Electrolyte Rebalancing Strategies in Flow Battery Systems
OCT 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flow Battery Electrolyte Rebalancing Background and Objectives
Flow batteries have emerged as a promising energy storage technology over the past several decades, with their development tracing back to NASA's research in the 1970s. These systems offer distinct advantages in grid-scale energy storage applications due to their ability to decouple power and energy capacity, long cycle life, and deep discharge capabilities. The evolution of flow battery technology has progressed from first-generation vanadium redox flow batteries (VRFBs) to more advanced chemistries including zinc-bromine, iron-chromium, and organic-based systems.
Electrolyte rebalancing represents a critical challenge that has persistently hindered the widespread commercial adoption of flow battery technology. This phenomenon occurs when the state of charge between the positive and negative electrolytes becomes imbalanced over time, leading to capacity fade, reduced efficiency, and ultimately system failure if left unaddressed. The primary causes include crossover of active species through imperfect membranes, side reactions with electrolyte components, and differential rates of water and solvent transport.
The technical objective of electrolyte rebalancing research is to develop cost-effective, energy-efficient, and reliable methods to maintain electrolyte balance throughout the operational lifetime of flow battery systems. This includes minimizing the root causes of imbalance while implementing corrective measures that can operate autonomously with minimal maintenance requirements. Successful rebalancing strategies must be compatible with existing flow battery architectures and should not introduce additional failure modes or significantly increase system complexity.
Current rebalancing approaches can be categorized into three main strategies: electrochemical methods, chemical rebalancing techniques, and hydraulic solutions. Each presents unique advantages and limitations in terms of energy efficiency, implementation complexity, and operational reliability. The evolution of these techniques has been driven by the need to reduce parasitic energy losses associated with rebalancing operations while ensuring long-term stability of the electrolyte chemistry.
The technological trajectory indicates a shift toward integrated rebalancing systems that combine multiple approaches to address the multifaceted nature of electrolyte imbalance. Recent innovations focus on in-situ monitoring and predictive analytics to enable preemptive rebalancing before significant capacity loss occurs. Additionally, advanced membrane materials with reduced crossover rates are being developed to minimize the fundamental causes of imbalance.
This technical investigation aims to comprehensively assess the state-of-the-art in electrolyte rebalancing strategies, identify key technological barriers, and outline promising research directions that could lead to breakthrough solutions in this critical aspect of flow battery technology.
Electrolyte rebalancing represents a critical challenge that has persistently hindered the widespread commercial adoption of flow battery technology. This phenomenon occurs when the state of charge between the positive and negative electrolytes becomes imbalanced over time, leading to capacity fade, reduced efficiency, and ultimately system failure if left unaddressed. The primary causes include crossover of active species through imperfect membranes, side reactions with electrolyte components, and differential rates of water and solvent transport.
The technical objective of electrolyte rebalancing research is to develop cost-effective, energy-efficient, and reliable methods to maintain electrolyte balance throughout the operational lifetime of flow battery systems. This includes minimizing the root causes of imbalance while implementing corrective measures that can operate autonomously with minimal maintenance requirements. Successful rebalancing strategies must be compatible with existing flow battery architectures and should not introduce additional failure modes or significantly increase system complexity.
Current rebalancing approaches can be categorized into three main strategies: electrochemical methods, chemical rebalancing techniques, and hydraulic solutions. Each presents unique advantages and limitations in terms of energy efficiency, implementation complexity, and operational reliability. The evolution of these techniques has been driven by the need to reduce parasitic energy losses associated with rebalancing operations while ensuring long-term stability of the electrolyte chemistry.
The technological trajectory indicates a shift toward integrated rebalancing systems that combine multiple approaches to address the multifaceted nature of electrolyte imbalance. Recent innovations focus on in-situ monitoring and predictive analytics to enable preemptive rebalancing before significant capacity loss occurs. Additionally, advanced membrane materials with reduced crossover rates are being developed to minimize the fundamental causes of imbalance.
This technical investigation aims to comprehensively assess the state-of-the-art in electrolyte rebalancing strategies, identify key technological barriers, and outline promising research directions that could lead to breakthrough solutions in this critical aspect of flow battery technology.
Market Analysis for Flow Battery Energy Storage Systems
The global flow battery energy storage market is experiencing significant growth, driven by increasing demand for long-duration energy storage solutions. As of 2023, the market was valued at approximately $290 million, with projections indicating a compound annual growth rate (CAGR) of 28.5% through 2030, potentially reaching $1.8 billion by the end of the decade. This remarkable growth trajectory is primarily fueled by the global transition to renewable energy sources and the inherent need for efficient energy storage systems to address intermittency issues.
Flow batteries have carved a distinct niche in the energy storage ecosystem, particularly for grid-scale applications requiring 4+ hours of discharge duration. Unlike lithium-ion batteries that dominate the short-duration storage market, flow batteries excel in applications requiring 6-12 hour discharge periods, with minimal capacity degradation even after thousands of cycles.
The utility sector represents the largest market segment, accounting for approximately 65% of flow battery deployments. These installations primarily serve grid stabilization, peak shaving, and renewable integration functions. Commercial and industrial applications constitute roughly 25% of the market, while remote microgrids and specialized applications make up the remaining 10%.
Geographically, North America leads the market with approximately 40% share, followed by Asia-Pacific at 35% and Europe at 20%. China, Japan, and Australia are showing particularly strong growth rates in the Asia-Pacific region, driven by aggressive renewable energy targets and supportive government policies.
Key market drivers include declining system costs (decreasing at approximately 15% annually), increasing renewable energy penetration, and growing recognition of flow batteries' advantages for long-duration applications. The total cost of ownership (TCO) analysis increasingly favors flow batteries for applications requiring 6+ hours of storage duration, particularly when factoring in their 20+ year operational lifespan compared to 7-10 years for lithium-ion alternatives.
Market challenges persist, including high upfront capital costs, limited manufacturing scale, and competition from rapidly improving alternative technologies. The electrolyte rebalancing issue specifically impacts market adoption by increasing maintenance requirements and operational complexity, potentially raising the levelized cost of storage by 8-12% compared to systems without such challenges.
Customer segments show varying priorities: utilities focus on reliability and duration, commercial users prioritize total cost of ownership, while remote applications value operational simplicity and minimal maintenance requirements. This market segmentation necessitates tailored electrolyte rebalancing solutions to address specific customer pain points across different application scenarios.
Flow batteries have carved a distinct niche in the energy storage ecosystem, particularly for grid-scale applications requiring 4+ hours of discharge duration. Unlike lithium-ion batteries that dominate the short-duration storage market, flow batteries excel in applications requiring 6-12 hour discharge periods, with minimal capacity degradation even after thousands of cycles.
The utility sector represents the largest market segment, accounting for approximately 65% of flow battery deployments. These installations primarily serve grid stabilization, peak shaving, and renewable integration functions. Commercial and industrial applications constitute roughly 25% of the market, while remote microgrids and specialized applications make up the remaining 10%.
Geographically, North America leads the market with approximately 40% share, followed by Asia-Pacific at 35% and Europe at 20%. China, Japan, and Australia are showing particularly strong growth rates in the Asia-Pacific region, driven by aggressive renewable energy targets and supportive government policies.
Key market drivers include declining system costs (decreasing at approximately 15% annually), increasing renewable energy penetration, and growing recognition of flow batteries' advantages for long-duration applications. The total cost of ownership (TCO) analysis increasingly favors flow batteries for applications requiring 6+ hours of storage duration, particularly when factoring in their 20+ year operational lifespan compared to 7-10 years for lithium-ion alternatives.
Market challenges persist, including high upfront capital costs, limited manufacturing scale, and competition from rapidly improving alternative technologies. The electrolyte rebalancing issue specifically impacts market adoption by increasing maintenance requirements and operational complexity, potentially raising the levelized cost of storage by 8-12% compared to systems without such challenges.
Customer segments show varying priorities: utilities focus on reliability and duration, commercial users prioritize total cost of ownership, while remote applications value operational simplicity and minimal maintenance requirements. This market segmentation necessitates tailored electrolyte rebalancing solutions to address specific customer pain points across different application scenarios.
Current Challenges in Electrolyte Management Technologies
Flow battery systems face significant challenges in electrolyte management, particularly in maintaining balanced electrolyte composition over extended operational periods. The primary issue stems from crossover phenomena, where active species migrate through the membrane separating the positive and negative electrolytes. This migration leads to capacity fade and efficiency losses, ultimately compromising the economic viability of these energy storage systems.
Membrane permeability presents a fundamental challenge, as no membrane material currently available offers perfect selectivity. Even state-of-the-art ion exchange membranes exhibit some degree of permeability to active species, resulting in gradual electrolyte imbalance. This issue is particularly pronounced in vanadium redox flow batteries (VRFBs), where vanadium ions of different oxidation states can traverse the membrane.
Temperature management compounds these challenges, as membrane permeability characteristics change significantly with temperature fluctuations. Systems operating in variable ambient conditions experience inconsistent crossover rates, making predictive management strategies difficult to implement. Current thermal management technologies often fail to maintain optimal temperature ranges without significant parasitic energy consumption.
Chemical degradation of electrolytes represents another critical challenge. Side reactions, particularly in the presence of oxygen, can alter the oxidation states of active species and introduce impurities that catalyze further unwanted reactions. Current purification technologies struggle to effectively remove these contaminants without disrupting system operation or incurring prohibitive costs.
Monitoring technologies for real-time electrolyte composition analysis remain inadequate. Existing sensors lack the precision, reliability, and longevity required for continuous operation in the harsh chemical environment of flow batteries. Without accurate compositional data, implementing effective rebalancing strategies becomes largely guesswork, leading to suboptimal system performance.
Hydraulic imbalances further complicate electrolyte management. Uneven pressure distributions across the cell stack can exacerbate crossover issues and create localized concentration gradients. Current flow field designs and pumping systems often fail to maintain uniform electrolyte distribution, particularly as systems scale up to commercial sizes.
Economic constraints limit the implementation of advanced rebalancing technologies. Many promising approaches require expensive materials, precise manufacturing, or energy-intensive processes that undermine the cost competitiveness of flow battery systems. The industry currently lacks cost-effective solutions that can be deployed at scale without significantly impacting system economics.
Integration challenges exist between rebalancing subsystems and the main flow battery architecture. Current designs often treat rebalancing as an afterthought rather than an integral component of the system, resulting in inefficient operation and maintenance difficulties. Standardized interfaces and control protocols for rebalancing subsystems remain underdeveloped.
Membrane permeability presents a fundamental challenge, as no membrane material currently available offers perfect selectivity. Even state-of-the-art ion exchange membranes exhibit some degree of permeability to active species, resulting in gradual electrolyte imbalance. This issue is particularly pronounced in vanadium redox flow batteries (VRFBs), where vanadium ions of different oxidation states can traverse the membrane.
Temperature management compounds these challenges, as membrane permeability characteristics change significantly with temperature fluctuations. Systems operating in variable ambient conditions experience inconsistent crossover rates, making predictive management strategies difficult to implement. Current thermal management technologies often fail to maintain optimal temperature ranges without significant parasitic energy consumption.
Chemical degradation of electrolytes represents another critical challenge. Side reactions, particularly in the presence of oxygen, can alter the oxidation states of active species and introduce impurities that catalyze further unwanted reactions. Current purification technologies struggle to effectively remove these contaminants without disrupting system operation or incurring prohibitive costs.
Monitoring technologies for real-time electrolyte composition analysis remain inadequate. Existing sensors lack the precision, reliability, and longevity required for continuous operation in the harsh chemical environment of flow batteries. Without accurate compositional data, implementing effective rebalancing strategies becomes largely guesswork, leading to suboptimal system performance.
Hydraulic imbalances further complicate electrolyte management. Uneven pressure distributions across the cell stack can exacerbate crossover issues and create localized concentration gradients. Current flow field designs and pumping systems often fail to maintain uniform electrolyte distribution, particularly as systems scale up to commercial sizes.
Economic constraints limit the implementation of advanced rebalancing technologies. Many promising approaches require expensive materials, precise manufacturing, or energy-intensive processes that undermine the cost competitiveness of flow battery systems. The industry currently lacks cost-effective solutions that can be deployed at scale without significantly impacting system economics.
Integration challenges exist between rebalancing subsystems and the main flow battery architecture. Current designs often treat rebalancing as an afterthought rather than an integral component of the system, resulting in inefficient operation and maintenance difficulties. Standardized interfaces and control protocols for rebalancing subsystems remain underdeveloped.
Contemporary Electrolyte Rebalancing Solutions
01 Chemical rebalancing methods for flow battery electrolytes
Various chemical methods can be employed to rebalance electrolytes in flow battery systems. These include precipitation reactions, oxidation-reduction processes, and the use of specific chemical additives to restore the proper ion concentration ratios. Chemical rebalancing helps maintain the electrochemical performance of the battery by addressing issues like crossover and side reactions that cause electrolyte imbalance over time.- Chemical rebalancing methods for flow battery electrolytes: Various chemical methods can be employed to rebalance electrolytes in flow battery systems. These include precipitation reactions, oxidation-reduction processes, and the use of specific chemical additives that selectively react with imbalanced species. These methods help restore the optimal ratio of active species in the electrolyte, thereby maintaining battery performance and extending operational life.
- Electrochemical rebalancing techniques: Electrochemical approaches for electrolyte rebalancing involve the use of auxiliary electrochemical cells or specialized electrodes to convert species from one oxidation state to another. These techniques can be integrated into the flow battery system to provide continuous or periodic rebalancing without requiring additional chemicals or significant system downtime, offering an efficient solution for maintaining electrolyte balance.
- Membrane-based separation for electrolyte rebalancing: Selective membranes and separation technologies can be utilized to rebalance flow battery electrolytes by allowing specific ions to pass while blocking others. These systems may employ ion-exchange membranes, nanofiltration, or other selective barriers to separate and recombine electrolyte components, effectively addressing imbalances that occur during battery operation.
- Automated monitoring and control systems for electrolyte management: Advanced monitoring and control systems can be implemented to continuously track electrolyte composition and automatically trigger rebalancing processes when needed. These systems typically include sensors for measuring key parameters such as ion concentration, pH, and redox potential, coupled with control algorithms that determine when and how to initiate rebalancing procedures, optimizing battery performance and reducing maintenance requirements.
- Hybrid rebalancing approaches and system integration: Hybrid approaches combine multiple rebalancing methods to address different aspects of electrolyte imbalance more effectively. These integrated solutions may incorporate chemical, electrochemical, and membrane-based techniques within a single system architecture. Such comprehensive approaches often include specialized subsystems designed specifically for electrolyte management, offering more robust and efficient rebalancing capabilities for flow battery installations.
02 Electrochemical rebalancing techniques
Electrochemical approaches to electrolyte rebalancing involve the use of additional electrochemical cells or specialized electrodes to selectively oxidize or reduce specific electrolyte species. These techniques can be integrated into the flow battery system to provide continuous or periodic rebalancing without requiring external chemical additions. Electrochemical rebalancing offers precise control over the state of charge and composition of the electrolyte solutions.Expand Specific Solutions03 Membrane and separator technologies for preventing imbalance
Advanced membrane and separator technologies can be implemented to minimize electrolyte imbalance in flow battery systems. These materials are designed to reduce crossover of active species between the positive and negative electrolytes while maintaining high ionic conductivity. Specialized ion-selective membranes can preferentially allow certain ions to pass while blocking others, thereby reducing the rate at which electrolyte imbalance occurs.Expand Specific Solutions04 Automated monitoring and control systems for electrolyte management
Sophisticated monitoring and control systems can be integrated into flow battery installations to continuously track electrolyte composition and automatically implement rebalancing procedures when needed. These systems typically include sensors for measuring parameters such as pH, conductivity, and redox potential, along with control algorithms that determine when and how to adjust the electrolyte composition to maintain optimal performance.Expand Specific Solutions05 Hydraulic rebalancing and electrolyte circulation strategies
Hydraulic approaches to electrolyte rebalancing involve the strategic management of electrolyte flow rates, mixing, and circulation patterns within the battery system. These methods can include controlled mixing of electrolyte streams, selective bypassing of certain cell stacks, or the implementation of specialized hydraulic circuits designed to maintain balanced electrolyte compositions. Proper hydraulic design can significantly reduce the frequency and extent of chemical or electrochemical rebalancing required.Expand Specific Solutions
Leading Companies and Research Institutions in Flow Battery Sector
The flow battery electrolyte rebalancing technology market is currently in a growth phase, with increasing adoption driven by renewable energy integration demands. The global market is projected to reach significant scale as grid-scale energy storage becomes critical infrastructure. Technologically, the field shows varying maturity levels across different approaches. Leading players include established corporations like Lockheed Martin Advanced Energy Storage and LG Chem developing proprietary systems, alongside specialized firms such as Primus Power, Imergy Power Systems, and EnerVault focusing exclusively on flow battery innovations. Academic institutions including Dalian Institute of Chemical Physics, Zhejiang University, and Case Western Reserve University are advancing fundamental research, while Chinese companies like Dalian Rongke Power have achieved commercial deployment at utility scale. The competitive landscape features both Western technology pioneers and rapidly advancing Chinese manufacturers.
EnerVault Corp.
Technical Solution: EnerVault has developed a proprietary electrolyte rebalancing system for iron-chromium flow batteries that addresses the unique challenges of this chemistry. Their approach focuses on managing the cross-contamination of electrolytes that occurs due to ion migration through the membrane. EnerVault's system employs a dedicated rebalancing circuit that operates in parallel with the main battery stack. This circuit incorporates specialized electrochemical cells with catalytically enhanced electrodes that selectively oxidize or reduce specific ions to restore proper electrolyte composition. Their technology also includes an innovative "electrolyte conditioning" subsystem that removes precipitates and impurities through a combination of filtration and electrochemical treatment. EnerVault's control system continuously monitors electrolyte properties including redox potential, pH, and metal ion concentrations, automatically adjusting the rebalancing process as needed. This integrated approach has enabled their iron-chromium flow batteries to achieve significantly longer cycle life than previous implementations of this chemistry, with demonstrated stable operation over thousands of cycles in field deployments.
Strengths: Their rebalancing technology is specifically optimized for iron-chromium chemistry, which offers cost advantages over vanadium-based systems. The parallel rebalancing circuit allows for continuous operation without interrupting the main battery function. Their approach effectively addresses the hydrogen evolution side reaction that has historically limited iron-chromium battery performance. Weaknesses: Iron-chromium chemistry has inherently lower energy density compared to some alternative flow battery chemistries. The system requires precise control of operating conditions to prevent chromium precipitation.
Largo Clean Energy Corp.
Technical Solution: Largo Clean Energy has developed an advanced electrolyte rebalancing system for their VCHARGE± vanadium redox flow battery technology. Their approach centers on a comprehensive electrolyte management strategy that addresses the primary causes of imbalance in vanadium flow batteries. The system incorporates a proprietary "dynamic rebalancing module" that continuously monitors electrolyte state of charge and vanadium ion distribution across half-cells. When imbalance is detected, their patented electrochemical rebalancing cells activate to selectively transfer ions between electrolyte circuits. This is complemented by an innovative hydraulic management system that periodically performs controlled mixing of electrolyte fractions to prevent stratification and localized imbalances. Largo's technology also includes advanced temperature management to prevent precipitation of vanadium compounds, which can exacerbate imbalance issues. Their electrolyte formulation incorporates stabilizing additives that extend the temperature operating window and reduce the frequency of required rebalancing operations. The effectiveness of this approach has been demonstrated in Largo's commercial VCHARGE± systems, which have shown stable performance over extended cycling with minimal capacity degradation.
Strengths: Their integrated approach addresses multiple causes of electrolyte imbalance simultaneously. The system operates autonomously with minimal operator intervention required. Largo leverages their position as a primary vanadium producer to optimize electrolyte formulations. Weaknesses: The comprehensive rebalancing system adds complexity and cost to the overall battery system. The technology is specifically designed for vanadium chemistry and cannot be easily adapted to other flow battery chemistries.
Critical Patents and Research on Electrolyte Management
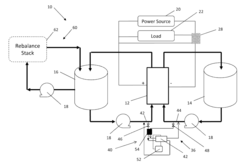
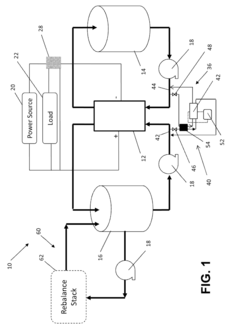
Method and system for rebalancing electrolytes in a redox flow battery system
PatentActiveUS12132241B2
Innovation
- A method involving directing hydrogen gas generated on the negative electrode to a catalyst surface where it reacts with the positive electrolyte, containing a metal ion, to balance the electrolyte state of charge and pH, using a catalyst such as graphite or precious metal-based catalysts to facilitate the reaction.
Systems and Methods for Rebalancing Redox Flow Battery Electrolytes
PatentInactiveUS20150147609A1
Innovation
- A system and method for rebalancing redox flow battery electrolytes using an electrochemical reaction cell with a catalyst-coated separator membrane, controlled hydrogen gas flow, and electric current application to adjust the charge balance between positive and negative electrolytes, minimizing contamination and energy consumption.
Environmental Impact and Sustainability of Electrolyte Systems
The environmental impact of electrolyte systems in flow batteries represents a critical consideration for sustainable energy storage development. Traditional electrolyte solutions often contain heavy metals and toxic compounds that pose significant environmental risks throughout their lifecycle. Vanadium-based systems, while offering excellent electrochemical performance, raise concerns regarding resource scarcity and extraction impacts. Mining operations for vanadium typically generate substantial carbon emissions and can lead to habitat destruction and water pollution in surrounding ecosystems.
Electrolyte leakage presents another environmental challenge, potentially contaminating soil and groundwater systems if containment measures fail. The energy-intensive production processes for electrolytes further contribute to the carbon footprint of flow battery manufacturing, with some estimates suggesting that electrolyte production accounts for up to 40% of the total embodied energy in certain flow battery systems.
Recent advancements in electrolyte rebalancing strategies have shown promising environmental benefits. Closed-loop rebalancing systems significantly reduce waste generation by extending electrolyte lifespan through continuous purification and regeneration. These systems can decrease the need for electrolyte replacement by up to 70%, substantially reducing the environmental burden associated with raw material extraction and processing.
Organic electrolytes derived from sustainable sources represent an emerging alternative to metal-based solutions. These biodegradable compounds demonstrate lower toxicity profiles and reduced environmental persistence compared to conventional options. Research indicates that quinone-based organic electrolytes can achieve comparable performance metrics while presenting minimal ecological hazards during production, operation, and end-of-life disposal.
Life cycle assessment (LCA) studies reveal that effective electrolyte rebalancing strategies can reduce the overall environmental impact of flow battery systems by 30-50% compared to systems without rebalancing capabilities. The extended operational lifetime achieved through proper electrolyte maintenance translates directly into improved sustainability metrics across carbon emissions, resource utilization, and waste generation parameters.
Water consumption represents another significant environmental consideration, particularly in regions facing water scarcity. Advanced electrolyte systems incorporating water recovery mechanisms can reduce freshwater requirements by up to 80% compared to conventional designs. This improvement addresses a critical sustainability challenge for large-scale deployment in water-stressed environments.
End-of-life management strategies for electrolyte systems have evolved substantially, with recycling technologies now capable of recovering over 95% of valuable components from spent electrolytes. These circular economy approaches minimize waste generation while reducing dependence on virgin material extraction, creating a more sustainable lifecycle for flow battery technology deployment.
Electrolyte leakage presents another environmental challenge, potentially contaminating soil and groundwater systems if containment measures fail. The energy-intensive production processes for electrolytes further contribute to the carbon footprint of flow battery manufacturing, with some estimates suggesting that electrolyte production accounts for up to 40% of the total embodied energy in certain flow battery systems.
Recent advancements in electrolyte rebalancing strategies have shown promising environmental benefits. Closed-loop rebalancing systems significantly reduce waste generation by extending electrolyte lifespan through continuous purification and regeneration. These systems can decrease the need for electrolyte replacement by up to 70%, substantially reducing the environmental burden associated with raw material extraction and processing.
Organic electrolytes derived from sustainable sources represent an emerging alternative to metal-based solutions. These biodegradable compounds demonstrate lower toxicity profiles and reduced environmental persistence compared to conventional options. Research indicates that quinone-based organic electrolytes can achieve comparable performance metrics while presenting minimal ecological hazards during production, operation, and end-of-life disposal.
Life cycle assessment (LCA) studies reveal that effective electrolyte rebalancing strategies can reduce the overall environmental impact of flow battery systems by 30-50% compared to systems without rebalancing capabilities. The extended operational lifetime achieved through proper electrolyte maintenance translates directly into improved sustainability metrics across carbon emissions, resource utilization, and waste generation parameters.
Water consumption represents another significant environmental consideration, particularly in regions facing water scarcity. Advanced electrolyte systems incorporating water recovery mechanisms can reduce freshwater requirements by up to 80% compared to conventional designs. This improvement addresses a critical sustainability challenge for large-scale deployment in water-stressed environments.
End-of-life management strategies for electrolyte systems have evolved substantially, with recycling technologies now capable of recovering over 95% of valuable components from spent electrolytes. These circular economy approaches minimize waste generation while reducing dependence on virgin material extraction, creating a more sustainable lifecycle for flow battery technology deployment.
Cost-Benefit Analysis of Rebalancing Strategies
The economic viability of electrolyte rebalancing strategies in flow battery systems requires comprehensive cost-benefit analysis to guide implementation decisions. Initial capital expenditure for rebalancing equipment varies significantly across methods, with chemical precipitation systems typically requiring investments of $50,000-100,000, while electrochemical methods may demand $75,000-150,000 depending on scale and sophistication. Membrane-based systems generally fall between $60,000-120,000, with costs heavily influenced by membrane quality and surface area requirements.
Operational expenses present another critical dimension, encompassing energy consumption, chemical reagents, maintenance, and labor costs. Electrochemical rebalancing methods consume approximately 3-5% of the battery's total energy output, representing a significant efficiency penalty. Chemical precipitation approaches require ongoing reagent costs of $5,000-15,000 annually for utility-scale installations, while membrane systems necessitate periodic replacement at intervals of 2-5 years, costing $10,000-30,000 per replacement cycle.
Performance benefits must be quantified against these costs. Effective rebalancing strategies typically extend battery cycle life by 30-50%, potentially translating to 3-7 additional years of operational lifespan. System efficiency improvements of 5-15% directly impact energy throughput economics, while capacity retention enhancements of 10-20% maintain revenue-generating capabilities over extended periods.
Return on investment calculations reveal varying payback periods across strategies. Chemical precipitation methods generally achieve payback within 2-3 years for large-scale systems, while electrochemical approaches may require 3-4 years despite higher initial costs. Membrane-based solutions typically fall between these ranges at 2.5-3.5 years, with performance highly dependent on specific implementation parameters.
Sensitivity analysis indicates that scale significantly impacts economic viability, with larger systems (>1MW/4MWh) achieving substantially better economics than smaller installations. Electricity pricing structures also dramatically influence returns, with high peak/off-peak differentials favoring more sophisticated rebalancing strategies despite higher implementation costs.
Long-term value proposition assessment reveals that rebalancing strategies deliver 15-25% improvement in levelized cost of storage (LCOS) over battery lifetime, with the most significant gains occurring in applications requiring deep cycling and frequent operation. This translates to potential lifetime savings of $200,000-500,000 for MW-scale systems, making rebalancing an essential consideration for flow battery deployment economics rather than an optional enhancement.
Operational expenses present another critical dimension, encompassing energy consumption, chemical reagents, maintenance, and labor costs. Electrochemical rebalancing methods consume approximately 3-5% of the battery's total energy output, representing a significant efficiency penalty. Chemical precipitation approaches require ongoing reagent costs of $5,000-15,000 annually for utility-scale installations, while membrane systems necessitate periodic replacement at intervals of 2-5 years, costing $10,000-30,000 per replacement cycle.
Performance benefits must be quantified against these costs. Effective rebalancing strategies typically extend battery cycle life by 30-50%, potentially translating to 3-7 additional years of operational lifespan. System efficiency improvements of 5-15% directly impact energy throughput economics, while capacity retention enhancements of 10-20% maintain revenue-generating capabilities over extended periods.
Return on investment calculations reveal varying payback periods across strategies. Chemical precipitation methods generally achieve payback within 2-3 years for large-scale systems, while electrochemical approaches may require 3-4 years despite higher initial costs. Membrane-based solutions typically fall between these ranges at 2.5-3.5 years, with performance highly dependent on specific implementation parameters.
Sensitivity analysis indicates that scale significantly impacts economic viability, with larger systems (>1MW/4MWh) achieving substantially better economics than smaller installations. Electricity pricing structures also dramatically influence returns, with high peak/off-peak differentials favoring more sophisticated rebalancing strategies despite higher implementation costs.
Long-term value proposition assessment reveals that rebalancing strategies deliver 15-25% improvement in levelized cost of storage (LCOS) over battery lifetime, with the most significant gains occurring in applications requiring deep cycling and frequent operation. This translates to potential lifetime savings of $200,000-500,000 for MW-scale systems, making rebalancing an essential consideration for flow battery deployment economics rather than an optional enhancement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!