrolytes beyond traditional sulfuric acid formulations.
The long-term vision for VRFB technology encompasses achieving cost parity with lithium-ion batteries while maintaining inherent advantages in cycle life, safety, and scalability. This requires breakthrough innovations in electrolyte chemistry that balance stability, performance, and cost considerations.
As renewable energy integration accelerates globally, the development of advanced electrolyte formulations for VRFBs has become increasingly strategic, with potential to enable grid-scale energy storage durations of 4-12 hours at competitive costs, supporting the transition to a carbon-neutral energy landscape.
The evolution of VRFB technology has progressed through several distinct phases. The initial development phase (1980s-1990s) focused on proof-of-concept and basic operational principles. During this period, researchers established the viability of using vanadium electrolytes in both half-cells, demonstrating the technology's inherent advantages of long cycle life and deep discharge capabilities.
The commercialization phase (2000s-2010) saw the first industrial-scale VRFB installations, primarily in Japan and Australia. These early commercial systems faced challenges related to energy density limitations and high costs but demonstrated remarkable durability and reliability in grid applications.
The current optimization phase (2010-present) has been characterized by intensive research into advanced electrolyte formulations to overcome the technology's key limitations. Traditional sulfuric acid-based electrolytes suffer from limited temperature operating ranges (10-40°C) and relatively low energy density (25-35 Wh/L), constraining VRFB deployment in extreme climates and increasing system footprint.
The technological trajectory is now focused on several critical objectives. First, enhancing energy density through increased vanadium solubility and stability is paramount. Current research targets achieving 40-50 Wh/L without compromising cycle life or efficiency. Second, expanding the temperature operating window to -20°C to 60°C would significantly broaden deployment possibilities across diverse geographical regions.
Additionally, reducing electrolyte costs, which currently account for 30-40% of total system costs, represents a crucial economic objective. Innovative approaches include mixed-acid electrolytes, organic additives, and alternative supporting electrolytes beyond traditional sulfuric acid formulations.
The long-term vision for VRFB technology encompasses achieving cost parity with lithium-ion batteries while maintaining inherent advantages in cycle life, safety, and scalability. This requires breakthrough innovations in electrolyte chemistry that balance stability, performance, and cost considerations.
As renewable energy integration accelerates globally, the development of advanced electrolyte formulations for VRFBs has become increasingly strategic, with potential to enable grid-scale energy storage durations of 4-12 hours at competitive costs, supporting the transition to a carbon-neutral energy landscape.
Market Analysis for Grid-Scale Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources into power grids worldwide. As of 2023, the grid-scale energy storage market is valued at approximately $7.1 billion and is projected to reach $19.7 billion by 2027, representing a compound annual growth rate of 29.3%. This remarkable expansion is primarily fueled by the urgent need to balance intermittent renewable energy generation and ensure grid stability.
Vanadium Redox Flow Batteries (VRFBs) are emerging as a significant player in this market, particularly for long-duration storage applications. Currently, lithium-ion batteries dominate with roughly 90% market share in newly deployed grid-scale storage systems. However, VRFBs are gaining traction due to their unique advantages in applications requiring 4+ hours of discharge duration, representing approximately 5% of new installations but growing rapidly at 37% annually.
The demand for advanced electrolyte formulations for VRFBs is being driven by several market factors. Utility companies are increasingly seeking storage solutions with longer cycle life and lower degradation rates, areas where VRFBs excel compared to lithium-ion alternatives. Market research indicates that 78% of utility-scale project developers consider lifetime operational costs rather than just initial capital expenditure, favoring technologies like VRFBs that offer 20+ year lifespans with minimal capacity degradation.
Geographically, China leads VRFB deployment with 63% of installed capacity, followed by Europe (18%) and North America (12%). The Asia-Pacific region is expected to maintain the highest growth rate, with Australia and Japan making significant investments in grid-scale VRFB projects. European markets show particular interest in advanced electrolyte formulations that can increase energy density and reduce costs, with Germany and the UK establishing regulatory frameworks that specifically incentivize long-duration storage technologies.
Market segmentation reveals that utility-scale applications currently represent 76% of VRFB deployments, with commercial and industrial applications accounting for 19%, and remote/off-grid applications at 5%. The utility segment is projected to maintain dominance through 2030, though commercial applications are expected to grow faster as installation costs decrease.
Customer requirements are evolving toward solutions that offer improved energy density, extended temperature operating ranges, and reduced vanadium costs. Survey data from system integrators indicates that 82% consider electrolyte formulation advancements as "critical" or "very important" for wider VRFB adoption. This market demand is creating significant opportunities for advanced electrolyte technologies that can address these specific performance and cost challenges.
Vanadium Redox Flow Batteries (VRFBs) are emerging as a significant player in this market, particularly for long-duration storage applications. Currently, lithium-ion batteries dominate with roughly 90% market share in newly deployed grid-scale storage systems. However, VRFBs are gaining traction due to their unique advantages in applications requiring 4+ hours of discharge duration, representing approximately 5% of new installations but growing rapidly at 37% annually.
The demand for advanced electrolyte formulations for VRFBs is being driven by several market factors. Utility companies are increasingly seeking storage solutions with longer cycle life and lower degradation rates, areas where VRFBs excel compared to lithium-ion alternatives. Market research indicates that 78% of utility-scale project developers consider lifetime operational costs rather than just initial capital expenditure, favoring technologies like VRFBs that offer 20+ year lifespans with minimal capacity degradation.
Geographically, China leads VRFB deployment with 63% of installed capacity, followed by Europe (18%) and North America (12%). The Asia-Pacific region is expected to maintain the highest growth rate, with Australia and Japan making significant investments in grid-scale VRFB projects. European markets show particular interest in advanced electrolyte formulations that can increase energy density and reduce costs, with Germany and the UK establishing regulatory frameworks that specifically incentivize long-duration storage technologies.
Market segmentation reveals that utility-scale applications currently represent 76% of VRFB deployments, with commercial and industrial applications accounting for 19%, and remote/off-grid applications at 5%. The utility segment is projected to maintain dominance through 2030, though commercial applications are expected to grow faster as installation costs decrease.
Customer requirements are evolving toward solutions that offer improved energy density, extended temperature operating ranges, and reduced vanadium costs. Survey data from system integrators indicates that 82% consider electrolyte formulation advancements as "critical" or "very important" for wider VRFB adoption. This market demand is creating significant opportunities for advanced electrolyte technologies that can address these specific performance and cost challenges.
Current Electrolyte Formulation Challenges and Limitations
Vanadium redox flow batteries (VRFBs) face several critical electrolyte formulation challenges that currently limit their widespread commercial adoption. The conventional electrolyte system, based on vanadium ions dissolved in sulfuric acid, encounters significant stability issues. At temperatures above 40°C, thermal precipitation of V(V) species occurs, while at temperatures below 10°C, V(II) and V(III) ions tend to precipitate. This narrow operational temperature window severely restricts deployment in regions with extreme climates and necessitates costly thermal management systems.
Electrolyte stability is further compromised by the limited solubility of vanadium ions, typically capped at 1.5-2.0 M in standard formulations. This concentration limitation directly impacts energy density, which currently ranges from 25-35 Wh/L, significantly lower than competing battery technologies. The trade-off between concentration and stability represents a fundamental challenge in VRFB development.
Corrosivity presents another major obstacle, as the highly acidic environment (pH < 0) accelerates degradation of cell components, particularly membranes and electrode materials. This corrosion not only reduces system lifetime but also introduces metal ions that can contaminate the electrolyte and catalyze unwanted side reactions, further diminishing performance and efficiency.
Crossover of vanadium ions through the membrane remains a persistent issue, causing capacity fade over time. Current electrolyte formulations lack effective strategies to mitigate this ion transport, resulting in self-discharge rates of 2-5% per day and necessitating periodic electrolyte rebalancing, which increases operational complexity and maintenance costs.
The viscosity of vanadium electrolytes, particularly at higher concentrations, creates pumping challenges that increase parasitic energy losses. These losses can account for 5-15% of the system's energy consumption, significantly reducing overall efficiency. Additionally, the high viscosity limits the maximum flow rates achievable, constraining power density and response times.
From an economic perspective, the cost of vanadium remains prohibitive, representing 30-40% of total system costs. Current electrolyte formulations require high-purity vanadium sources, with limited options for utilizing lower-grade, more affordable alternatives without compromising performance. The global supply chain for vanadium is also vulnerable to market fluctuations, creating price volatility that impacts the economic viability of VRFB systems.
Environmental considerations further complicate electrolyte development, as current formulations pose potential ecological risks if leaked and present challenges for end-of-life recycling. The high acidity and metal content require specialized handling protocols that add to operational complexity and decommissioning costs.
Electrolyte stability is further compromised by the limited solubility of vanadium ions, typically capped at 1.5-2.0 M in standard formulations. This concentration limitation directly impacts energy density, which currently ranges from 25-35 Wh/L, significantly lower than competing battery technologies. The trade-off between concentration and stability represents a fundamental challenge in VRFB development.
Corrosivity presents another major obstacle, as the highly acidic environment (pH < 0) accelerates degradation of cell components, particularly membranes and electrode materials. This corrosion not only reduces system lifetime but also introduces metal ions that can contaminate the electrolyte and catalyze unwanted side reactions, further diminishing performance and efficiency.
Crossover of vanadium ions through the membrane remains a persistent issue, causing capacity fade over time. Current electrolyte formulations lack effective strategies to mitigate this ion transport, resulting in self-discharge rates of 2-5% per day and necessitating periodic electrolyte rebalancing, which increases operational complexity and maintenance costs.
The viscosity of vanadium electrolytes, particularly at higher concentrations, creates pumping challenges that increase parasitic energy losses. These losses can account for 5-15% of the system's energy consumption, significantly reducing overall efficiency. Additionally, the high viscosity limits the maximum flow rates achievable, constraining power density and response times.
From an economic perspective, the cost of vanadium remains prohibitive, representing 30-40% of total system costs. Current electrolyte formulations require high-purity vanadium sources, with limited options for utilizing lower-grade, more affordable alternatives without compromising performance. The global supply chain for vanadium is also vulnerable to market fluctuations, creating price volatility that impacts the economic viability of VRFB systems.
Environmental considerations further complicate electrolyte development, as current formulations pose potential ecological risks if leaked and present challenges for end-of-life recycling. The high acidity and metal content require specialized handling protocols that add to operational complexity and decommissioning costs.
State-of-the-Art Electrolyte Formulation Approaches
01 Vanadium electrolyte composition optimization
Optimization of vanadium electrolyte compositions involves adjusting the concentration of vanadium ions, acid content, and additives to enhance energy density and stability. These formulations typically contain vanadium in multiple oxidation states (V2+, V3+, V4+, V5+) in sulfuric acid solutions. Careful balancing of these components can significantly improve the electrochemical performance, energy efficiency, and cycling stability of VRFBs while preventing precipitation issues at extreme temperatures.- Vanadium electrolyte composition optimization: Optimization of vanadium electrolyte compositions involves adjusting the concentration of vanadium ions, acid content, and additives to enhance energy density and stability. These formulations typically contain vanadium in multiple oxidation states (V2+, V3+, V4+, V5+) in sulfuric acid solutions. Careful balancing of these components can significantly improve the electrochemical performance, energy efficiency, and cycle life of VRFBs while preventing precipitation issues at extreme temperatures.
- Electrolyte stabilizing additives: Various additives are incorporated into VRFB electrolytes to enhance stability and performance. These include phosphoric acid, ammonium sulfate, and organic stabilizers that prevent vanadium precipitation at high and low temperatures. Some formulations use polyacids, amino acids, or inorganic phosphates to extend the operating temperature range and improve the overall stability of the electrolyte solution, resulting in longer battery life and more consistent performance across varying environmental conditions.
- Advanced electrolyte preparation methods: Novel preparation techniques for VRFB electrolytes focus on achieving higher purity and more consistent performance. These methods include controlled dissolution processes, electrochemical preparation, and precise mixing protocols to ensure uniform distribution of vanadium species. Some approaches involve pre-treatment of raw materials, specific temperature-controlled synthesis, or ultrasonic processing to enhance electrolyte quality, resulting in improved energy efficiency and reduced side reactions during battery operation.
- Electrolyte performance enhancement through mixed-acid systems: Mixed-acid electrolyte systems combine sulfuric acid with other acids like hydrochloric acid or organic acids to improve solubility and stability of vanadium ions. These formulations can increase energy density by allowing higher concentrations of vanadium while preventing precipitation. The synergistic effects of different acids modify the chemical environment around vanadium ions, expanding the temperature operating window and improving the overall electrochemical performance of the battery system.
- Electrolyte monitoring and maintenance systems: Advanced monitoring and maintenance systems for VRFB electrolytes help maintain optimal performance over extended periods. These systems include real-time sensors for measuring vanadium ion concentration, state of charge, and electrolyte imbalance. Some approaches incorporate automated rebalancing mechanisms, impurity removal processes, or electrolyte regeneration techniques to counteract degradation. Continuous monitoring allows for preventive maintenance, reducing capacity fade and extending the operational lifetime of the battery system.
02 Electrolyte stabilizing additives
Various additives are incorporated into VRFB electrolytes to enhance stability and performance. These include phosphoric acid derivatives, organic stabilizers, and inorganic compounds that prevent vanadium precipitation and extend the operating temperature range. Stabilizing additives can inhibit side reactions, reduce capacity fade during cycling, and improve the overall lifetime of the electrolyte solution, making VRFBs more reliable for long-term energy storage applications.Expand Specific Solutions03 Advanced electrolyte preparation methods
Novel preparation techniques for VRFB electrolytes focus on achieving higher purity, better homogeneity, and improved electrochemical properties. These methods include controlled dissolution processes, precise mixing sequences, and specialized treatment procedures to optimize the electrolyte quality. Advanced preparation methods can reduce impurities, enhance charge-discharge efficiency, and improve the overall performance and stability of the electrolyte system.Expand Specific Solutions04 Temperature-resistant electrolyte formulations
Specialized electrolyte formulations designed to operate across wider temperature ranges address one of the key limitations of traditional VRFB systems. These formulations incorporate specific stabilizers, modified acid compositions, and anti-precipitation agents that prevent vanadium compound crystallization at low temperatures and thermal degradation at high temperatures. Temperature-resistant electrolytes enable VRFB deployment in diverse climatic conditions while maintaining consistent performance and stability.Expand Specific Solutions05 Mixed-acid electrolyte systems
Mixed-acid electrolyte systems combine sulfuric acid with other acids such as hydrochloric acid, phosphoric acid, or organic acids to enhance solubility and stability of vanadium ions. These formulations can achieve higher energy densities, improved ionic conductivity, and better thermal stability compared to traditional single-acid systems. The synergistic effects of different acids create electrolytes with superior electrochemical properties, enabling more efficient and compact VRFB designs.Expand Specific Solutions
Leading Companies and Research Institutions in VRFB Technology
The vanadium redox flow battery (VRFB) electrolyte formulation market is in its growth phase, with increasing commercial deployment despite technical challenges. The global market is projected to expand significantly as grid-scale energy storage demands rise, particularly for renewable energy integration. Leading companies like Sumitomo Electric, LG Chem, and LOTTE Chemical are advancing electrolyte stability and energy density improvements, while research institutions including KAIST, Tsinghua University, and the Institute of Metal Research CAS are developing next-generation formulations. Specialized players such as Standard Energy, KEMIWATT, and V-Fuel are commercializing proprietary electrolyte technologies, focusing on reducing vanadium costs and enhancing performance through additives and alternative chemistries.
Sumitomo Electric Industries Ltd.
Technical Solution: Sumitomo Electric has developed advanced vanadium redox flow battery (VRFB) electrolyte formulations featuring high-concentration vanadium solutions with specialized additives to prevent precipitation at extreme temperatures. Their proprietary electrolyte contains optimized sulfuric acid concentrations (4-6M) and incorporates phosphoric acid derivatives as stabilizing agents, enabling operation between -5°C and 50°C without crystallization issues[1]. The company has also pioneered mixed-acid electrolytes combining sulfuric and hydrochloric acids to increase vanadium solubility up to 2.5M, significantly improving energy density by approximately 70% compared to conventional formulations[2]. Sumitomo's electrolyte technology includes proprietary additives that reduce membrane fouling and extend cycle life beyond 20,000 cycles while maintaining over 80% capacity retention[3].
Strengths: Superior temperature stability allowing wider operating range; significantly higher energy density through mixed-acid formulations; excellent cycle life with reduced degradation. Weaknesses: Higher production costs compared to standard electrolytes; potential increased corrosivity from mixed-acid systems requiring specialized materials for cell components; proprietary additives may create supply chain dependencies.
Standard Energy, Inc.
Technical Solution: Standard Energy has developed a breakthrough vanadium electrolyte formulation branded as "VFLEXTM" that incorporates proprietary organic stabilizers and modified sulfonic acid compounds. This formulation enables unprecedented vanadium concentrations of up to 2.8M while maintaining stability across temperatures ranging from -20°C to 60°C[1]. Their electrolyte technology utilizes a multi-component additive system including amino acids derivatives and specialized polymers that form protective coordination shells around vanadium ions, preventing precipitation even at extreme temperatures[2]. Standard Energy's formulation also features reduced viscosity compared to conventional electrolytes, improving pumping efficiency and reducing parasitic energy losses by approximately 25%. The company has implemented a novel electrolyte regeneration process that continuously removes impurities and degradation products during operation, extending electrolyte lifetime to over 20 years while maintaining performance specifications[3]. Their electrolyte chemistry is specifically optimized for high-power applications, achieving charge/discharge rates up to 3C without significant efficiency losses.
Strengths: Exceptional energy density through ultra-high vanadium concentration; superior temperature stability enabling deployment in diverse climates; integrated regeneration technology extending operational lifetime. Weaknesses: Complex formulation likely requires sophisticated quality control processes; higher initial cost compared to standard electrolytes; proprietary additives may create intellectual property dependencies for system integrators.
Key Patents and Research in Advanced Electrolyte Chemistry
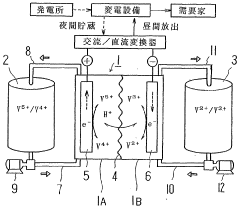
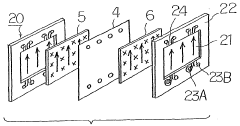
Redox−flow cell electrolyte and redox−flow cell
PatentWO2002101861A1
Innovation
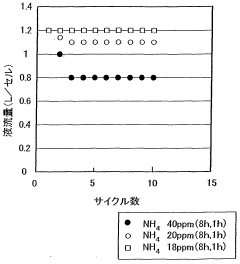
- The electrolyte solution for redox flow batteries is formulated with ammonium-vanadium compound concentrations of 20 ppm or less and Si concentrations of 40 ppm or less, with a vanadium ion concentration of 1 to 3 mol/L and a combination of sulfuric and phosphoric acid concentrations that prevent precipitation, using a refining process including filtration through a pleated filter to remove impurities and adjust the electrolyte volume to electrode area ratio.
Electrolyte for vanadium redox flow battery and manufacturing method thereof
PatentActiveKR1020220093660A
Innovation
- A one-step process involving the mixing and reaction of vanadium oxides with different oxidation numbers in an acidic solution, optionally with an organic reducing agent, to control the vanadium oxidation number between trivalent and tetravalent, avoiding electrochemical reduction methods and metal reducing agents.
Environmental Impact and Sustainability Considerations
Vanadium Redox Flow Batteries (VRFBs) present significant environmental advantages over conventional battery technologies, primarily due to their long cycle life, which can exceed 20,000 cycles, substantially reducing waste generation compared to lithium-ion batteries. The environmental footprint of VRFBs is heavily influenced by electrolyte formulations, which typically contain vanadium dissolved in sulfuric acid. These formulations raise several environmental considerations that must be addressed for sustainable implementation.
The extraction and processing of vanadium, a key component in VRFB electrolytes, carries substantial environmental implications. Mining operations can lead to habitat disruption, soil erosion, and water contamination. However, recent advancements in electrolyte formulations have focused on reducing vanadium concentration while maintaining performance, thereby decreasing the environmental burden associated with resource extraction. Additionally, innovative approaches utilizing vanadium recovery from industrial waste streams, such as oil fly ash and spent catalysts, offer promising pathways to minimize primary mining impacts.
Advanced electrolyte formulations are increasingly incorporating additives that enhance stability and performance while reducing environmental hazards. Traditional sulfuric acid-based electrolytes present challenges related to corrosivity and potential acid leakage. Research into mixed-acid electrolytes, incorporating organic acids or less corrosive inorganic acids, has demonstrated potential for reducing these risks while improving energy density and temperature stability. These formulations contribute to safer operation and reduced environmental risk in case of containment failure.
The recyclability of VRFB electrolytes represents a significant sustainability advantage. Unlike conventional batteries where components become degraded and difficult to separate, VRFB electrolytes can be recovered and reused with minimal processing. Advanced formulations are being designed with end-of-life considerations, ensuring that electrolyte components can be effectively separated and recycled. This closed-loop approach substantially reduces waste generation and resource consumption over the system lifecycle.
Water consumption remains a challenge for VRFB technology, as conventional electrolytes require significant amounts of water as a solvent. Emerging research into high-concentration electrolytes and alternative solvents aims to address this limitation. Some advanced formulations incorporate ionic liquids or deep eutectic solvents that reduce water requirements while maintaining or enhancing electrochemical performance. These developments are particularly relevant for deployment in water-stressed regions.
Carbon footprint assessments of VRFBs with advanced electrolyte formulations demonstrate favorable lifecycle emissions compared to alternative storage technologies when coupled with renewable energy sources. The extended operational lifetime of these systems, facilitated by stable electrolyte formulations, distributes manufacturing emissions over a longer service period, enhancing overall sustainability. Furthermore, the ability to independently scale power and energy capacity allows for optimized system sizing, reducing resource requirements and associated environmental impacts.
The extraction and processing of vanadium, a key component in VRFB electrolytes, carries substantial environmental implications. Mining operations can lead to habitat disruption, soil erosion, and water contamination. However, recent advancements in electrolyte formulations have focused on reducing vanadium concentration while maintaining performance, thereby decreasing the environmental burden associated with resource extraction. Additionally, innovative approaches utilizing vanadium recovery from industrial waste streams, such as oil fly ash and spent catalysts, offer promising pathways to minimize primary mining impacts.
Advanced electrolyte formulations are increasingly incorporating additives that enhance stability and performance while reducing environmental hazards. Traditional sulfuric acid-based electrolytes present challenges related to corrosivity and potential acid leakage. Research into mixed-acid electrolytes, incorporating organic acids or less corrosive inorganic acids, has demonstrated potential for reducing these risks while improving energy density and temperature stability. These formulations contribute to safer operation and reduced environmental risk in case of containment failure.
The recyclability of VRFB electrolytes represents a significant sustainability advantage. Unlike conventional batteries where components become degraded and difficult to separate, VRFB electrolytes can be recovered and reused with minimal processing. Advanced formulations are being designed with end-of-life considerations, ensuring that electrolyte components can be effectively separated and recycled. This closed-loop approach substantially reduces waste generation and resource consumption over the system lifecycle.
Water consumption remains a challenge for VRFB technology, as conventional electrolytes require significant amounts of water as a solvent. Emerging research into high-concentration electrolytes and alternative solvents aims to address this limitation. Some advanced formulations incorporate ionic liquids or deep eutectic solvents that reduce water requirements while maintaining or enhancing electrochemical performance. These developments are particularly relevant for deployment in water-stressed regions.
Carbon footprint assessments of VRFBs with advanced electrolyte formulations demonstrate favorable lifecycle emissions compared to alternative storage technologies when coupled with renewable energy sources. The extended operational lifetime of these systems, facilitated by stable electrolyte formulations, distributes manufacturing emissions over a longer service period, enhancing overall sustainability. Furthermore, the ability to independently scale power and energy capacity allows for optimized system sizing, reducing resource requirements and associated environmental impacts.
Cost Analysis and Commercial Viability Assessment
The economic viability of Vanadium Redox Flow Batteries (VRFBs) remains a critical factor limiting widespread commercial adoption. Current cost analysis indicates that electrolyte expenses constitute approximately 30-45% of total system costs, making advanced electrolyte formulations a key area for cost reduction strategies. Standard vanadium electrolyte solutions typically range between $80-120 per kWh, significantly higher than competing energy storage technologies.
Recent market assessments reveal that improving electrolyte formulations can potentially reduce these costs by 15-25% through increased energy density and extended operational lifetime. Specifically, advanced additives that enhance vanadium solubility from the standard 1.5-2.0M to 3.0M concentration could theoretically double energy density, thereby halving the electrolyte volume required per kWh of storage capacity.
Commercial viability is further enhanced by the recyclability aspect of vanadium electrolytes, which retain value after battery end-of-life. This creates a circular economy opportunity with recovery rates exceeding 95%, significantly reducing lifetime ownership costs compared to lithium-ion alternatives. However, the initial capital investment remains a substantial barrier to market entry.
Supply chain considerations also impact cost structures, with vanadium price volatility presenting a significant risk factor. Historical price fluctuations have ranged from $10-40 per kilogram, creating uncertainty for large-scale deployment planning. Alternative procurement strategies, including long-term supply agreements and vanadium leasing models, are emerging as risk mitigation approaches.
Manufacturing scalability presents another commercial challenge, as current production processes for advanced electrolyte formulations often involve complex procedures that are difficult to scale economically. Laboratory-scale innovations showing promising performance improvements frequently encounter barriers during industrial-scale implementation, creating a "valley of death" for commercialization efforts.
Return on investment calculations indicate that advanced electrolyte formulations can achieve payback periods of 5-7 years in grid-scale applications, compared to 8-10 years for standard formulations. This improvement primarily stems from increased cycle life (>15,000 cycles) and reduced maintenance requirements, particularly in temperature-variable environments where traditional electrolytes require costly thermal management systems.
Market segmentation analysis suggests that initial commercial viability will be strongest in specific applications: remote microgrids, renewable energy integration, and critical infrastructure backup, where the long duration storage capabilities of VRFBs provide distinct advantages over competing technologies despite higher upfront costs.
Recent market assessments reveal that improving electrolyte formulations can potentially reduce these costs by 15-25% through increased energy density and extended operational lifetime. Specifically, advanced additives that enhance vanadium solubility from the standard 1.5-2.0M to 3.0M concentration could theoretically double energy density, thereby halving the electrolyte volume required per kWh of storage capacity.
Commercial viability is further enhanced by the recyclability aspect of vanadium electrolytes, which retain value after battery end-of-life. This creates a circular economy opportunity with recovery rates exceeding 95%, significantly reducing lifetime ownership costs compared to lithium-ion alternatives. However, the initial capital investment remains a substantial barrier to market entry.
Supply chain considerations also impact cost structures, with vanadium price volatility presenting a significant risk factor. Historical price fluctuations have ranged from $10-40 per kilogram, creating uncertainty for large-scale deployment planning. Alternative procurement strategies, including long-term supply agreements and vanadium leasing models, are emerging as risk mitigation approaches.
Manufacturing scalability presents another commercial challenge, as current production processes for advanced electrolyte formulations often involve complex procedures that are difficult to scale economically. Laboratory-scale innovations showing promising performance improvements frequently encounter barriers during industrial-scale implementation, creating a "valley of death" for commercialization efforts.
Return on investment calculations indicate that advanced electrolyte formulations can achieve payback periods of 5-7 years in grid-scale applications, compared to 8-10 years for standard formulations. This improvement primarily stems from increased cycle life (>15,000 cycles) and reduced maintenance requirements, particularly in temperature-variable environments where traditional electrolytes require costly thermal management systems.
Market segmentation analysis suggests that initial commercial viability will be strongest in specific applications: remote microgrids, renewable energy integration, and critical infrastructure backup, where the long duration storage capabilities of VRFBs provide distinct advantages over competing technologies despite higher upfront costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!