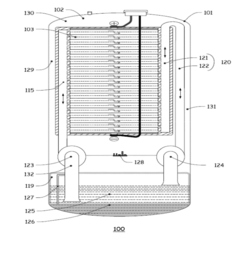
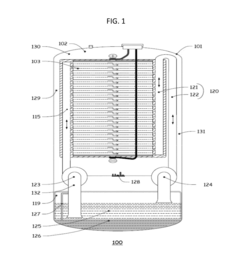
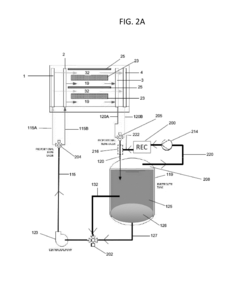
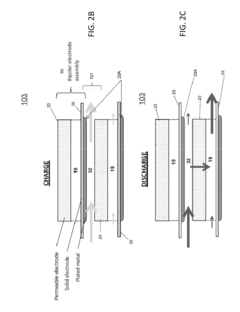
Flow Battery Electrolytes Design for Long Term Durability
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flow Battery Electrolytes Evolution and Durability Goals
Flow batteries have evolved significantly since their inception in the 1970s, with electrolyte development being a central focus for improving performance and durability. Initially, these systems utilized simple metal salts dissolved in aqueous solutions, primarily vanadium-based compounds. The first-generation flow batteries suffered from limited energy density, significant crossover issues, and rapid capacity fade, with operational lifetimes rarely exceeding 1,000 cycles.
The 1990s marked a pivotal advancement with the introduction of all-vanadium redox flow batteries (VRFBs), which eliminated cross-contamination issues by using the same element in different oxidation states. This innovation extended cycle life to approximately 5,000-10,000 cycles, representing a substantial improvement in durability. However, these systems still faced challenges with membrane degradation and electrolyte instability under extended operation.
By the early 2000s, research shifted toward enhancing electrolyte stability through additives and supporting electrolytes. Scientists discovered that specific acid concentrations and stabilizing compounds could significantly reduce vanadium precipitation and side reactions. These modifications extended operational temperature ranges and improved cycling efficiency, pushing durability boundaries toward 15,000 cycles under controlled conditions.
The 2010s witnessed the emergence of non-aqueous and hybrid electrolyte systems, offering higher cell voltages and energy densities. Organic redox-active materials and metal coordination complexes expanded the chemical diversity of flow battery electrolytes. Despite these advances, these newer systems typically demonstrated shorter lifetimes than mature vanadium systems, highlighting the inherent trade-off between energy density and long-term stability.
Current durability goals for flow battery electrolytes are ambitious yet essential for commercial viability. The U.S. Department of Energy has established targets of 20+ year operational lifetimes (equivalent to >10,000 full cycles) with less than 20% capacity degradation. These benchmarks require electrolytes that maintain chemical stability under repeated cycling, resist degradation from oxygen exposure, and minimize side reactions at extreme states of charge.
Industry standards are increasingly focusing on total cost of ownership metrics, where electrolyte durability directly impacts replacement costs and system economics. For grid-scale applications, achieving levelized cost of storage below $0.05/kWh-cycle necessitates electrolytes with exceptional chemical resilience and minimal maintenance requirements. The ultimate goal is developing self-healing electrolyte formulations that can maintain performance characteristics throughout the entire system lifetime without significant intervention.
The 1990s marked a pivotal advancement with the introduction of all-vanadium redox flow batteries (VRFBs), which eliminated cross-contamination issues by using the same element in different oxidation states. This innovation extended cycle life to approximately 5,000-10,000 cycles, representing a substantial improvement in durability. However, these systems still faced challenges with membrane degradation and electrolyte instability under extended operation.
By the early 2000s, research shifted toward enhancing electrolyte stability through additives and supporting electrolytes. Scientists discovered that specific acid concentrations and stabilizing compounds could significantly reduce vanadium precipitation and side reactions. These modifications extended operational temperature ranges and improved cycling efficiency, pushing durability boundaries toward 15,000 cycles under controlled conditions.
The 2010s witnessed the emergence of non-aqueous and hybrid electrolyte systems, offering higher cell voltages and energy densities. Organic redox-active materials and metal coordination complexes expanded the chemical diversity of flow battery electrolytes. Despite these advances, these newer systems typically demonstrated shorter lifetimes than mature vanadium systems, highlighting the inherent trade-off between energy density and long-term stability.
Current durability goals for flow battery electrolytes are ambitious yet essential for commercial viability. The U.S. Department of Energy has established targets of 20+ year operational lifetimes (equivalent to >10,000 full cycles) with less than 20% capacity degradation. These benchmarks require electrolytes that maintain chemical stability under repeated cycling, resist degradation from oxygen exposure, and minimize side reactions at extreme states of charge.
Industry standards are increasingly focusing on total cost of ownership metrics, where electrolyte durability directly impacts replacement costs and system economics. For grid-scale applications, achieving levelized cost of storage below $0.05/kWh-cycle necessitates electrolytes with exceptional chemical resilience and minimal maintenance requirements. The ultimate goal is developing self-healing electrolyte formulations that can maintain performance characteristics throughout the entire system lifetime without significant intervention.
Market Analysis for Long-Duration Energy Storage Solutions
The long-duration energy storage (LDES) market is experiencing unprecedented growth, driven by the global transition to renewable energy sources and the increasing need for grid stability solutions. Current market valuations place the LDES sector at approximately $14 billion globally, with projections indicating potential growth to $58 billion by 2030, representing a compound annual growth rate of 19.7% over the decade.
Flow batteries, particularly those with advanced electrolyte designs focused on durability, are positioned as a critical segment within this expanding market. Unlike conventional lithium-ion batteries that typically provide 2-4 hours of storage, flow batteries can deliver 6-12 hours or more of continuous power, making them ideally suited for grid-scale applications requiring extended discharge periods.
Market demand for flow battery technology is being fueled by several converging factors. Utility companies are increasingly seeking storage solutions that can provide multi-day backup capabilities to compensate for the intermittency of renewable energy sources. Additionally, commercial and industrial customers are looking to flow batteries as a means to reduce peak demand charges and enhance energy resilience.
Regional analysis reveals significant market variations. North America currently leads in flow battery adoption, accounting for approximately 38% of global installations, followed by Asia-Pacific at 32% and Europe at 25%. China has emerged as a particularly aggressive market, with government initiatives supporting domestic flow battery manufacturing and deployment.
Customer segmentation within the LDES market shows utilities as the primary adopters (52%), followed by commercial/industrial users (28%), remote/off-grid applications (12%), and military/defense sectors (8%). This distribution highlights the technology's versatility across different application scenarios.
The economic proposition of flow batteries with durable electrolytes is becoming increasingly compelling. While initial capital costs remain higher than lithium-ion alternatives ($400-700/kWh versus $200-300/kWh), the extended cycle life (15,000+ cycles compared to 1,000-3,000 for lithium-ion) and lower degradation rates result in a more favorable levelized cost of storage (LCOS) over the system lifetime.
Market barriers include high upfront costs, limited manufacturing scale, and competition from emerging alternative technologies. However, the value proposition of flow batteries strengthens as electrolyte durability improves, directly addressing one of the technology's historical limitations and potentially unlocking new market segments previously considered economically unfeasible.
Flow batteries, particularly those with advanced electrolyte designs focused on durability, are positioned as a critical segment within this expanding market. Unlike conventional lithium-ion batteries that typically provide 2-4 hours of storage, flow batteries can deliver 6-12 hours or more of continuous power, making them ideally suited for grid-scale applications requiring extended discharge periods.
Market demand for flow battery technology is being fueled by several converging factors. Utility companies are increasingly seeking storage solutions that can provide multi-day backup capabilities to compensate for the intermittency of renewable energy sources. Additionally, commercial and industrial customers are looking to flow batteries as a means to reduce peak demand charges and enhance energy resilience.
Regional analysis reveals significant market variations. North America currently leads in flow battery adoption, accounting for approximately 38% of global installations, followed by Asia-Pacific at 32% and Europe at 25%. China has emerged as a particularly aggressive market, with government initiatives supporting domestic flow battery manufacturing and deployment.
Customer segmentation within the LDES market shows utilities as the primary adopters (52%), followed by commercial/industrial users (28%), remote/off-grid applications (12%), and military/defense sectors (8%). This distribution highlights the technology's versatility across different application scenarios.
The economic proposition of flow batteries with durable electrolytes is becoming increasingly compelling. While initial capital costs remain higher than lithium-ion alternatives ($400-700/kWh versus $200-300/kWh), the extended cycle life (15,000+ cycles compared to 1,000-3,000 for lithium-ion) and lower degradation rates result in a more favorable levelized cost of storage (LCOS) over the system lifetime.
Market barriers include high upfront costs, limited manufacturing scale, and competition from emerging alternative technologies. However, the value proposition of flow batteries strengthens as electrolyte durability improves, directly addressing one of the technology's historical limitations and potentially unlocking new market segments previously considered economically unfeasible.
Current Challenges in Flow Battery Electrolyte Stability
Flow battery electrolytes face significant stability challenges that impede their long-term durability and commercial viability. One primary issue is chemical degradation, where electrolyte components undergo unwanted side reactions during cycling. These reactions can be accelerated by exposure to oxygen, which causes oxidation of active species, particularly in vanadium redox flow batteries (VRFBs) where V(II) and V(III) are highly sensitive to air. Temperature fluctuations further exacerbate degradation, with elevated temperatures accelerating decomposition reactions while low temperatures can cause precipitation of active materials.
Membrane crossover represents another critical challenge, where active ionic species migrate through the separator membrane between positive and negative electrolytes. This phenomenon leads to capacity fade and efficiency loss over time, requiring periodic rebalancing of the electrolyte composition. In VRFBs, vanadium ion crossover results in self-discharge and reduced energy efficiency, while in organic flow batteries, crossover can lead to irreversible side reactions between incompatible species.
Corrosion of cell components presents a significant materials challenge. The highly acidic nature of many flow battery electrolytes (pH < 1 in sulfuric acid-based VRFBs) accelerates corrosion of metallic components, releasing contaminants that can catalyze unwanted side reactions and further degrade electrolyte performance. This necessitates expensive corrosion-resistant materials, increasing system costs.
Electrolyte stability is also compromised by precipitation issues. Limited solubility of active species restricts energy density and operational temperature ranges. In VRFBs, V(V) species can precipitate as V2O5 at elevated temperatures or high concentrations, while zinc-bromine flow batteries face zinc dendrite formation during cycling, potentially causing short circuits and safety hazards.
Additives introduced to mitigate these issues often create secondary complications. Stabilizing agents may reduce active species concentration, limiting energy density, while anti-corrosion additives can interfere with electrode reactions. Furthermore, the long-term stability of these additives themselves remains questionable under the harsh electrochemical conditions of flow batteries.
Environmental factors further complicate electrolyte stability. Exposure to light can degrade certain organic electrolytes, while impurities in industrial-grade chemicals used for large-scale production can catalyze unwanted side reactions. The cumulative effect of these challenges manifests as capacity fade, reduced coulombic efficiency, and increased maintenance requirements, significantly impacting the economic viability of flow battery systems for grid-scale energy storage applications.
Membrane crossover represents another critical challenge, where active ionic species migrate through the separator membrane between positive and negative electrolytes. This phenomenon leads to capacity fade and efficiency loss over time, requiring periodic rebalancing of the electrolyte composition. In VRFBs, vanadium ion crossover results in self-discharge and reduced energy efficiency, while in organic flow batteries, crossover can lead to irreversible side reactions between incompatible species.
Corrosion of cell components presents a significant materials challenge. The highly acidic nature of many flow battery electrolytes (pH < 1 in sulfuric acid-based VRFBs) accelerates corrosion of metallic components, releasing contaminants that can catalyze unwanted side reactions and further degrade electrolyte performance. This necessitates expensive corrosion-resistant materials, increasing system costs.
Electrolyte stability is also compromised by precipitation issues. Limited solubility of active species restricts energy density and operational temperature ranges. In VRFBs, V(V) species can precipitate as V2O5 at elevated temperatures or high concentrations, while zinc-bromine flow batteries face zinc dendrite formation during cycling, potentially causing short circuits and safety hazards.
Additives introduced to mitigate these issues often create secondary complications. Stabilizing agents may reduce active species concentration, limiting energy density, while anti-corrosion additives can interfere with electrode reactions. Furthermore, the long-term stability of these additives themselves remains questionable under the harsh electrochemical conditions of flow batteries.
Environmental factors further complicate electrolyte stability. Exposure to light can degrade certain organic electrolytes, while impurities in industrial-grade chemicals used for large-scale production can catalyze unwanted side reactions. The cumulative effect of these challenges manifests as capacity fade, reduced coulombic efficiency, and increased maintenance requirements, significantly impacting the economic viability of flow battery systems for grid-scale energy storage applications.
State-of-the-Art Electrolyte Formulations and Protection Strategies
01 Additives for enhancing electrolyte stability
Various additives can be incorporated into flow battery electrolytes to enhance their stability and durability. These additives can include stabilizing agents, antioxidants, and metal ion chelators that prevent degradation of the electrolyte components. By reducing side reactions and preventing precipitation of active materials, these additives significantly extend the operational lifetime of flow battery electrolytes under cycling conditions.- Additives for enhancing electrolyte stability: Various additives can be incorporated into flow battery electrolytes to enhance their stability and durability. These additives can include stabilizing agents, antioxidants, and metal ion chelators that prevent degradation of the electrolyte components. By reducing side reactions and preventing precipitation of active materials, these additives significantly extend the operational lifetime of flow battery electrolytes under cycling conditions.
- Advanced membrane technologies for electrolyte separation: Specialized membrane technologies play a crucial role in maintaining electrolyte durability by preventing cross-contamination between positive and negative electrolytes. These membranes feature enhanced ion selectivity, chemical resistance, and mechanical strength to withstand the harsh chemical environment of flow batteries. Improved membrane designs reduce capacity fade and extend the operational lifetime of the electrolyte systems by minimizing unwanted ion transport.
- pH-controlled electrolyte formulations: Controlling the pH of flow battery electrolytes is essential for maintaining their durability. Specific pH buffers and regulators can be incorporated to prevent degradation of active materials and supporting electrolytes. These formulations help maintain optimal operating conditions, prevent precipitation of active species, and reduce side reactions that would otherwise lead to capacity fade and reduced cycle life of the battery system.
- Redox-active organic compounds for enhanced durability: Novel redox-active organic compounds can be used as alternatives to traditional metal-based electrolytes to improve durability. These organic compounds offer advantages such as tunable redox potentials, reduced environmental impact, and resistance to degradation. By carefully selecting and modifying these organic structures, flow battery electrolytes can achieve extended cycle life and improved stability under various operating conditions.
- Temperature management systems for electrolyte preservation: Implementing effective temperature management systems helps preserve electrolyte durability in flow batteries. Controlling the operating temperature prevents accelerated degradation of electrolyte components and reduces unwanted side reactions. These systems can include thermal regulation units, insulation materials, and heat exchangers designed specifically to maintain optimal temperature ranges for maximum electrolyte stability and battery performance.
02 Advanced membrane technologies for electrolyte separation
Specialized membrane technologies play a crucial role in maintaining electrolyte durability by effectively separating the positive and negative electrolytes while minimizing crossover. These membranes can be modified with functional groups or coatings to enhance selectivity and reduce contamination between electrolyte compartments. Improved membrane performance directly contributes to extended electrolyte lifetime and overall battery durability.Expand Specific Solutions03 pH and temperature control mechanisms
Maintaining optimal pH levels and temperature conditions is essential for flow battery electrolyte durability. Systems incorporating pH buffers and temperature regulation mechanisms can prevent accelerated degradation of electrolyte components. These control strategies help maintain electrolyte stability during extended operation and storage periods, significantly improving the overall durability and performance consistency of flow battery systems.Expand Specific Solutions04 Novel electrolyte compositions with enhanced durability
Innovative electrolyte formulations have been developed specifically to address durability challenges in flow batteries. These compositions may include alternative redox couples, supporting electrolytes with higher stability, or hybrid organic-inorganic systems. Such novel electrolyte compositions demonstrate improved resistance to degradation mechanisms like oxidation, hydrolysis, and precipitation, resulting in significantly extended cycle life and calendar life for flow battery systems.Expand Specific Solutions05 Electrolyte purification and regeneration systems
Continuous or periodic purification and regeneration of flow battery electrolytes can substantially extend their operational lifetime. These systems may employ techniques such as filtration, electrochemical regeneration, or chemical treatment to remove impurities and restore degraded active species. By implementing these maintenance processes, the durability of flow battery electrolytes can be significantly improved, reducing the need for complete electrolyte replacement and lowering operational costs.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Flow Battery Sector
The flow battery electrolytes market is currently in a growth phase, with increasing adoption driven by the need for long-duration energy storage solutions. Market size is projected to expand significantly as renewable energy integration accelerates, with an estimated CAGR of 15-20% through 2030. Technologically, the sector shows varying maturity levels, with companies like Lockheed Martin Advanced Energy Storage, Primus Power, and Dalian Rongke Power leading commercial deployment of vanadium-based systems. Research institutions including MIT, Case Western Reserve University, and Chinese academic centers are advancing next-generation electrolyte chemistries. Japanese corporations (Sumitomo Electric, Toshiba) and American players are competing to overcome durability challenges, while collaboration between industry and academia is accelerating innovation in membrane technology and electrolyte formulations to extend operational lifetimes beyond current limitations.
Massachusetts Institute of Technology
Technical Solution: MIT has developed revolutionary organic flow battery electrolytes designed specifically for exceptional long-term durability. Their approach centers on synthesizing novel redox-active organic molecules that are inherently stable in both oxidized and reduced states. MIT researchers have created quinone-based compounds with carefully positioned functional groups that resist degradation mechanisms common in flow batteries. Their electrolyte design incorporates pH-buffering components that maintain optimal operating conditions throughout battery cycling, preventing capacity fade caused by chemical decomposition. A significant innovation is their development of asymmetric electrolyte systems that pair complementary organic molecules with different redox potentials, enabling higher cell voltages while maintaining stability. MIT has also pioneered computational screening methods that identify molecular structures resistant to nucleophilic attack and radical-induced decomposition, the primary failure modes in organic flow batteries. Their electrolyte formulations have demonstrated remarkable cycling stability, maintaining over 90% capacity after thousands of cycles in laboratory testing.
Strengths: Cutting-edge molecular engineering capabilities that enable precise tuning of electrolyte properties for optimal performance and durability. Their organic electrolyte designs potentially offer lower cost and environmental impact than metal-based alternatives. Weaknesses: Organic flow battery technology remains less mature than vanadium-based systems, with challenges in achieving comparable power density and proving long-term reliability at commercial scale.
Primus Power Corp.
Technical Solution: Primus Power has developed a proprietary zinc-bromine flow battery technology with innovative electrolyte formulations designed specifically for long-term durability. Their single-flow design eliminates the need for a membrane separator, removing a common failure point in traditional flow batteries. Primus's electrolyte system incorporates proprietary metal complexing agents that stabilize the zinc bromide chemistry, preventing dendrite formation and electrode deterioration that typically limit cycle life. Their EnergyPod system utilizes a titanium electrode design with specialized surface treatments that resist corrosion from the bromine-rich environment, enabling extended operational lifetimes. A key innovation in their electrolyte formulation is the inclusion of phase-transfer catalysts that enhance reaction kinetics while maintaining chemical stability during thousands of charge-discharge cycles. Primus has demonstrated field deployments with their systems showing minimal capacity degradation after years of operation in various environmental conditions, validating their approach to electrolyte durability.
Strengths: Membrane-less design significantly reduces system complexity and eliminates a major failure point, while their zinc-bromine chemistry offers higher energy density than vanadium-based alternatives. Weaknesses: Zinc-based systems historically face challenges with metal plating uniformity during cycling, which can affect long-term performance consistency despite Primus's innovations to address these issues.
Critical Patents and Research on Electrolyte Degradation Mechanisms
Flow battery electrolyte compositions containing a chelating agent and a metal plating enhancer
PatentActiveUS20160276691A1
Innovation
- An aqueous electrolyte composition comprising zinc bromide, a chelating agent, a metal plating enhancer, and a bromine sequestering agent is used, which reduces dendrite formation, corrosion, and improves the morphology of zinc deposits, enhancing energy capacity and efficiency by up to 400%.
Flow battery
PatentWO2019031033A1
Innovation
- The flow battery design incorporates a gas supply system that generates bubbles within the electrolyte, promoting circulation and maintaining uniform electrolyte concentration, while a dense diaphragm with high hydroxide ion conductivity prevents metal ion permeation and reduces dendrite growth, ensuring stable electrode conduction.
Environmental Impact and Sustainability of Electrolyte Materials
The environmental impact of flow battery electrolytes represents a critical consideration in the development of sustainable energy storage technologies. Traditional electrolytes often contain heavy metals such as vanadium or toxic organic compounds that pose significant environmental risks throughout their lifecycle. When evaluating flow battery electrolytes for long-term durability, their environmental footprint must be assessed from raw material extraction through manufacturing, operation, and end-of-life disposal.
Water-based electrolytes generally demonstrate lower environmental impact compared to organic solvent-based alternatives, which often involve volatile organic compounds (VOCs) that contribute to air pollution and potential groundwater contamination. However, aqueous systems typically contain metal ions that require careful management to prevent ecological damage. Recent advances in bio-inspired electrolytes derived from quinones and other naturally occurring compounds show promise for reducing environmental burden while maintaining performance metrics.
The sustainability of electrolyte materials depends significantly on their sourcing and abundance. Vanadium-based systems, while offering excellent electrochemical stability, rely on a relatively scarce metal with geographically concentrated mining operations, raising concerns about supply chain resilience and extraction impacts. Alternatives utilizing more abundant elements such as iron, zinc, or organic compounds derived from biomass present more sustainable pathways, though often with trade-offs in energy density or cycle life.
Electrolyte recyclability emerges as a paramount factor in long-term environmental sustainability. Systems designed with reclamation in mind can significantly reduce waste and resource consumption. Research indicates that vanadium electrolytes can achieve recycling rates exceeding 95%, substantially reducing lifecycle environmental impact. Emerging organic electrolytes face greater recycling challenges due to degradation products but offer advantages in biodegradability when properly designed.
Energy and water consumption during electrolyte production represents another critical environmental consideration. Manufacturing processes for sophisticated organic electrolytes often require multiple synthesis steps with significant energy inputs and potential for hazardous waste generation. Simplified formulations that minimize processing steps while maintaining durability characteristics can substantially reduce embodied energy and associated carbon emissions.
Leakage prevention and containment systems play vital roles in mitigating environmental risks during operation. Even small electrolyte leaks can result in soil or water contamination, particularly with metal-ion containing solutions. Advanced membrane technologies and improved sealing systems not only extend operational durability but also enhance environmental protection by minimizing release potential throughout the battery lifecycle.
Water-based electrolytes generally demonstrate lower environmental impact compared to organic solvent-based alternatives, which often involve volatile organic compounds (VOCs) that contribute to air pollution and potential groundwater contamination. However, aqueous systems typically contain metal ions that require careful management to prevent ecological damage. Recent advances in bio-inspired electrolytes derived from quinones and other naturally occurring compounds show promise for reducing environmental burden while maintaining performance metrics.
The sustainability of electrolyte materials depends significantly on their sourcing and abundance. Vanadium-based systems, while offering excellent electrochemical stability, rely on a relatively scarce metal with geographically concentrated mining operations, raising concerns about supply chain resilience and extraction impacts. Alternatives utilizing more abundant elements such as iron, zinc, or organic compounds derived from biomass present more sustainable pathways, though often with trade-offs in energy density or cycle life.
Electrolyte recyclability emerges as a paramount factor in long-term environmental sustainability. Systems designed with reclamation in mind can significantly reduce waste and resource consumption. Research indicates that vanadium electrolytes can achieve recycling rates exceeding 95%, substantially reducing lifecycle environmental impact. Emerging organic electrolytes face greater recycling challenges due to degradation products but offer advantages in biodegradability when properly designed.
Energy and water consumption during electrolyte production represents another critical environmental consideration. Manufacturing processes for sophisticated organic electrolytes often require multiple synthesis steps with significant energy inputs and potential for hazardous waste generation. Simplified formulations that minimize processing steps while maintaining durability characteristics can substantially reduce embodied energy and associated carbon emissions.
Leakage prevention and containment systems play vital roles in mitigating environmental risks during operation. Even small electrolyte leaks can result in soil or water contamination, particularly with metal-ion containing solutions. Advanced membrane technologies and improved sealing systems not only extend operational durability but also enhance environmental protection by minimizing release potential throughout the battery lifecycle.
Techno-Economic Assessment of Flow Battery Electrolyte Solutions
The techno-economic assessment of flow battery electrolyte solutions reveals critical insights into the financial viability and market potential of different electrolyte formulations. Current market analysis indicates that vanadium-based electrolytes dominate with approximately 70% market share, despite their high cost ranging from $25-40/kWh. This cost factor significantly impacts the overall capital expenditure of flow battery systems, with electrolytes typically representing 30-45% of total system costs.
Alternative electrolyte chemistries such as zinc-bromine, iron-chromium, and organic compounds offer potential cost advantages, with projected costs as low as $10-15/kWh for organic electrolytes at scale. However, these alternatives present trade-offs in energy density, operational lifetime, and system complexity that must be factored into comprehensive economic models.
Lifecycle cost analysis demonstrates that electrolyte durability directly correlates with long-term operational economics. For instance, a 20% improvement in electrolyte stability can reduce lifetime operational costs by 15-18% through decreased replacement and maintenance requirements. Sensitivity analysis reveals that electrolyte degradation rates below 2% annually represent a critical threshold for achieving competitive levelized cost of storage (LCOS) below $0.10/kWh-cycle.
Manufacturing scale presents another crucial economic factor. Current production methods for high-purity electrolytes involve significant processing costs, but economies of scale could reduce production expenses by 30-40% at gigawatt-hour production levels. This scaling effect is particularly pronounced for organic and metal-organic electrolyte solutions, where precursor costs can be significantly reduced through bulk purchasing and optimized synthesis pathways.
Regional variations in raw material availability create substantial cost differentials. For example, vanadium prices fluctuate by up to 300% based on geographic sourcing, while organic electrolyte precursors show more stable global pricing with variations under 50%. These regional factors significantly impact optimal electrolyte selection for specific deployment locations.
Recycling and circular economy approaches offer promising pathways to improve long-term economics. Advanced electrolyte recycling processes can recover 85-95% of active materials, potentially reducing lifetime electrolyte costs by 25-35%. However, these processes require additional capital investment that must be balanced against raw material savings in comprehensive economic models.
Alternative electrolyte chemistries such as zinc-bromine, iron-chromium, and organic compounds offer potential cost advantages, with projected costs as low as $10-15/kWh for organic electrolytes at scale. However, these alternatives present trade-offs in energy density, operational lifetime, and system complexity that must be factored into comprehensive economic models.
Lifecycle cost analysis demonstrates that electrolyte durability directly correlates with long-term operational economics. For instance, a 20% improvement in electrolyte stability can reduce lifetime operational costs by 15-18% through decreased replacement and maintenance requirements. Sensitivity analysis reveals that electrolyte degradation rates below 2% annually represent a critical threshold for achieving competitive levelized cost of storage (LCOS) below $0.10/kWh-cycle.
Manufacturing scale presents another crucial economic factor. Current production methods for high-purity electrolytes involve significant processing costs, but economies of scale could reduce production expenses by 30-40% at gigawatt-hour production levels. This scaling effect is particularly pronounced for organic and metal-organic electrolyte solutions, where precursor costs can be significantly reduced through bulk purchasing and optimized synthesis pathways.
Regional variations in raw material availability create substantial cost differentials. For example, vanadium prices fluctuate by up to 300% based on geographic sourcing, while organic electrolyte precursors show more stable global pricing with variations under 50%. These regional factors significantly impact optimal electrolyte selection for specific deployment locations.
Recycling and circular economy approaches offer promising pathways to improve long-term economics. Advanced electrolyte recycling processes can recover 85-95% of active materials, potentially reducing lifetime electrolyte costs by 25-35%. However, these processes require additional capital investment that must be balanced against raw material savings in comprehensive economic models.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!