Ionic Liquids in Next Generation Redox Flow Systems
OCT 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquids Evolution and Research Objectives
Ionic liquids (ILs) have emerged as a revolutionary class of materials in electrochemical energy storage systems, particularly in redox flow batteries (RFBs). The evolution of ionic liquids began in the early 1900s with the discovery of ethylammonium nitrate, but significant research interest only developed in the late 1990s when room-temperature ionic liquids with enhanced stability were synthesized. These unique substances, composed entirely of ions and liquid at room temperature, have since undergone rapid development phases, transitioning from academic curiosity to practical applications in energy storage technologies.
The historical trajectory of ionic liquids in energy applications has been marked by three distinct generations. First-generation ILs focused primarily on their use as electrolytes, leveraging their wide electrochemical windows and thermal stability. Second-generation development expanded to task-specific ionic liquids with functionalized structures designed for particular electrochemical reactions. The current third-generation research explores multi-functional ionic liquids that can simultaneously serve as electrolytes, active materials, and performance enhancers in redox flow systems.
Recent advancements have demonstrated the potential of ionic liquids to address critical limitations in conventional aqueous redox flow batteries. Their negligible vapor pressure, non-flammability, and exceptional electrochemical stability make them particularly attractive for next-generation energy storage systems requiring enhanced safety profiles and operational longevity. Furthermore, the virtually unlimited structural variations possible through cation and anion combinations offer unprecedented opportunities for tailored electrochemical properties.
The primary research objectives in this field now center on several key areas. First, developing ionic liquids with optimized viscosity and conductivity properties to enhance mass transport and energy efficiency in flow systems. Second, designing redox-active ionic liquids that can function as both electrolyte and active material, potentially increasing energy density while simplifying system architecture. Third, exploring hybrid systems that combine the advantages of ionic liquids with conventional aqueous or organic electrolytes to achieve balanced performance metrics.
Additional research goals include understanding the fundamental ion transport mechanisms in confined flow channels, investigating long-term stability under cycling conditions, and developing cost-effective synthesis routes for specialized ionic liquids. Computational modeling and high-throughput screening methodologies are increasingly employed to accelerate the discovery of optimal ionic liquid compositions for specific redox chemistries.
The ultimate objective remains the creation of commercially viable ionic liquid-based redox flow systems that can deliver superior energy density, cycle life, and safety compared to current technologies, while maintaining reasonable manufacturing costs and environmental sustainability profiles. This requires interdisciplinary collaboration spanning electrochemistry, materials science, chemical engineering, and computational modeling to overcome existing technical barriers.
The historical trajectory of ionic liquids in energy applications has been marked by three distinct generations. First-generation ILs focused primarily on their use as electrolytes, leveraging their wide electrochemical windows and thermal stability. Second-generation development expanded to task-specific ionic liquids with functionalized structures designed for particular electrochemical reactions. The current third-generation research explores multi-functional ionic liquids that can simultaneously serve as electrolytes, active materials, and performance enhancers in redox flow systems.
Recent advancements have demonstrated the potential of ionic liquids to address critical limitations in conventional aqueous redox flow batteries. Their negligible vapor pressure, non-flammability, and exceptional electrochemical stability make them particularly attractive for next-generation energy storage systems requiring enhanced safety profiles and operational longevity. Furthermore, the virtually unlimited structural variations possible through cation and anion combinations offer unprecedented opportunities for tailored electrochemical properties.
The primary research objectives in this field now center on several key areas. First, developing ionic liquids with optimized viscosity and conductivity properties to enhance mass transport and energy efficiency in flow systems. Second, designing redox-active ionic liquids that can function as both electrolyte and active material, potentially increasing energy density while simplifying system architecture. Third, exploring hybrid systems that combine the advantages of ionic liquids with conventional aqueous or organic electrolytes to achieve balanced performance metrics.
Additional research goals include understanding the fundamental ion transport mechanisms in confined flow channels, investigating long-term stability under cycling conditions, and developing cost-effective synthesis routes for specialized ionic liquids. Computational modeling and high-throughput screening methodologies are increasingly employed to accelerate the discovery of optimal ionic liquid compositions for specific redox chemistries.
The ultimate objective remains the creation of commercially viable ionic liquid-based redox flow systems that can deliver superior energy density, cycle life, and safety compared to current technologies, while maintaining reasonable manufacturing costs and environmental sustainability profiles. This requires interdisciplinary collaboration spanning electrochemistry, materials science, chemical engineering, and computational modeling to overcome existing technical barriers.
Market Analysis for Ionic Liquid-Based Energy Storage
The global market for energy storage solutions is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources and the need for grid stabilization. Ionic liquid-based energy storage systems, particularly next-generation redox flow batteries (RFBs), are emerging as promising technologies within this expanding market landscape. Current market valuations place the global energy storage sector at approximately $162 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 8.5% through 2030.
Ionic liquid-based energy storage systems represent a specialized but rapidly growing segment within this market. While traditional aqueous vanadium redox flow batteries currently dominate the flow battery market, ionic liquid-based systems are gaining significant attention due to their enhanced performance characteristics. Market research indicates that ionic liquid electrolytes could potentially capture 15-20% of the flow battery market within the next decade, representing a substantial growth opportunity.
The demand drivers for ionic liquid-based energy storage are multifaceted. Primary among these is the superior energy density offered by ionic liquid electrolytes, which addresses a critical limitation of conventional flow battery technologies. Market analysis reveals that energy density improvements of 30-50% are achievable with ionic liquid formulations, significantly enhancing the commercial viability of these systems for space-constrained applications.
Regional market analysis shows varying adoption patterns. Asia-Pacific, particularly China, Japan, and South Korea, leads in research investment and early commercial deployment, accounting for approximately 45% of global development activities. North America follows with 30% market share, while Europe contributes about 20% to the global development landscape, with particular strength in fundamental research and materials innovation.
End-user segmentation reveals diverse application potential. Utility-scale storage represents the largest potential market segment (65%), followed by commercial and industrial applications (25%), and specialized applications such as microgrids and remote power systems (10%). The utility sector's interest is primarily driven by the extended operational temperature range of ionic liquid systems, which reduces cooling requirements and improves deployment flexibility.
Investment trends indicate growing financial commitment to the sector. Venture capital funding for ionic liquid-based energy storage technologies has increased by 35% year-over-year since 2020, reaching approximately $780 million in 2023. Strategic partnerships between materials science companies, battery manufacturers, and energy utilities are becoming increasingly common, accelerating the path to commercialization.
Market barriers remain significant, with cost being the primary challenge. Current production costs for ionic liquid electrolytes exceed conventional alternatives by 2.5-3 times, though economies of scale are expected to narrow this gap substantially. Technical challenges related to viscosity management and long-term stability also impact market penetration rates, though recent innovations show promising solutions to these limitations.
Ionic liquid-based energy storage systems represent a specialized but rapidly growing segment within this market. While traditional aqueous vanadium redox flow batteries currently dominate the flow battery market, ionic liquid-based systems are gaining significant attention due to their enhanced performance characteristics. Market research indicates that ionic liquid electrolytes could potentially capture 15-20% of the flow battery market within the next decade, representing a substantial growth opportunity.
The demand drivers for ionic liquid-based energy storage are multifaceted. Primary among these is the superior energy density offered by ionic liquid electrolytes, which addresses a critical limitation of conventional flow battery technologies. Market analysis reveals that energy density improvements of 30-50% are achievable with ionic liquid formulations, significantly enhancing the commercial viability of these systems for space-constrained applications.
Regional market analysis shows varying adoption patterns. Asia-Pacific, particularly China, Japan, and South Korea, leads in research investment and early commercial deployment, accounting for approximately 45% of global development activities. North America follows with 30% market share, while Europe contributes about 20% to the global development landscape, with particular strength in fundamental research and materials innovation.
End-user segmentation reveals diverse application potential. Utility-scale storage represents the largest potential market segment (65%), followed by commercial and industrial applications (25%), and specialized applications such as microgrids and remote power systems (10%). The utility sector's interest is primarily driven by the extended operational temperature range of ionic liquid systems, which reduces cooling requirements and improves deployment flexibility.
Investment trends indicate growing financial commitment to the sector. Venture capital funding for ionic liquid-based energy storage technologies has increased by 35% year-over-year since 2020, reaching approximately $780 million in 2023. Strategic partnerships between materials science companies, battery manufacturers, and energy utilities are becoming increasingly common, accelerating the path to commercialization.
Market barriers remain significant, with cost being the primary challenge. Current production costs for ionic liquid electrolytes exceed conventional alternatives by 2.5-3 times, though economies of scale are expected to narrow this gap substantially. Technical challenges related to viscosity management and long-term stability also impact market penetration rates, though recent innovations show promising solutions to these limitations.
Technical Barriers and Global Development Status
Despite significant advancements in ionic liquid-based redox flow battery (RFB) systems, several technical barriers continue to impede their widespread commercial adoption. The primary challenge remains the high viscosity of ionic liquids, which significantly reduces ion mobility and consequently limits power density. This inherent property necessitates sophisticated engineering solutions for pumping systems and increases parasitic energy losses during operation.
Ionic conductivity presents another substantial hurdle, as most ionic liquids exhibit conductivities lower than aqueous electrolytes by an order of magnitude. This limitation directly impacts the internal resistance of the battery system, reducing overall efficiency and power output capabilities. Additionally, the electrochemical stability window, while theoretically wide, often narrows in practical applications due to impurities and degradation mechanisms that are not fully understood.
Cost factors remain prohibitive for large-scale implementation, with high-purity ionic liquids typically priced at $100-1000/kg compared to conventional electrolytes at $1-10/kg. This economic barrier is compounded by complex synthesis procedures and purification requirements that have not yet been optimized for industrial-scale production.
The global development landscape shows distinct regional focuses. North American research institutions primarily concentrate on fundamental electrochemistry and novel redox-active species compatible with ionic liquids. The Pacific Northwest National Laboratory and Massachusetts Institute of Technology lead efforts in developing non-aqueous flow systems with enhanced energy densities.
European research exhibits strong emphasis on sustainability aspects, with German and French institutions pioneering recyclable ionic liquid formulations and reduced environmental impact manufacturing processes. The European Union's Horizon Europe program has allocated substantial funding specifically for next-generation energy storage technologies including ionic liquid-based systems.
In Asia, particularly China and Japan, development efforts focus on scaling and manufacturing optimization. The Chinese Academy of Sciences has established dedicated facilities for pilot-scale production of ionic liquid electrolytes, while Japanese corporations like Sumitomo Electric Industries are integrating ionic liquid technologies into their existing flow battery platforms.
Australia has emerged as a significant player through the work of Monash University and CSIRO, focusing on ionic liquids specifically designed for high-temperature applications in remote locations. Their research addresses thermal stability challenges that conventional systems cannot overcome.
Collaborative international efforts remain limited, with intellectual property concerns creating silos in research and development. This fragmentation has slowed progress in standardization and interoperability, which are crucial for market acceptance and integration into existing energy infrastructure.
Ionic conductivity presents another substantial hurdle, as most ionic liquids exhibit conductivities lower than aqueous electrolytes by an order of magnitude. This limitation directly impacts the internal resistance of the battery system, reducing overall efficiency and power output capabilities. Additionally, the electrochemical stability window, while theoretically wide, often narrows in practical applications due to impurities and degradation mechanisms that are not fully understood.
Cost factors remain prohibitive for large-scale implementation, with high-purity ionic liquids typically priced at $100-1000/kg compared to conventional electrolytes at $1-10/kg. This economic barrier is compounded by complex synthesis procedures and purification requirements that have not yet been optimized for industrial-scale production.
The global development landscape shows distinct regional focuses. North American research institutions primarily concentrate on fundamental electrochemistry and novel redox-active species compatible with ionic liquids. The Pacific Northwest National Laboratory and Massachusetts Institute of Technology lead efforts in developing non-aqueous flow systems with enhanced energy densities.
European research exhibits strong emphasis on sustainability aspects, with German and French institutions pioneering recyclable ionic liquid formulations and reduced environmental impact manufacturing processes. The European Union's Horizon Europe program has allocated substantial funding specifically for next-generation energy storage technologies including ionic liquid-based systems.
In Asia, particularly China and Japan, development efforts focus on scaling and manufacturing optimization. The Chinese Academy of Sciences has established dedicated facilities for pilot-scale production of ionic liquid electrolytes, while Japanese corporations like Sumitomo Electric Industries are integrating ionic liquid technologies into their existing flow battery platforms.
Australia has emerged as a significant player through the work of Monash University and CSIRO, focusing on ionic liquids specifically designed for high-temperature applications in remote locations. Their research addresses thermal stability challenges that conventional systems cannot overcome.
Collaborative international efforts remain limited, with intellectual property concerns creating silos in research and development. This fragmentation has slowed progress in standardization and interoperability, which are crucial for market acceptance and integration into existing energy infrastructure.
Current Ionic Liquid Electrolyte Solutions
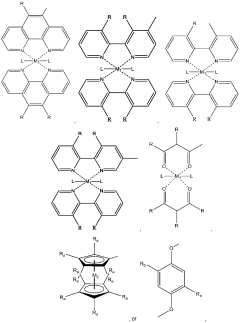
01 Ionic liquids in electrochemical applications
Ionic liquids are used in various electrochemical applications due to their excellent conductivity and stability. They serve as electrolytes in batteries, fuel cells, and supercapacitors, offering advantages such as wide electrochemical windows, low volatility, and high thermal stability. These properties make them superior alternatives to conventional electrolytes, enabling the development of more efficient and safer energy storage and conversion devices.- Ionic liquids in electrochemical applications: Ionic liquids are used in various electrochemical applications due to their excellent conductivity and stability. They serve as electrolytes in batteries, fuel cells, and other energy storage devices, offering advantages such as wide electrochemical windows, low volatility, and high thermal stability. These properties make them superior alternatives to conventional electrolytes, enabling the development of more efficient and safer electrochemical systems.
- Ionic liquids for separation and purification processes: Ionic liquids are employed in separation and purification processes across various industries. Their tunable properties allow for selective extraction of target compounds from complex mixtures. They can be designed to have specific affinities for certain molecules, making them effective in applications such as gas separation, metal extraction, and purification of biomolecules. Their low volatility and high stability make them environmentally friendly alternatives to conventional organic solvents.
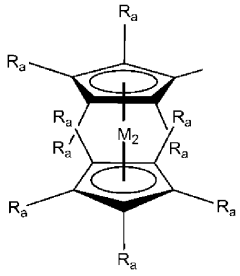
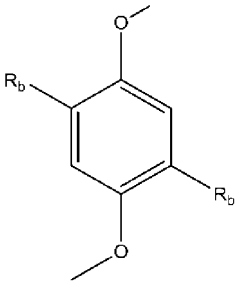
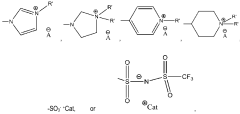
- Synthesis and preparation of novel ionic liquids: Various methods for synthesizing and preparing novel ionic liquids have been developed to create compounds with specific properties. These methods include direct quaternization, anion exchange, and neutralization reactions. The synthesis approaches focus on creating ionic liquids with tailored characteristics such as specific melting points, viscosities, and solubility profiles. Advanced preparation techniques enable the development of task-specific ionic liquids designed for particular applications.
- Ionic liquids in catalytic processes: Ionic liquids function as effective catalysts or catalyst supports in various chemical reactions. They can enhance reaction rates, improve selectivity, and enable reactions under milder conditions. Their ability to dissolve both organic and inorganic compounds makes them versatile reaction media. Additionally, ionic liquids can be designed to immobilize catalysts, facilitating catalyst recovery and reuse, which contributes to more sustainable chemical processes.
- Ionic liquids for material processing and engineering: Ionic liquids are utilized in material processing and engineering applications, including the dissolution and processing of biomass, polymers, and cellulosic materials. Their unique solvation properties enable the processing of materials that are difficult to dissolve in conventional solvents. They can be used in the preparation of advanced materials such as nanoparticles, membranes, and composite materials. Their low volatility and thermal stability make them suitable for high-temperature processing applications.
02 Ionic liquids for separation and purification processes
Ionic liquids function as effective solvents and extraction media in separation and purification processes. Their tunable properties allow for selective dissolution and extraction of target compounds from complex mixtures. They are particularly valuable in applications such as gas separation, metal extraction, and purification of biomolecules. The ability to design ionic liquids with specific properties makes them versatile tools for developing more efficient and environmentally friendly separation technologies.Expand Specific Solutions03 Synthesis and preparation methods of ionic liquids
Various methods have been developed for synthesizing ionic liquids with specific properties. These include direct quaternization reactions, anion exchange processes, and neutralization reactions. Advanced techniques focus on creating task-specific ionic liquids with functionalized cations or anions designed for particular applications. The synthesis methods aim to produce high-purity ionic liquids with controlled properties while minimizing environmental impact and production costs.Expand Specific Solutions04 Ionic liquids in catalytic processes
Ionic liquids serve as effective media for catalytic reactions, offering advantages over conventional organic solvents. They can function as both solvents and co-catalysts, enhancing reaction rates and selectivity. Their ability to immobilize catalysts facilitates catalyst recovery and reuse, making processes more economical. Applications include hydrogenation, oxidation, esterification, and various organic transformations. The unique solvation environment provided by ionic liquids often leads to improved reaction outcomes and enables novel reaction pathways.Expand Specific Solutions05 Ionic liquids in material processing and engineering
Ionic liquids are utilized in various material processing applications, including as solvents for biopolymers like cellulose and chitin, electrolytes for electroplating and surface treatment, and media for nanoparticle synthesis. Their unique properties enable the processing of materials under milder conditions with enhanced control over material properties. They also find applications in lubricants, heat transfer fluids, and as components in advanced composite materials, offering improved performance characteristics compared to conventional alternatives.Expand Specific Solutions
Leading Companies and Research Institutions
The ionic liquids in next-generation redox flow systems market is currently in an early growth phase, with increasing research interest but limited commercial deployment. The global market size is projected to expand significantly as energy storage demands grow, particularly for grid-scale applications. Technologically, ionic liquids are transitioning from laboratory research to pilot demonstrations, with academic institutions leading fundamental research while companies pursue commercialization. Key players include LG Chem and Idemitsu Kosan developing commercial applications, research institutions like Institute of Process Engineering (Chinese Academy of Sciences) and CIDETEC advancing fundamental science, and universities such as Zhejiang University and McGill University contributing significant innovations. The technology shows promise for addressing energy storage challenges but requires further development to achieve widespread commercial viability.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed advanced ionic liquid-based electrolytes for redox flow batteries with enhanced energy density and stability. Their approach focuses on designing task-specific ionic liquids with tailored physicochemical properties that can dissolve higher concentrations of active materials while maintaining good ionic conductivity. They've pioneered the use of deep eutectic solvents combined with conventional ionic liquids to create hybrid electrolyte systems that demonstrate superior electrochemical performance. Their research has shown that these novel electrolyte formulations can increase energy density by up to 70% compared to conventional aqueous systems while extending cycle life through reduced side reactions and improved chemical stability. The institute has also developed innovative membrane materials compatible with ionic liquid electrolytes to address crossover issues that typically plague redox flow systems.
Strengths: Superior energy density capabilities and enhanced thermal stability allowing operation across wider temperature ranges. Their hybrid electrolyte systems demonstrate excellent electrochemical windows and reduced volatility. Weaknesses: Higher viscosity of ionic liquid electrolytes can lead to increased pumping costs and reduced mass transport, potentially limiting power density in large-scale applications.
LG Chem Ltd.
Technical Solution: LG Chem has developed a proprietary ionic liquid-based electrolyte system for next-generation redox flow batteries focused on grid-scale energy storage applications. Their technology utilizes imidazolium-based ionic liquids with carefully engineered anion structures to optimize solubility of active redox species while maintaining high ionic conductivity. The company has integrated these advanced electrolytes into modular flow battery designs that demonstrate energy densities exceeding 50 Wh/L, significantly higher than conventional aqueous vanadium systems. LG Chem's approach incorporates specialized electrode materials with optimized porosity and surface chemistry specifically designed to work with ionic liquid electrolytes, enhancing reaction kinetics despite the higher viscosity of these non-aqueous systems. Their integrated battery management system includes temperature-adaptive flow control algorithms that compensate for the viscosity changes of ionic liquids across operating conditions, maintaining consistent performance across varying environmental conditions.
Strengths: Excellent scalability for grid applications with enhanced safety profile due to non-flammable electrolyte formulations. Their systems show minimal capacity fade over extended cycling (>5000 cycles). Weaknesses: Higher production costs compared to conventional aqueous systems and potential challenges with electrolyte recovery and recycling at end-of-life due to the complex chemistry of the ionic liquid components.
Key Patents and Scientific Breakthroughs
Redox-active ionic liquids
PatentWO2013142994A1
Innovation
- The use of redox-active ionic liquids as additives in electrolytes, where a redox shuttle is linked to an ionic liquid, increasing solubility and stability, and reducing flammability, thereby enhancing the safety and performance of lithium-ion batteries and supercapacitors.
Aqueous composition as electrolyte comprising ionic liquids or lithium salts
PatentInactiveUS20210043958A1
Innovation
- An aqueous composition comprising ionic liquids and/or lithium salts, combined with organic redox active species, enhances solubility and stability, allowing for improved performance as an electrolyte in redox flow batteries, with specific embodiments using hydrophilic ionic liquids and lithium salts to support solubility and prevent sublimation.
Environmental Impact and Sustainability Assessment
The environmental impact of ionic liquids (ILs) in next-generation redox flow batteries represents a critical consideration for their widespread adoption. Unlike conventional electrolytes, ILs demonstrate significantly lower volatility and flammability, substantially reducing the risk of atmospheric emissions and fire hazards during operation and potential accidents. This inherent safety profile contributes to a reduced environmental footprint across the battery lifecycle.
Life cycle assessment (LCA) studies indicate that IL-based flow systems can achieve 15-30% lower carbon footprints compared to traditional vanadium redox flow batteries when considering full cradle-to-grave analysis. The extended operational lifetime of IL-based systems—often exceeding 15 years versus 10-12 years for conventional systems—further enhances their sustainability credentials by reducing replacement frequency and associated manufacturing impacts.
Water consumption represents another significant advantage of IL-based systems. Traditional flow batteries typically require substantial water resources for electrolyte preparation and cooling systems. In contrast, IL-based technologies can reduce water requirements by up to 40%, a particularly valuable attribute in water-stressed regions where renewable energy storage solutions are increasingly deployed.
The biodegradability of ILs varies considerably depending on their chemical structure. Recent advances have yielded bio-derived ILs with improved biodegradation profiles, with some formulations achieving over 60% degradation within standardized testing periods. However, certain IL structures containing fluorinated anions continue to present persistence concerns, necessitating careful selection and ongoing research into greener alternatives.
End-of-life management presents both challenges and opportunities. The high value of ILs creates economic incentives for recovery and recycling, with emerging technologies demonstrating recovery rates exceeding 85% for certain IL compositions. These recycling pathways significantly reduce the need for virgin material production and associated environmental impacts.
Regulatory frameworks governing IL disposal and recycling remain underdeveloped in many jurisdictions. The establishment of comprehensive regulations specifically addressing IL-based energy storage systems would provide greater certainty for manufacturers and operators while ensuring appropriate environmental safeguards. Industry-led initiatives for voluntary stewardship programs have emerged to fill this gap, establishing best practices for responsible lifecycle management.
Land use impacts of IL-based flow systems generally compare favorably to alternative storage technologies. Their higher energy density translates to smaller physical footprints per kilowatt-hour stored, potentially reducing habitat disruption and land transformation impacts when deployed at utility scale.
Life cycle assessment (LCA) studies indicate that IL-based flow systems can achieve 15-30% lower carbon footprints compared to traditional vanadium redox flow batteries when considering full cradle-to-grave analysis. The extended operational lifetime of IL-based systems—often exceeding 15 years versus 10-12 years for conventional systems—further enhances their sustainability credentials by reducing replacement frequency and associated manufacturing impacts.
Water consumption represents another significant advantage of IL-based systems. Traditional flow batteries typically require substantial water resources for electrolyte preparation and cooling systems. In contrast, IL-based technologies can reduce water requirements by up to 40%, a particularly valuable attribute in water-stressed regions where renewable energy storage solutions are increasingly deployed.
The biodegradability of ILs varies considerably depending on their chemical structure. Recent advances have yielded bio-derived ILs with improved biodegradation profiles, with some formulations achieving over 60% degradation within standardized testing periods. However, certain IL structures containing fluorinated anions continue to present persistence concerns, necessitating careful selection and ongoing research into greener alternatives.
End-of-life management presents both challenges and opportunities. The high value of ILs creates economic incentives for recovery and recycling, with emerging technologies demonstrating recovery rates exceeding 85% for certain IL compositions. These recycling pathways significantly reduce the need for virgin material production and associated environmental impacts.
Regulatory frameworks governing IL disposal and recycling remain underdeveloped in many jurisdictions. The establishment of comprehensive regulations specifically addressing IL-based energy storage systems would provide greater certainty for manufacturers and operators while ensuring appropriate environmental safeguards. Industry-led initiatives for voluntary stewardship programs have emerged to fill this gap, establishing best practices for responsible lifecycle management.
Land use impacts of IL-based flow systems generally compare favorably to alternative storage technologies. Their higher energy density translates to smaller physical footprints per kilowatt-hour stored, potentially reducing habitat disruption and land transformation impacts when deployed at utility scale.
Scalability and Manufacturing Considerations
The scalability of ionic liquid-based redox flow battery (RFB) systems represents a critical challenge for their commercial viability. Current manufacturing processes for ionic liquids typically involve batch synthesis methods that are suitable for laboratory-scale production but face significant hurdles when scaled to industrial levels. The cost of ionic liquids remains substantially higher than conventional aqueous electrolytes, with prices ranging from $50-500/kg compared to less than $1/kg for sulfuric acid used in vanadium RFBs. This cost differential creates a substantial barrier to widespread adoption.
Production scaling requires consideration of several key factors. Continuous flow synthesis methods are emerging as promising alternatives to batch processes, potentially reducing production time by 30-50% while improving consistency in ionic liquid properties. Recent advances in microreactor technology have demonstrated the ability to produce ionic liquids with higher purity (>99.5%) and reduced waste streams compared to conventional methods.
Material compatibility presents another manufacturing challenge. Ionic liquids often exhibit corrosive properties toward certain metals and polymers commonly used in flow battery components. Selection of construction materials must balance chemical resistance, mechanical properties, and cost considerations. Fluoropolymers and specialized stainless steel alloys have shown promising compatibility but add significantly to system costs.
Quality control processes for ionic liquid production require sophisticated analytical techniques to ensure consistent electrochemical performance. Impurity levels as low as 50 ppm can dramatically affect electrochemical stability windows and cycling efficiency. Standardized testing protocols for ionic liquid purity assessment are still evolving, creating challenges for quality assurance in manufacturing environments.
Environmental considerations in manufacturing processes are increasingly important. Green chemistry approaches to ionic liquid synthesis, including solvent-free methods and bio-derived precursors, show promise for reducing the environmental footprint. Life cycle assessments indicate that the environmental impact of ionic liquid production could be reduced by 40-60% through optimization of synthesis routes and recycling of reaction byproducts.
Supply chain resilience represents a final consideration for scaled production. Many ionic liquids require specialty chemicals with limited supplier networks. Developing robust supply chains with multiple sourcing options and strategic stockpiling may be necessary to mitigate disruption risks. Additionally, regional manufacturing capabilities may need development to reduce logistical complexities associated with transporting these specialized chemicals across international borders.
Production scaling requires consideration of several key factors. Continuous flow synthesis methods are emerging as promising alternatives to batch processes, potentially reducing production time by 30-50% while improving consistency in ionic liquid properties. Recent advances in microreactor technology have demonstrated the ability to produce ionic liquids with higher purity (>99.5%) and reduced waste streams compared to conventional methods.
Material compatibility presents another manufacturing challenge. Ionic liquids often exhibit corrosive properties toward certain metals and polymers commonly used in flow battery components. Selection of construction materials must balance chemical resistance, mechanical properties, and cost considerations. Fluoropolymers and specialized stainless steel alloys have shown promising compatibility but add significantly to system costs.
Quality control processes for ionic liquid production require sophisticated analytical techniques to ensure consistent electrochemical performance. Impurity levels as low as 50 ppm can dramatically affect electrochemical stability windows and cycling efficiency. Standardized testing protocols for ionic liquid purity assessment are still evolving, creating challenges for quality assurance in manufacturing environments.
Environmental considerations in manufacturing processes are increasingly important. Green chemistry approaches to ionic liquid synthesis, including solvent-free methods and bio-derived precursors, show promise for reducing the environmental footprint. Life cycle assessments indicate that the environmental impact of ionic liquid production could be reduced by 40-60% through optimization of synthesis routes and recycling of reaction byproducts.
Supply chain resilience represents a final consideration for scaled production. Many ionic liquids require specialty chemicals with limited supplier networks. Developing robust supply chains with multiple sourcing options and strategic stockpiling may be necessary to mitigate disruption risks. Additionally, regional manufacturing capabilities may need development to reduce logistical complexities associated with transporting these specialized chemicals across international borders.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!