Flow Battery Electrolytes for Large Scale Grid Applications
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flow Battery Electrolyte Evolution and Objectives
Flow batteries have evolved significantly since their inception in the 1970s, transitioning from experimental concepts to commercially viable energy storage solutions. The initial development focused on iron-chromium chemistries, which offered modest energy densities but suffered from cross-contamination issues. The 1980s witnessed the emergence of vanadium redox flow batteries (VRFBs), pioneered by Maria Skyllas-Kazacos at the University of New South Wales, representing a breakthrough in addressing cross-contamination through the use of the same element in different oxidation states.
The 1990s and early 2000s saw expanded research into alternative chemistries, including zinc-bromine, polysulfide-bromine, and organic-based electrolytes. These developments aimed to overcome the limitations of vanadium-based systems, particularly their relatively high cost and limited energy density. Concurrently, improvements in membrane technology enhanced ion selectivity while reducing resistance, addressing key performance bottlenecks.
Recent advancements have focused on developing aqueous organic flow batteries, which utilize abundant, low-cost organic molecules as active materials. These systems promise significant cost reductions compared to metal-based alternatives, though challenges in stability and energy density persist. Additionally, non-aqueous electrolytes have gained attention for their wider electrochemical windows, potentially enabling higher energy densities, albeit with increased complexity and cost.
The primary objective of flow battery electrolyte development is to achieve cost-effective, long-duration energy storage for grid applications. This necessitates electrolytes with high energy density (>40 Wh/L), extended cycle life (>10,000 cycles), minimal capacity degradation (<0.01% per cycle), and competitive costs (<$100/kWh). These parameters are essential for economic viability in grid-scale applications, where systems must operate reliably for decades.
Secondary objectives include enhancing operational safety through non-flammable, environmentally benign chemistries, and improving temperature tolerance to reduce auxiliary system requirements. Sustainability considerations have also gained prominence, driving research toward electrolytes composed of earth-abundant elements with established recycling pathways.
The evolution trajectory suggests a convergence toward hybrid systems that combine the advantages of multiple chemistries. Future development will likely prioritize electrolytes with multi-electron transfer capabilities to maximize energy density, while incorporating advanced additives to enhance stability and conductivity. Computational modeling and high-throughput screening methodologies are accelerating this development process, enabling rapid evaluation of novel electrolyte formulations before experimental validation.
The 1990s and early 2000s saw expanded research into alternative chemistries, including zinc-bromine, polysulfide-bromine, and organic-based electrolytes. These developments aimed to overcome the limitations of vanadium-based systems, particularly their relatively high cost and limited energy density. Concurrently, improvements in membrane technology enhanced ion selectivity while reducing resistance, addressing key performance bottlenecks.
Recent advancements have focused on developing aqueous organic flow batteries, which utilize abundant, low-cost organic molecules as active materials. These systems promise significant cost reductions compared to metal-based alternatives, though challenges in stability and energy density persist. Additionally, non-aqueous electrolytes have gained attention for their wider electrochemical windows, potentially enabling higher energy densities, albeit with increased complexity and cost.
The primary objective of flow battery electrolyte development is to achieve cost-effective, long-duration energy storage for grid applications. This necessitates electrolytes with high energy density (>40 Wh/L), extended cycle life (>10,000 cycles), minimal capacity degradation (<0.01% per cycle), and competitive costs (<$100/kWh). These parameters are essential for economic viability in grid-scale applications, where systems must operate reliably for decades.
Secondary objectives include enhancing operational safety through non-flammable, environmentally benign chemistries, and improving temperature tolerance to reduce auxiliary system requirements. Sustainability considerations have also gained prominence, driving research toward electrolytes composed of earth-abundant elements with established recycling pathways.
The evolution trajectory suggests a convergence toward hybrid systems that combine the advantages of multiple chemistries. Future development will likely prioritize electrolytes with multi-electron transfer capabilities to maximize energy density, while incorporating advanced additives to enhance stability and conductivity. Computational modeling and high-throughput screening methodologies are accelerating this development process, enabling rapid evaluation of novel electrolyte formulations before experimental validation.
Grid-Scale Energy Storage Market Analysis
The global grid-scale energy storage market is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources and the need for grid stability. As of 2023, the market size has reached approximately $7.9 billion and is projected to grow at a CAGR of 28.1% through 2030, potentially reaching $31.2 billion. This remarkable expansion reflects the critical role that energy storage systems, particularly flow batteries, play in modern electrical grid infrastructure.
Demand for grid-scale energy storage is primarily fueled by three key factors. First, the rapid deployment of intermittent renewable energy sources such as solar and wind necessitates complementary storage solutions to manage supply fluctuations. Second, aging grid infrastructure in developed nations requires modernization to accommodate distributed energy resources. Third, regulatory policies and incentives worldwide are increasingly favoring clean energy technologies, including storage systems.
Regional analysis reveals distinct market characteristics across different geographies. North America currently leads the market with approximately 38% share, driven by substantial investments in grid modernization and favorable regulatory frameworks. The Asia-Pacific region, particularly China, South Korea, and Australia, represents the fastest-growing market segment with annual growth exceeding 30%, primarily due to aggressive renewable energy targets and manufacturing capabilities. Europe follows closely, with significant deployments in Germany, UK, and Spain.
Flow battery technology occupies a growing segment within this market, currently accounting for about 9% of grid-scale storage deployments but expected to reach 15% by 2028. The value proposition of flow batteries centers on their long duration discharge capabilities (4-12 hours), extended cycle life (15,000+ cycles), and scalability advantages for large installations above 10MWh.
Market segmentation by application shows utility-scale integration leading with 56% market share, followed by commercial and industrial applications (28%), microgrids (11%), and other specialized applications (5%). When analyzed by ownership model, utility-owned systems dominate at 62%, while third-party ownership models are growing rapidly at 34% annual rate.
Key market drivers include declining battery costs (approximately 12% annual reduction for flow battery systems), increasing renewable penetration targets (with over 140 countries having renewable energy goals), and growing grid resilience concerns due to climate change impacts and cybersecurity threats. However, market barriers persist, including high upfront capital costs, regulatory uncertainties in emerging markets, and competition from alternative storage technologies.
Demand for grid-scale energy storage is primarily fueled by three key factors. First, the rapid deployment of intermittent renewable energy sources such as solar and wind necessitates complementary storage solutions to manage supply fluctuations. Second, aging grid infrastructure in developed nations requires modernization to accommodate distributed energy resources. Third, regulatory policies and incentives worldwide are increasingly favoring clean energy technologies, including storage systems.
Regional analysis reveals distinct market characteristics across different geographies. North America currently leads the market with approximately 38% share, driven by substantial investments in grid modernization and favorable regulatory frameworks. The Asia-Pacific region, particularly China, South Korea, and Australia, represents the fastest-growing market segment with annual growth exceeding 30%, primarily due to aggressive renewable energy targets and manufacturing capabilities. Europe follows closely, with significant deployments in Germany, UK, and Spain.
Flow battery technology occupies a growing segment within this market, currently accounting for about 9% of grid-scale storage deployments but expected to reach 15% by 2028. The value proposition of flow batteries centers on their long duration discharge capabilities (4-12 hours), extended cycle life (15,000+ cycles), and scalability advantages for large installations above 10MWh.
Market segmentation by application shows utility-scale integration leading with 56% market share, followed by commercial and industrial applications (28%), microgrids (11%), and other specialized applications (5%). When analyzed by ownership model, utility-owned systems dominate at 62%, while third-party ownership models are growing rapidly at 34% annual rate.
Key market drivers include declining battery costs (approximately 12% annual reduction for flow battery systems), increasing renewable penetration targets (with over 140 countries having renewable energy goals), and growing grid resilience concerns due to climate change impacts and cybersecurity threats. However, market barriers persist, including high upfront capital costs, regulatory uncertainties in emerging markets, and competition from alternative storage technologies.
Current Flow Battery Electrolyte Technologies and Barriers
Flow battery technology has evolved significantly over the past decades, with various electrolyte systems being developed to address the growing demand for large-scale energy storage solutions. Currently, the market is dominated by several key electrolyte technologies, each with distinct advantages and limitations that affect their commercial viability for grid applications.
Vanadium redox flow batteries (VRFBs) represent the most mature technology, utilizing vanadium ions in different oxidation states. These systems offer excellent cycling stability (>20,000 cycles) and long operational lifetimes (15-20 years). However, they face significant barriers including high vanadium material costs ($20-30/kg), limited energy density (25-35 Wh/L), and temperature sensitivity that typically restricts operation between 10-40°C without additional thermal management systems.
Zinc-bromine flow batteries present a cost-effective alternative with higher energy density (60-70 Wh/L) and utilize more abundant materials. Their limitations include dendrite formation during zinc plating, bromine volatility concerns, and membrane degradation issues that collectively reduce cycle life to approximately 2,000-3,000 cycles.
Iron-chromium systems have reemerged as potential low-cost options, utilizing earth-abundant materials and simpler designs. However, these systems suffer from sluggish kinetics at the chromium electrode, hydrogen evolution side reactions, and cross-contamination through membranes, resulting in lower efficiency (65-75%) compared to vanadium systems (>80%).
Organic electrolytes represent an emerging frontier, utilizing carbon-based redox-active molecules that can be synthesized from abundant resources. While promising in terms of material cost reduction and environmental impact, these systems currently face stability challenges, limited solubility, and membrane compatibility issues that restrict their practical energy density and cycle life.
Hybrid flow batteries, particularly zinc-polyiodide and hydrogen-bromine systems, offer exceptional energy densities (>100 Wh/L) but face commercialization barriers related to material corrosion, complex balance-of-plant requirements, and safety concerns associated with reactive components.
The membrane remains a critical component across all technologies, with current ion-exchange membranes contributing significantly to system costs ($300-500/m²) while still suffering from selectivity limitations that lead to capacity fade through active species crossover.
Scalability presents another universal challenge, as electrolyte production capacity, particularly for high-purity vanadium, remains insufficient to meet projected grid storage demands. Additionally, the environmental impact of electrolyte manufacturing and end-of-life management requires further attention to ensure sustainable deployment at grid scale.
Vanadium redox flow batteries (VRFBs) represent the most mature technology, utilizing vanadium ions in different oxidation states. These systems offer excellent cycling stability (>20,000 cycles) and long operational lifetimes (15-20 years). However, they face significant barriers including high vanadium material costs ($20-30/kg), limited energy density (25-35 Wh/L), and temperature sensitivity that typically restricts operation between 10-40°C without additional thermal management systems.
Zinc-bromine flow batteries present a cost-effective alternative with higher energy density (60-70 Wh/L) and utilize more abundant materials. Their limitations include dendrite formation during zinc plating, bromine volatility concerns, and membrane degradation issues that collectively reduce cycle life to approximately 2,000-3,000 cycles.
Iron-chromium systems have reemerged as potential low-cost options, utilizing earth-abundant materials and simpler designs. However, these systems suffer from sluggish kinetics at the chromium electrode, hydrogen evolution side reactions, and cross-contamination through membranes, resulting in lower efficiency (65-75%) compared to vanadium systems (>80%).
Organic electrolytes represent an emerging frontier, utilizing carbon-based redox-active molecules that can be synthesized from abundant resources. While promising in terms of material cost reduction and environmental impact, these systems currently face stability challenges, limited solubility, and membrane compatibility issues that restrict their practical energy density and cycle life.
Hybrid flow batteries, particularly zinc-polyiodide and hydrogen-bromine systems, offer exceptional energy densities (>100 Wh/L) but face commercialization barriers related to material corrosion, complex balance-of-plant requirements, and safety concerns associated with reactive components.
The membrane remains a critical component across all technologies, with current ion-exchange membranes contributing significantly to system costs ($300-500/m²) while still suffering from selectivity limitations that lead to capacity fade through active species crossover.
Scalability presents another universal challenge, as electrolyte production capacity, particularly for high-purity vanadium, remains insufficient to meet projected grid storage demands. Additionally, the environmental impact of electrolyte manufacturing and end-of-life management requires further attention to ensure sustainable deployment at grid scale.
State-of-the-Art Flow Battery Electrolyte Solutions
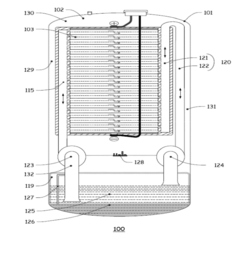
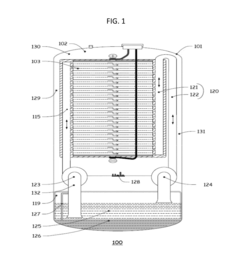
01 Vanadium-based electrolytes for flow batteries
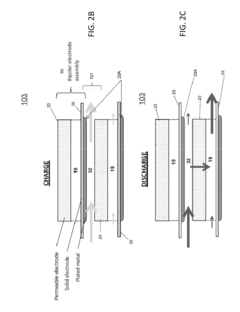
Vanadium-based electrolytes are widely used in flow batteries due to their stability and efficiency. These electrolytes typically contain vanadium ions in different oxidation states dissolved in acidic solutions. The use of vanadium in both the positive and negative electrolytes eliminates cross-contamination issues and extends battery life. Various additives and stabilizers can be incorporated to improve the performance and stability of vanadium electrolytes across different operating temperatures.- Vanadium-based electrolytes for flow batteries: Vanadium-based electrolytes are widely used in flow battery systems due to their stability and electrochemical properties. These electrolytes typically consist of vanadium ions in different oxidation states dissolved in acidic solutions. The use of the same element in different oxidation states helps prevent cross-contamination issues between the positive and negative electrolytes, extending the battery life and improving overall performance. Various additives and supporting electrolytes can be incorporated to enhance stability and conductivity.
- Organic electrolytes for flow batteries: Organic compounds can be used as active materials in flow battery electrolytes, offering advantages such as lower cost, environmental friendliness, and tunable properties. These organic electrolytes typically consist of redox-active organic molecules dissolved in suitable solvents. The molecular structure of these compounds can be modified to achieve desired electrochemical properties, including redox potential, solubility, and stability. Organic electrolytes provide alternatives to traditional metal-based systems and can potentially reduce the environmental impact of flow battery technology.
- Electrolyte additives for improved stability and performance: Various additives can be incorporated into flow battery electrolytes to enhance their stability, conductivity, and overall performance. These additives may include stabilizing agents that prevent unwanted side reactions, conductivity enhancers that improve ionic transport, and compounds that modify the viscosity or other physical properties of the electrolyte. By carefully selecting and optimizing these additives, the cycle life, energy efficiency, and power density of flow batteries can be significantly improved.
- Non-aqueous electrolyte systems: Non-aqueous electrolyte systems offer expanded voltage windows compared to water-based electrolytes, potentially increasing the energy density of flow batteries. These systems typically use organic solvents or ionic liquids as the electrolyte medium instead of water. The choice of solvent affects the solubility of active materials, operating temperature range, and safety characteristics of the battery. Non-aqueous systems can enable the use of redox couples that would otherwise be incompatible with aqueous environments, opening up new possibilities for high-energy flow battery designs.
- Hybrid and novel electrolyte configurations: Innovative electrolyte configurations combine different types of redox couples or battery architectures to overcome limitations of traditional flow battery designs. These may include hybrid systems that use solid electrodes with flowing electrolytes, semi-solid suspensions, or combinations of different redox chemistries. Novel electrolyte configurations can address challenges related to energy density, power density, and system cost. These approaches often involve engineering the electrolyte composition and flow patterns to optimize the electrochemical performance and practical utility of flow battery systems.
02 Organic electrolytes for flow batteries
Organic electrolytes offer alternatives to metal-based systems for flow batteries. These electrolytes utilize organic compounds as active materials, which can be dissolved in various solvents including water and non-aqueous media. Organic electrolytes often provide benefits such as lower cost, reduced environmental impact, and potentially higher energy density. Redox-active organic molecules can be designed with specific properties to enhance battery performance, including improved solubility and voltage windows.Expand Specific Solutions03 Electrolyte additives and stabilizers
Various additives and stabilizers can be incorporated into flow battery electrolytes to enhance performance and longevity. These additives may prevent precipitation of active materials, improve thermal stability, reduce side reactions, or enhance ionic conductivity. Common additives include acid stabilizers, anti-oxidants, chelating agents, and surfactants. The proper selection and concentration of additives can significantly extend the operational temperature range and cycle life of flow batteries.Expand Specific Solutions04 Non-aqueous electrolyte systems
Non-aqueous electrolyte systems expand the voltage window and operating temperature range of flow batteries beyond what is possible with water-based systems. These electrolytes typically use organic solvents such as acetonitrile, propylene carbonate, or ionic liquids as the carrier medium. Non-aqueous systems can accommodate active materials that are unstable or insoluble in water, potentially enabling higher energy density batteries. However, challenges include higher cost, lower conductivity, and safety concerns related to flammability.Expand Specific Solutions05 Hybrid and novel electrolyte compositions
Hybrid and novel electrolyte compositions combine different types of active materials or use unconventional redox couples to achieve improved performance. These may include zinc-bromine, iron-chromium, or hydrogen-bromine systems, as well as newer approaches using metal-organic frameworks or polymeric active materials. Such hybrid systems often aim to leverage the advantages of multiple chemistries while mitigating their individual limitations. Research in this area focuses on achieving higher energy density, better stability, and lower cost compared to conventional flow battery electrolytes.Expand Specific Solutions
Leading Companies and Research Institutions in Flow Battery Sector
Flow battery technology for large-scale grid applications is currently in a growth phase, with market size expected to expand significantly due to increasing demand for renewable energy storage solutions. The competitive landscape features established players like Lockheed Martin Advanced Energy Storage and Sumitomo Electric Industries developing proprietary electrolyte technologies, alongside emerging companies such as Primus Power and Largo Clean Energy focusing on zinc-bromine and vanadium-based systems respectively. Research institutions including MIT, Harvard, and China's State Grid Corporation are advancing electrolyte chemistry innovations, particularly in aqueous organic and hybrid systems. Technical maturity varies across electrolyte types, with vanadium flow batteries being most commercially developed while newer organic and hybrid electrolytes show promising cost and performance improvements but require further validation for grid-scale deployment.
State Grid Corp. of China
Technical Solution: State Grid Corporation of China has developed comprehensive flow battery electrolyte technologies through their extensive research programs focused on large-scale grid energy storage. Their approach encompasses multiple chemistries, with particular emphasis on vanadium redox flow batteries (VRFBs) optimized for grid-scale deployment. State Grid has engineered advanced electrolyte formulations that incorporate stabilizing additives to extend the temperature operating range from -5°C to 50°C, addressing precipitation issues that typically limit vanadium systems in extreme environments. Their technology includes proprietary electrolyte purification processes that remove contaminants that can degrade performance over time, enabling demonstrated cycle life exceeding 20,000 cycles in laboratory testing. State Grid has developed integrated electrolyte management systems that continuously monitor and maintain optimal chemical balance, automatically compensating for crossover effects that occur during long-term operation. Their grid-scale implementations feature modular designs that can be scaled from hundreds of kilowatts to hundreds of megawatts, with several large-scale demonstration projects deployed across China's electrical grid network, including a 200MW/800MWh installation in Dalian that represents one of the world's largest flow battery systems.
Strengths: State Grid's massive scale enables comprehensive testing and validation across diverse grid environments, accelerating technology maturation. Their integration of flow battery systems with advanced grid management software provides superior control capabilities for complex grid applications. Weaknesses: The technology remains heavily dependent on vanadium availability and pricing, creating potential supply chain vulnerabilities. The system architecture prioritizes reliability over energy density, resulting in larger physical footprints compared to some competing technologies.
Primus Power Corp.
Technical Solution: Primus Power has developed a zinc-bromine flow battery technology with several innovative electrolyte advancements for grid-scale applications. Their EnergyPod system utilizes a single-flow design where both the zinc and bromine species are contained within a single electrolyte solution, eliminating the need for an ion-exchange membrane that typically represents a failure point in conventional flow batteries. Primus's proprietary electrolyte formulation incorporates specialized complexing agents that sequester bromine, reducing its volatility and enhancing safety while maintaining electrochemical availability. Their technology employs metal electrodes with proprietary surface treatments that enhance zinc plating efficiency and uniformity during charging cycles, addressing dendrite formation issues that have historically limited zinc-based flow batteries. The electrolyte chemistry achieves energy densities approaching 70 Wh/L, significantly higher than vanadium-based alternatives. Primus has demonstrated system lifetimes exceeding 10,000 cycles with minimal capacity degradation in field deployments, including installations with major utilities for grid stabilization and renewable energy integration applications.
Strengths: Primus's membrane-free design significantly reduces system complexity and eliminates a common failure point in flow batteries. Their zinc-bromine chemistry achieves higher energy density than vanadium systems, reducing footprint and electrolyte volume requirements. Weaknesses: The zinc-bromine chemistry presents greater safety challenges than some alternative electrolytes due to bromine's inherent toxicity. The metal electrode approach may face scaling limitations for very large installations compared to carbon-based electrodes used in other flow battery designs.
Key Patents and Scientific Breakthroughs in Electrolyte Chemistry
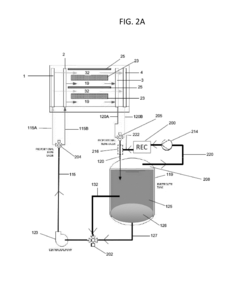
Flow battery electrolyte compositions containing a chelating agent and a metal plating enhancer
PatentActiveUS20160276691A1
Innovation
- An aqueous electrolyte composition comprising zinc bromide, a chelating agent, a metal plating enhancer, and a bromine sequestering agent is used, which reduces dendrite formation, corrosion, and improves the morphology of zinc deposits, enhancing energy capacity and efficiency by up to 400%.
Zinc-iron flow battery
PatentActiveUS20190363387A1
Innovation
- A zinc-iron chloride electrolyte system with equimolar concentrations of zinc and iron ions, using a microporous polymeric separator and complexing agents to prevent hydroxide precipitation, promoting anomalous co-deposition and reducing costs and complexity, while maintaining low pH to inhibit iron hydroxide formation and hydrogen evolution.
Environmental Impact and Sustainability of Flow Battery Materials
The environmental impact of flow battery materials is a critical consideration for large-scale grid applications, particularly as deployment increases globally. Vanadium redox flow batteries (VRFBs), while offering excellent cycling stability, raise sustainability concerns due to vanadium's limited global reserves and energy-intensive mining processes. The extraction of vanadium typically generates significant carbon emissions and can lead to soil and water contamination if not properly managed.
Alternative electrolyte chemistries such as organic compounds and iron-based solutions present more environmentally friendly options. These materials often derive from abundant resources and can be synthesized through less energy-intensive processes. For instance, quinone-based organic electrolytes can be sourced from renewable feedstocks, significantly reducing the carbon footprint compared to metal-based alternatives.
Life cycle assessment (LCA) studies indicate that the environmental impact of flow batteries extends beyond raw material extraction to include manufacturing, operation, and end-of-life management. The energy return on investment (EROI) for flow batteries varies considerably depending on electrolyte chemistry, with some newer formulations showing promising improvements in sustainability metrics.
Water usage represents another significant environmental consideration, particularly for aqueous electrolyte systems. While water-based electrolytes offer safety advantages, their production and maintenance can strain local water resources in water-scarce regions. Non-aqueous electrolytes may reduce water consumption but often introduce other environmental challenges through the use of organic solvents.
Recycling and material recovery processes are increasingly important for mitigating the environmental impact of flow battery deployment. Advanced separation techniques now enable the recovery of up to 95% of certain electrolyte materials, substantially extending resource availability and reducing waste. However, these processes remain energy-intensive and economically challenging at scale.
Regulatory frameworks worldwide are evolving to address the environmental implications of energy storage technologies. The European Union's Battery Directive and similar regulations in other regions increasingly emphasize sustainable sourcing, reduced toxicity, and end-of-life management for battery materials. These regulations are driving innovation toward more environmentally benign electrolyte formulations.
Future research directions include the development of bio-derived electrolytes with minimal environmental footprint, closed-loop manufacturing systems that eliminate waste streams, and advanced recycling technologies that reduce the energy requirements for material recovery. These innovations will be crucial for ensuring that flow batteries contribute positively to global sustainability goals while supporting grid decarbonization efforts.
Alternative electrolyte chemistries such as organic compounds and iron-based solutions present more environmentally friendly options. These materials often derive from abundant resources and can be synthesized through less energy-intensive processes. For instance, quinone-based organic electrolytes can be sourced from renewable feedstocks, significantly reducing the carbon footprint compared to metal-based alternatives.
Life cycle assessment (LCA) studies indicate that the environmental impact of flow batteries extends beyond raw material extraction to include manufacturing, operation, and end-of-life management. The energy return on investment (EROI) for flow batteries varies considerably depending on electrolyte chemistry, with some newer formulations showing promising improvements in sustainability metrics.
Water usage represents another significant environmental consideration, particularly for aqueous electrolyte systems. While water-based electrolytes offer safety advantages, their production and maintenance can strain local water resources in water-scarce regions. Non-aqueous electrolytes may reduce water consumption but often introduce other environmental challenges through the use of organic solvents.
Recycling and material recovery processes are increasingly important for mitigating the environmental impact of flow battery deployment. Advanced separation techniques now enable the recovery of up to 95% of certain electrolyte materials, substantially extending resource availability and reducing waste. However, these processes remain energy-intensive and economically challenging at scale.
Regulatory frameworks worldwide are evolving to address the environmental implications of energy storage technologies. The European Union's Battery Directive and similar regulations in other regions increasingly emphasize sustainable sourcing, reduced toxicity, and end-of-life management for battery materials. These regulations are driving innovation toward more environmentally benign electrolyte formulations.
Future research directions include the development of bio-derived electrolytes with minimal environmental footprint, closed-loop manufacturing systems that eliminate waste streams, and advanced recycling technologies that reduce the energy requirements for material recovery. These innovations will be crucial for ensuring that flow batteries contribute positively to global sustainability goals while supporting grid decarbonization efforts.
Regulatory Framework for Grid-Scale Energy Storage Systems
The regulatory landscape for grid-scale energy storage systems, particularly flow battery technologies utilizing advanced electrolytes, has evolved significantly in recent years. In the United States, FERC Order 841 marked a watershed moment by requiring regional transmission organizations to establish market rules enabling energy storage participation in wholesale electricity markets. This regulatory shift has created substantial opportunities for flow battery deployment, especially those with innovative electrolyte formulations designed for large-scale applications.
The European Union has implemented the Clean Energy Package, which explicitly recognizes energy storage as a distinct asset class within the electricity value chain. This classification has removed previous double-charging barriers where storage systems were taxed both as consumers when charging and producers when discharging. For flow battery technologies, these regulatory changes have significantly improved economic viability, particularly for vanadium and organic electrolyte systems designed for long-duration storage.
Safety standards and certification requirements present another critical regulatory dimension. UL 1973 and IEC 62619 standards govern battery safety for stationary applications, while NFPA 855 addresses installation requirements specifically for stationary energy storage systems. Flow batteries with aqueous electrolytes often receive favorable treatment under these frameworks due to their inherently lower fire risks compared to lithium-ion alternatives, though regulations regarding chemical handling and containment remain stringent.
Environmental regulations significantly impact flow battery deployment, particularly concerning electrolyte materials. The EU's REACH regulation and similar frameworks globally require thorough documentation of chemical properties and potential environmental impacts. Vanadium electrolytes face scrutiny due to mining impacts, while organic electrolytes must demonstrate biodegradability and low toxicity. These requirements are driving innovation toward more environmentally benign electrolyte formulations.
Grid interconnection standards present technical regulatory hurdles that flow battery systems must overcome. IEEE 1547 and regional grid codes establish requirements for frequency regulation, voltage support, and fault response capabilities. Flow battery systems with advanced power electronics and control systems can leverage these requirements as competitive advantages, particularly when their electrolyte chemistry enables rapid response capabilities alongside long-duration storage.
Emerging regulatory frameworks are increasingly focusing on lifecycle management. The EU Battery Directive's recent revisions and similar initiatives globally are establishing requirements for electrolyte recycling and recovery. This regulatory direction favors flow battery technologies where electrolytes can be fully recovered and reused, creating potential competitive advantages for systems designed with circular economy principles in mind.
The European Union has implemented the Clean Energy Package, which explicitly recognizes energy storage as a distinct asset class within the electricity value chain. This classification has removed previous double-charging barriers where storage systems were taxed both as consumers when charging and producers when discharging. For flow battery technologies, these regulatory changes have significantly improved economic viability, particularly for vanadium and organic electrolyte systems designed for long-duration storage.
Safety standards and certification requirements present another critical regulatory dimension. UL 1973 and IEC 62619 standards govern battery safety for stationary applications, while NFPA 855 addresses installation requirements specifically for stationary energy storage systems. Flow batteries with aqueous electrolytes often receive favorable treatment under these frameworks due to their inherently lower fire risks compared to lithium-ion alternatives, though regulations regarding chemical handling and containment remain stringent.
Environmental regulations significantly impact flow battery deployment, particularly concerning electrolyte materials. The EU's REACH regulation and similar frameworks globally require thorough documentation of chemical properties and potential environmental impacts. Vanadium electrolytes face scrutiny due to mining impacts, while organic electrolytes must demonstrate biodegradability and low toxicity. These requirements are driving innovation toward more environmentally benign electrolyte formulations.
Grid interconnection standards present technical regulatory hurdles that flow battery systems must overcome. IEEE 1547 and regional grid codes establish requirements for frequency regulation, voltage support, and fault response capabilities. Flow battery systems with advanced power electronics and control systems can leverage these requirements as competitive advantages, particularly when their electrolyte chemistry enables rapid response capabilities alongside long-duration storage.
Emerging regulatory frameworks are increasingly focusing on lifecycle management. The EU Battery Directive's recent revisions and similar initiatives globally are establishing requirements for electrolyte recycling and recovery. This regulatory direction favors flow battery technologies where electrolytes can be fully recovered and reused, creating potential competitive advantages for systems designed with circular economy principles in mind.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!