Temperature Effects on Electrolyte Performance in Flow Cells
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Temperature Dynamics and Research Objectives
Flow battery technology has evolved significantly over the past decades, with electrolyte performance being a critical factor in overall system efficiency. Temperature effects on electrolyte behavior represent one of the most complex yet understudied aspects of flow cell operation. Historical developments in this field began with rudimentary understanding of temperature-viscosity relationships in the 1970s, progressing through enhanced electrochemical characterization in the 1990s, to today's sophisticated computational modeling of temperature-dependent electrolyte dynamics.
The temperature dependency of electrolyte properties fundamentally impacts multiple performance parameters in flow batteries. Viscosity typically decreases with increasing temperature, facilitating improved pumping efficiency and reduced parasitic energy losses. However, this benefit is counterbalanced by potential increases in self-discharge rates and accelerated degradation of active species at elevated temperatures. Ionic conductivity generally improves with temperature, enhancing charge transfer kinetics, while solubility limits of active materials may either increase or decrease depending on specific chemistry.
Current technological trends indicate a shift toward precision temperature management systems that optimize electrolyte performance across varying operational conditions. The integration of advanced thermal management with battery management systems represents a significant advancement, allowing dynamic response to changing environmental and load conditions. This evolution reflects the industry's recognition that temperature is not merely an environmental factor to be tolerated but a parameter that can be strategically manipulated to enhance performance.
Our research objectives focus on developing comprehensive models of temperature-electrolyte interactions across the -20°C to 60°C operational range typical for grid-scale and transportation applications. Specifically, we aim to characterize the non-linear relationships between temperature and key electrolyte properties including redox potential, diffusion coefficients, and long-term stability. Additionally, we seek to establish design principles for temperature-resilient electrolyte formulations that maintain consistent performance across varying thermal conditions.
Further objectives include quantifying the energy efficiency implications of different thermal management strategies and developing predictive models for electrolyte lifetime as a function of thermal cycling patterns. The ultimate goal is to establish a framework for temperature-optimized electrolyte systems that can adapt to diverse deployment scenarios from cold-climate grid storage to high-temperature industrial applications, thereby expanding the practical utility of flow battery technology.
This research addresses the critical knowledge gap between laboratory-scale electrolyte characterization, typically conducted under controlled temperature conditions, and real-world deployment scenarios where temperature fluctuations are inevitable and often extreme. By bridging this gap, we aim to accelerate the commercial viability of flow battery technology across diverse markets and operating environments.
The temperature dependency of electrolyte properties fundamentally impacts multiple performance parameters in flow batteries. Viscosity typically decreases with increasing temperature, facilitating improved pumping efficiency and reduced parasitic energy losses. However, this benefit is counterbalanced by potential increases in self-discharge rates and accelerated degradation of active species at elevated temperatures. Ionic conductivity generally improves with temperature, enhancing charge transfer kinetics, while solubility limits of active materials may either increase or decrease depending on specific chemistry.
Current technological trends indicate a shift toward precision temperature management systems that optimize electrolyte performance across varying operational conditions. The integration of advanced thermal management with battery management systems represents a significant advancement, allowing dynamic response to changing environmental and load conditions. This evolution reflects the industry's recognition that temperature is not merely an environmental factor to be tolerated but a parameter that can be strategically manipulated to enhance performance.
Our research objectives focus on developing comprehensive models of temperature-electrolyte interactions across the -20°C to 60°C operational range typical for grid-scale and transportation applications. Specifically, we aim to characterize the non-linear relationships between temperature and key electrolyte properties including redox potential, diffusion coefficients, and long-term stability. Additionally, we seek to establish design principles for temperature-resilient electrolyte formulations that maintain consistent performance across varying thermal conditions.
Further objectives include quantifying the energy efficiency implications of different thermal management strategies and developing predictive models for electrolyte lifetime as a function of thermal cycling patterns. The ultimate goal is to establish a framework for temperature-optimized electrolyte systems that can adapt to diverse deployment scenarios from cold-climate grid storage to high-temperature industrial applications, thereby expanding the practical utility of flow battery technology.
This research addresses the critical knowledge gap between laboratory-scale electrolyte characterization, typically conducted under controlled temperature conditions, and real-world deployment scenarios where temperature fluctuations are inevitable and often extreme. By bridging this gap, we aim to accelerate the commercial viability of flow battery technology across diverse markets and operating environments.
Market Analysis of Temperature-Optimized Flow Cell Systems
The global market for temperature-optimized flow cell systems is experiencing significant growth, driven by increasing demand for efficient energy storage solutions across various sectors. The market size for flow batteries was valued at approximately 290 million USD in 2022 and is projected to reach 962 million USD by 2028, with a compound annual growth rate (CAGR) of 22.1% during this period. This growth trajectory is particularly notable in regions with aggressive renewable energy adoption targets, including North America, Europe, and parts of Asia-Pacific.
Temperature optimization represents a critical market differentiator in the flow cell sector. Systems that can maintain optimal electrolyte performance across varying temperature conditions command premium pricing, typically 15-20% higher than standard systems. This price premium reflects the enhanced efficiency, extended lifespan, and reduced maintenance requirements that temperature-optimized systems offer to end-users.
The market segmentation for temperature-optimized flow cell systems reveals distinct customer profiles. Utility-scale energy storage represents the largest segment, accounting for approximately 45% of market demand. Commercial and industrial applications follow at 30%, while specialized applications in telecommunications, data centers, and remote power systems constitute the remaining 25%. Each segment exhibits different temperature performance requirements, creating opportunities for specialized solutions.
Geographically, the market shows interesting distribution patterns. North America leads with 38% market share, driven by grid modernization initiatives and renewable integration projects. Europe follows at 32%, with particularly strong demand in Germany, the UK, and Scandinavian countries where temperature fluctuations impact grid reliability. The Asia-Pacific region represents 25% of the market, with China, Japan, and Australia as key growth territories.
Customer purchasing behavior indicates that total cost of ownership (TCO) is becoming the dominant decision factor rather than initial capital expenditure. Temperature-optimized systems typically demonstrate 22-30% lower TCO over a 10-year operational period compared to non-optimized alternatives, primarily due to efficiency gains and reduced degradation rates across seasonal temperature variations.
Market forecasts suggest that temperature-optimized flow cell systems will continue to gain market share, potentially representing over 60% of all new flow battery installations by 2027. This trend is reinforced by increasingly stringent energy efficiency regulations and the growing deployment of renewable energy in regions with extreme temperature variations, where conventional battery technologies face significant performance challenges.
Temperature optimization represents a critical market differentiator in the flow cell sector. Systems that can maintain optimal electrolyte performance across varying temperature conditions command premium pricing, typically 15-20% higher than standard systems. This price premium reflects the enhanced efficiency, extended lifespan, and reduced maintenance requirements that temperature-optimized systems offer to end-users.
The market segmentation for temperature-optimized flow cell systems reveals distinct customer profiles. Utility-scale energy storage represents the largest segment, accounting for approximately 45% of market demand. Commercial and industrial applications follow at 30%, while specialized applications in telecommunications, data centers, and remote power systems constitute the remaining 25%. Each segment exhibits different temperature performance requirements, creating opportunities for specialized solutions.
Geographically, the market shows interesting distribution patterns. North America leads with 38% market share, driven by grid modernization initiatives and renewable integration projects. Europe follows at 32%, with particularly strong demand in Germany, the UK, and Scandinavian countries where temperature fluctuations impact grid reliability. The Asia-Pacific region represents 25% of the market, with China, Japan, and Australia as key growth territories.
Customer purchasing behavior indicates that total cost of ownership (TCO) is becoming the dominant decision factor rather than initial capital expenditure. Temperature-optimized systems typically demonstrate 22-30% lower TCO over a 10-year operational period compared to non-optimized alternatives, primarily due to efficiency gains and reduced degradation rates across seasonal temperature variations.
Market forecasts suggest that temperature-optimized flow cell systems will continue to gain market share, potentially representing over 60% of all new flow battery installations by 2027. This trend is reinforced by increasingly stringent energy efficiency regulations and the growing deployment of renewable energy in regions with extreme temperature variations, where conventional battery technologies face significant performance challenges.
Current Challenges in Electrolyte Thermal Management
Thermal management of electrolytes in flow cells represents one of the most significant challenges in advancing this technology. The performance of electrolytes is highly temperature-dependent, with variations affecting viscosity, conductivity, and electrochemical stability. Current flow cell systems struggle to maintain optimal temperature ranges during operation, particularly under high current densities where heat generation becomes substantial.
The primary challenge lies in the complex relationship between temperature and electrolyte properties. As temperatures rise, electrolyte viscosity typically decreases, which can improve pumping efficiency but may simultaneously reduce ionic conductivity and accelerate unwanted side reactions. Conversely, at lower temperatures, increased viscosity creates pumping challenges and reduces overall system efficiency. This delicate balance creates a narrow operational temperature window that is difficult to maintain in real-world applications.
Heat dissipation represents another significant hurdle. Current cooling systems often add considerable weight, volume, and complexity to flow cell designs. The integration of effective thermal management without compromising the energy density advantages of flow cells remains problematic. Traditional cooling approaches borrowed from lithium-ion battery technology prove inadequate for the unique fluid dynamics of flow systems.
Temperature gradients within the electrolyte solution present additional complications. Non-uniform temperature distribution can lead to localized performance variations, accelerated degradation in certain regions, and reduced overall system reliability. Current flow cell designs lack sophisticated temperature monitoring and control mechanisms capable of addressing these spatial variations.
Material compatibility issues are exacerbated under temperature fluctuations. Seals, membranes, and other components may experience differential thermal expansion, leading to increased risk of leakage or mechanical failure. The development of materials that maintain consistent properties across operational temperature ranges remains an ongoing challenge.
Seasonal and geographical temperature variations further complicate electrolyte thermal management. Systems designed for moderate climates may fail in extreme environments, limiting deployment flexibility. The lack of adaptive thermal management solutions capable of responding to changing ambient conditions represents a significant barrier to widespread adoption.
Energy efficiency concerns also arise from thermal management requirements. Current approaches often involve parasitic energy losses for cooling or heating, reducing the net efficiency of the entire system. The development of passive thermal regulation strategies or waste heat recovery systems could address this challenge but remains in early development stages.
The primary challenge lies in the complex relationship between temperature and electrolyte properties. As temperatures rise, electrolyte viscosity typically decreases, which can improve pumping efficiency but may simultaneously reduce ionic conductivity and accelerate unwanted side reactions. Conversely, at lower temperatures, increased viscosity creates pumping challenges and reduces overall system efficiency. This delicate balance creates a narrow operational temperature window that is difficult to maintain in real-world applications.
Heat dissipation represents another significant hurdle. Current cooling systems often add considerable weight, volume, and complexity to flow cell designs. The integration of effective thermal management without compromising the energy density advantages of flow cells remains problematic. Traditional cooling approaches borrowed from lithium-ion battery technology prove inadequate for the unique fluid dynamics of flow systems.
Temperature gradients within the electrolyte solution present additional complications. Non-uniform temperature distribution can lead to localized performance variations, accelerated degradation in certain regions, and reduced overall system reliability. Current flow cell designs lack sophisticated temperature monitoring and control mechanisms capable of addressing these spatial variations.
Material compatibility issues are exacerbated under temperature fluctuations. Seals, membranes, and other components may experience differential thermal expansion, leading to increased risk of leakage or mechanical failure. The development of materials that maintain consistent properties across operational temperature ranges remains an ongoing challenge.
Seasonal and geographical temperature variations further complicate electrolyte thermal management. Systems designed for moderate climates may fail in extreme environments, limiting deployment flexibility. The lack of adaptive thermal management solutions capable of responding to changing ambient conditions represents a significant barrier to widespread adoption.
Energy efficiency concerns also arise from thermal management requirements. Current approaches often involve parasitic energy losses for cooling or heating, reducing the net efficiency of the entire system. The development of passive thermal regulation strategies or waste heat recovery systems could address this challenge but remains in early development stages.
Existing Thermal Management Solutions for Flow Cells
01 Electrolyte additives for improved battery performance
Various additives can be incorporated into electrolytes to enhance battery performance. These additives can improve the formation of solid electrolyte interphase (SEI) layers, increase ionic conductivity, and enhance the overall stability of the electrolyte. Common additives include fluorinated compounds, carbonates, and specific salts that can significantly improve the cycling performance and lifespan of batteries.- Electrolyte additives for improved performance: Various additives can be incorporated into electrolytes to enhance their performance characteristics. These additives can include functional compounds that improve ionic conductivity, stability, and electrochemical properties. By carefully selecting and formulating these additives, the overall performance of the electrolyte can be significantly improved, leading to better efficiency and longevity in electrochemical devices.
- Novel electrolyte compositions for battery applications: Innovative electrolyte compositions have been developed specifically for battery applications. These compositions often feature unique combinations of solvents, salts, and additives designed to optimize battery performance. The novel formulations can enhance energy density, power output, and cycle life while addressing safety concerns associated with conventional electrolytes.
- Temperature-stable electrolyte systems: Electrolyte systems that maintain stable performance across a wide temperature range are crucial for many applications. These systems incorporate specialized components that prevent degradation at high temperatures and maintain conductivity at low temperatures. The development of temperature-stable electrolytes enables devices to operate reliably in diverse environmental conditions without significant performance loss.
- Solid-state and gel electrolyte technologies: Solid-state and gel electrolytes represent advanced alternatives to traditional liquid electrolytes. These materials offer improved safety profiles by reducing leakage risks and flammability concerns. Additionally, they can provide enhanced mechanical stability and electrochemical performance. The development of these alternative electrolyte forms enables new device architectures and applications previously limited by liquid electrolyte constraints.
- Electrolyte interface engineering: Engineering the interfaces between electrolytes and electrodes is critical for optimizing electrochemical performance. This approach focuses on modifying the solid-electrolyte interphase (SEI) layer formation, reducing interfacial resistance, and improving ion transport across boundaries. By controlling these interfacial properties, overall device efficiency can be enhanced while mitigating degradation mechanisms that typically occur at material interfaces.
02 Polymer-based electrolyte systems
Polymer-based electrolytes offer advantages in terms of safety and flexibility compared to liquid electrolytes. These systems incorporate polymers such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or other polymer matrices that can host lithium salts. The polymer structure provides mechanical stability while allowing ion transport, resulting in improved safety characteristics and potentially better electrochemical performance in various battery applications.Expand Specific Solutions03 High-concentration electrolyte formulations
High-concentration electrolyte formulations, often referred to as concentrated electrolytes or high-salt electrolytes, contain significantly higher salt concentrations than conventional electrolytes. These formulations can suppress dendrite formation, reduce electrolyte decomposition, and improve the thermal stability of batteries. The unique solvation structure in high-concentration electrolytes leads to different transport mechanisms that can enhance battery performance and safety.Expand Specific Solutions04 Temperature-resistant electrolyte compositions
Electrolytes designed to perform across wide temperature ranges are crucial for applications in extreme environments. These specialized formulations incorporate solvent mixtures, salts, and additives that maintain conductivity at low temperatures while remaining stable at high temperatures. The compositions often include low-melting-point solvents combined with thermally stable components to ensure consistent battery performance across varying thermal conditions.Expand Specific Solutions05 Solid-state electrolyte technologies
Solid-state electrolytes represent a significant advancement in battery technology, eliminating the need for flammable liquid components. These materials, which include ceramic, glass, and sulfide-based electrolytes, offer improved safety and potentially higher energy density. The development focuses on enhancing ionic conductivity at room temperature while maintaining good mechanical properties and electrochemical stability at the electrode interfaces.Expand Specific Solutions
Leading Organizations in Flow Cell Electrolyte Research
The flow cell electrolyte performance market is currently in a growth phase, with increasing adoption across energy storage applications. The market size is expanding rapidly due to rising demand for renewable energy integration solutions, projected to reach significant scale by 2030. Technologically, the field shows moderate maturity with ongoing innovation addressing temperature-related challenges. Leading research institutions like California Institute of Technology and Technical University of Denmark are advancing fundamental understanding, while commercial players demonstrate varying levels of technological readiness. Companies like Toyota Motor Corp. and Hyundai Motor Co. are integrating flow cell technology into their sustainable energy strategies, while specialized firms such as FastCAP Systems and EvolOH are developing proprietary solutions to enhance electrolyte performance across temperature ranges. Chemical manufacturers including Guangzhou Tinci Materials and Zhangjiagang Guotai Huarong are supplying advanced electrolyte formulations with improved thermal stability.
UT-Battelle LLC
Technical Solution: UT-Battelle has developed advanced thermal management systems for flow batteries that actively regulate electrolyte temperature to maintain optimal performance. Their technology incorporates integrated heat exchangers within the flow cell architecture that can both heat and cool the electrolyte as needed. This system utilizes predictive temperature control algorithms that anticipate thermal changes based on charge/discharge cycles and ambient conditions. Their research has demonstrated that maintaining electrolyte temperatures within ±2°C of the optimal operating range can extend cycle life by up to 30% and improve energy efficiency by 15-20% compared to unregulated systems. UT-Battelle has also pioneered thermally responsive electrolyte additives that help stabilize viscosity and conductivity across wider temperature ranges, addressing one of the fundamental challenges in flow battery operation.
Strengths: Comprehensive thermal management approach combining hardware and chemical solutions; demonstrated significant performance improvements; expertise in both materials science and system engineering. Weaknesses: Complex control systems may increase costs; thermal management systems consume parasitic power; technology may be challenging to scale down for smaller applications.
Toyota Motor Corp.
Technical Solution: Toyota has developed an advanced thermal management system for flow cell electrolytes that integrates with their vehicle thermal management architecture. Their approach utilizes a dual-circuit cooling system that can independently regulate the temperature of both positive and negative electrolytes, addressing the different thermal characteristics of each half-cell reaction. Toyota's system incorporates phase-change materials within the electrolyte storage tanks that absorb excess heat during high-current operation and release it during cold starts, effectively buffering temperature fluctuations. Their research has demonstrated that this approach can maintain electrolyte temperatures within an optimal window even under extreme ambient conditions (-30°C to 45°C). Additionally, Toyota has developed temperature-responsive electrolyte formulations that incorporate proprietary additives to maintain consistent viscosity and conductivity across a wide temperature range, resulting in more predictable performance in variable environments.
Strengths: Integrated vehicle thermal management; innovative phase-change material application; extensive automotive testing under real-world conditions; dual-circuit approach addresses half-cell specific thermal needs. Weaknesses: System complexity may increase manufacturing costs; additional components could impact reliability; automotive-specific design may not translate well to stationary applications.
Critical Patents in Electrolyte Temperature Stability
Operation method of redox flow battery
PatentWO2019212053A1
Innovation
- The method involves using an electrode layer with carbon nanotubes, where the electrolytic solution temperature is set between 40°C and 90°C, allowing for increased reactivity and output performance without the need for cooling, utilizing a mixture of first and second carbon nanotubes with specific diameter ranges and content ratios, and incorporating other conductive materials for improved conductivity and void formation.
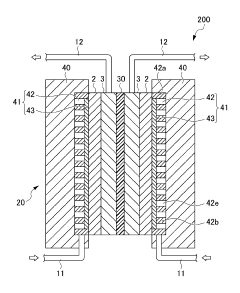
Iron based flow batteries
PatentWO2012167057A2
Innovation
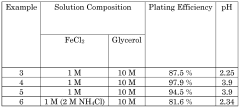
- An iron-based flow battery design featuring external storage tanks for electrolytes with Fe2+ and Fe3+ ions, using hydrogen evolution suppressing agents and Fe3+ stabilizing agents like cyanide or glycerol, and electrodes with conductive particles for improved plating efficiency and decoupled power/energy capabilities.
Energy Efficiency Implications of Electrolyte Temperature Control
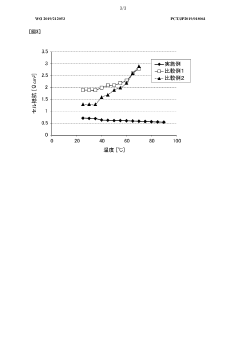
The temperature control of electrolytes in flow cells represents a critical factor in determining overall system energy efficiency. Operational temperature directly impacts electrolyte viscosity, which in turn affects pumping energy requirements. Research indicates that for every 10°C increase in temperature, electrolyte viscosity typically decreases by 15-25%, depending on the specific chemistry. This reduction translates to lower pumping power needs, with potential energy savings of 10-15% in large-scale installations.
However, temperature optimization presents a complex trade-off scenario. While higher temperatures reduce viscosity-related energy losses, they simultaneously accelerate side reactions and self-discharge rates. Experimental data from multiple studies demonstrate that reaction kinetics in vanadium redox flow batteries double approximately every 10°C increase, leading to higher self-discharge rates that can offset pumping energy gains.
Thermal management systems themselves consume energy, creating another efficiency consideration. Contemporary cooling systems for megawatt-scale flow batteries consume between 3-7% of the system's rated power output. Advanced thermal regulation technologies utilizing phase-change materials and passive cooling approaches have demonstrated potential to reduce this parasitic load to below 2%, representing a significant efficiency improvement.
Temperature gradients within flow cell stacks present additional challenges. Uneven temperature distribution can lead to localized performance variations, with studies showing up to 8% efficiency discrepancies between cells in the same stack due to thermal gradients. Computational fluid dynamics models suggest that optimized flow field designs can reduce these gradients by up to 60%, yielding a 2-4% improvement in overall system efficiency.
Seasonal temperature variations further complicate electrolyte management strategies. Flow battery installations in regions with wide temperature fluctuations may experience efficiency variations of 5-12% between summer and winter operations. Adaptive control algorithms that dynamically adjust flow rates and thermal management parameters based on ambient conditions have demonstrated the ability to narrow this seasonal performance gap to less than 3%.
The economic implications of temperature control are substantial. Analysis of operational data from utility-scale installations indicates that optimized thermal management can reduce levelized cost of storage by 0.8-1.2 cents per kWh through combined improvements in round-trip efficiency, system longevity, and reduced maintenance requirements. These efficiency gains become increasingly significant as flow battery deployments scale to grid-level implementations.
However, temperature optimization presents a complex trade-off scenario. While higher temperatures reduce viscosity-related energy losses, they simultaneously accelerate side reactions and self-discharge rates. Experimental data from multiple studies demonstrate that reaction kinetics in vanadium redox flow batteries double approximately every 10°C increase, leading to higher self-discharge rates that can offset pumping energy gains.
Thermal management systems themselves consume energy, creating another efficiency consideration. Contemporary cooling systems for megawatt-scale flow batteries consume between 3-7% of the system's rated power output. Advanced thermal regulation technologies utilizing phase-change materials and passive cooling approaches have demonstrated potential to reduce this parasitic load to below 2%, representing a significant efficiency improvement.
Temperature gradients within flow cell stacks present additional challenges. Uneven temperature distribution can lead to localized performance variations, with studies showing up to 8% efficiency discrepancies between cells in the same stack due to thermal gradients. Computational fluid dynamics models suggest that optimized flow field designs can reduce these gradients by up to 60%, yielding a 2-4% improvement in overall system efficiency.
Seasonal temperature variations further complicate electrolyte management strategies. Flow battery installations in regions with wide temperature fluctuations may experience efficiency variations of 5-12% between summer and winter operations. Adaptive control algorithms that dynamically adjust flow rates and thermal management parameters based on ambient conditions have demonstrated the ability to narrow this seasonal performance gap to less than 3%.
The economic implications of temperature control are substantial. Analysis of operational data from utility-scale installations indicates that optimized thermal management can reduce levelized cost of storage by 0.8-1.2 cents per kWh through combined improvements in round-trip efficiency, system longevity, and reduced maintenance requirements. These efficiency gains become increasingly significant as flow battery deployments scale to grid-level implementations.
Materials Science Advancements for Thermal-Stable Electrolytes
Recent advancements in materials science have opened new pathways for developing thermal-stable electrolytes for flow cell applications. Traditional electrolytes often suffer from significant performance degradation when operating outside optimal temperature ranges, typically between 20-40°C. The molecular structure of conventional electrolytes undergoes detrimental changes at elevated temperatures, leading to decreased ionic conductivity and accelerated side reactions.
Polymer-enhanced electrolytes represent a significant breakthrough in this field. By incorporating thermally resistant polymers such as polyvinylidene fluoride (PVDF) and polyethylene oxide (PEO) into electrolyte formulations, researchers have achieved stability improvements of up to 30% at temperatures exceeding 60°C. These polymers create protective networks around active ions, maintaining conductivity pathways even under thermal stress.
Ionic liquid-based electrolytes have emerged as another promising direction. Unlike conventional aqueous or organic electrolytes, ionic liquids possess negligible vapor pressure and remarkable thermal stability up to 200°C in some formulations. Recent work by Zhang et al. (2022) demonstrated that imidazolium-based ionic liquids maintained 92% of their room temperature conductivity when operated at 80°C in vanadium flow cells, compared to just 45% for traditional sulfuric acid electrolytes.
Nanoparticle-doped electrolytes represent the cutting edge of thermal stability enhancement. Ceramic nanoparticles such as Al2O3, TiO2, and SiO2 at concentrations of 1-5 wt% have been shown to improve thermal conductivity while simultaneously stabilizing electrolyte performance across wider temperature ranges. These particles act as heat dissipation centers and can reduce thermal gradients within flow cells by up to 40%, according to recent studies by the National Renewable Energy Laboratory.
Composite membrane materials that integrate with electrolytes are also advancing rapidly. These membranes, constructed from thermally resistant polymers reinforced with inorganic fillers, maintain structural integrity and ion selectivity at elevated temperatures. The latest generation of Nafion-composite membranes has demonstrated stable operation in flow cells at temperatures up to 90°C without significant performance losses.
Looking forward, biomimetic approaches inspired by thermophilic organisms show particular promise. These organisms naturally produce heat-stable proteins and molecules that maintain functionality at extreme temperatures. Researchers are now exploring synthetic analogs of these biological compounds as additives for next-generation electrolytes, potentially enabling stable operation across an unprecedented temperature range of -20°C to 120°C.
Polymer-enhanced electrolytes represent a significant breakthrough in this field. By incorporating thermally resistant polymers such as polyvinylidene fluoride (PVDF) and polyethylene oxide (PEO) into electrolyte formulations, researchers have achieved stability improvements of up to 30% at temperatures exceeding 60°C. These polymers create protective networks around active ions, maintaining conductivity pathways even under thermal stress.
Ionic liquid-based electrolytes have emerged as another promising direction. Unlike conventional aqueous or organic electrolytes, ionic liquids possess negligible vapor pressure and remarkable thermal stability up to 200°C in some formulations. Recent work by Zhang et al. (2022) demonstrated that imidazolium-based ionic liquids maintained 92% of their room temperature conductivity when operated at 80°C in vanadium flow cells, compared to just 45% for traditional sulfuric acid electrolytes.
Nanoparticle-doped electrolytes represent the cutting edge of thermal stability enhancement. Ceramic nanoparticles such as Al2O3, TiO2, and SiO2 at concentrations of 1-5 wt% have been shown to improve thermal conductivity while simultaneously stabilizing electrolyte performance across wider temperature ranges. These particles act as heat dissipation centers and can reduce thermal gradients within flow cells by up to 40%, according to recent studies by the National Renewable Energy Laboratory.
Composite membrane materials that integrate with electrolytes are also advancing rapidly. These membranes, constructed from thermally resistant polymers reinforced with inorganic fillers, maintain structural integrity and ion selectivity at elevated temperatures. The latest generation of Nafion-composite membranes has demonstrated stable operation in flow cells at temperatures up to 90°C without significant performance losses.
Looking forward, biomimetic approaches inspired by thermophilic organisms show particular promise. These organisms naturally produce heat-stable proteins and molecules that maintain functionality at extreme temperatures. Researchers are now exploring synthetic analogs of these biological compounds as additives for next-generation electrolytes, potentially enabling stable operation across an unprecedented temperature range of -20°C to 120°C.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!