Vanadium Alternatives in Redox Flow Electrolyte Development
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vanadium-Free Electrolyte Development Background and Objectives
Redox flow batteries (RFBs) have emerged as promising large-scale energy storage solutions due to their unique ability to decouple power and energy capacity, long cycle life, and rapid response times. Since their inception in the 1970s, vanadium-based systems have dominated the commercial RFB landscape, with the all-vanadium redox flow battery (VRFB) becoming the industry standard following breakthrough developments in the 1980s by researchers at the University of New South Wales.
The evolution of RFB technology has been primarily driven by the need for grid-scale energy storage solutions to complement the growing deployment of intermittent renewable energy sources. VRFBs have demonstrated excellent electrochemical reversibility, relatively high energy density, and exceptional cycle stability exceeding 20,000 cycles. However, the widespread adoption of this technology faces significant barriers related to the use of vanadium as the primary electroactive species.
Vanadium's limited global reserves, concentrated production in a few countries (primarily China, Russia, and South Africa), and price volatility present substantial challenges to the scalability and economic viability of VRFBs. Historical vanadium price fluctuations have ranged from $10-80/kg, creating uncertainty for long-term project planning. Additionally, the environmental impact of vanadium mining and processing raises sustainability concerns for a technology intended to support clean energy transitions.
These limitations have catalyzed intensive research into alternative electrolyte chemistries that maintain the advantages of flow batteries while addressing the economic and supply chain vulnerabilities associated with vanadium. The primary technical objectives for vanadium-free electrolyte development include: achieving comparable or superior energy density (>25 Wh/L), maintaining long cycle life (>10,000 cycles), ensuring chemical stability, reducing system costs below $150/kWh, and utilizing abundant, environmentally benign materials.
Recent technological trends indicate growing interest in organic compounds, earth-abundant metals (iron, zinc, manganese), and hybrid systems that combine multiple redox couples. These alternatives aim to leverage more sustainable and cost-effective materials while potentially offering improved performance characteristics such as wider operating temperature ranges and higher cell voltages.
The development of vanadium-free electrolytes represents a critical pathway toward realizing the full potential of flow battery technology for grid-scale energy storage applications. Success in this domain could significantly accelerate the integration of renewable energy sources into electrical grids worldwide, supporting the transition to a more sustainable and resilient energy infrastructure.
The evolution of RFB technology has been primarily driven by the need for grid-scale energy storage solutions to complement the growing deployment of intermittent renewable energy sources. VRFBs have demonstrated excellent electrochemical reversibility, relatively high energy density, and exceptional cycle stability exceeding 20,000 cycles. However, the widespread adoption of this technology faces significant barriers related to the use of vanadium as the primary electroactive species.
Vanadium's limited global reserves, concentrated production in a few countries (primarily China, Russia, and South Africa), and price volatility present substantial challenges to the scalability and economic viability of VRFBs. Historical vanadium price fluctuations have ranged from $10-80/kg, creating uncertainty for long-term project planning. Additionally, the environmental impact of vanadium mining and processing raises sustainability concerns for a technology intended to support clean energy transitions.
These limitations have catalyzed intensive research into alternative electrolyte chemistries that maintain the advantages of flow batteries while addressing the economic and supply chain vulnerabilities associated with vanadium. The primary technical objectives for vanadium-free electrolyte development include: achieving comparable or superior energy density (>25 Wh/L), maintaining long cycle life (>10,000 cycles), ensuring chemical stability, reducing system costs below $150/kWh, and utilizing abundant, environmentally benign materials.
Recent technological trends indicate growing interest in organic compounds, earth-abundant metals (iron, zinc, manganese), and hybrid systems that combine multiple redox couples. These alternatives aim to leverage more sustainable and cost-effective materials while potentially offering improved performance characteristics such as wider operating temperature ranges and higher cell voltages.
The development of vanadium-free electrolytes represents a critical pathway toward realizing the full potential of flow battery technology for grid-scale energy storage applications. Success in this domain could significantly accelerate the integration of renewable energy sources into electrical grids worldwide, supporting the transition to a more sustainable and resilient energy infrastructure.
Market Analysis for Alternative Redox Flow Battery Electrolytes
The global market for redox flow batteries (RFBs) is experiencing significant growth, driven by increasing demand for long-duration energy storage solutions to support renewable energy integration. Currently valued at approximately $290 million in 2023, the market is projected to reach $1.1 billion by 2030, representing a compound annual growth rate (CAGR) of 20.8%. This growth trajectory is particularly notable in regions with aggressive renewable energy targets, including Europe, North America, and parts of Asia-Pacific.
Alternative electrolytes to vanadium are gaining market attention due to several economic and technical factors. Vanadium-based systems, while technically mature, face supply chain vulnerabilities with over 73% of global vanadium production concentrated in China and Russia. Price volatility has been substantial, with vanadium pentoxide prices fluctuating between $5-16 per pound over the past five years, creating significant cost uncertainties for manufacturers and end-users.
The market for alternative electrolytes is segmented by chemistry type, with organic compounds, iron-based solutions, and zinc-bromine systems emerging as the most commercially promising alternatives. Organic electrolytes, particularly quinone-based systems, are attracting substantial investment with funding exceeding $150 million in the past three years. These alternatives offer potential cost reductions of 30-50% compared to vanadium systems.
End-user demand is primarily driven by utility-scale applications, which account for 68% of the current market. Commercial and industrial applications represent 24%, with the remaining 8% distributed across microgrids and specialized applications. Geographically, China leads adoption with 42% market share, followed by North America (27%), Europe (21%), and other regions (10%).
Key market drivers include declining renewable energy costs, with solar PV and wind energy now cheaper than fossil fuel alternatives in many markets. This has created increased demand for storage solutions that can address intermittency issues. Additionally, policy support through initiatives like the U.S. Inflation Reduction Act, which provides investment tax credits for energy storage, and the EU's Green Deal are accelerating market growth.
Market barriers include technology readiness levels, with most alternative electrolytes at TRL 5-7 compared to vanadium's TRL 9. Scaling challenges and limited field demonstration data create adoption hesitancy among conservative utility customers. Furthermore, the lack of standardized testing protocols for new electrolyte chemistries complicates performance comparisons and risk assessments.
Customer requirements are evolving toward longer duration storage (8+ hours), improved round-trip efficiency (target >80%), and reduced levelized cost of storage (target <$0.05/kWh-cycle). These market demands are creating specific opportunities for alternative electrolytes that can deliver on these metrics while avoiding vanadium's supply chain and cost challenges.
Alternative electrolytes to vanadium are gaining market attention due to several economic and technical factors. Vanadium-based systems, while technically mature, face supply chain vulnerabilities with over 73% of global vanadium production concentrated in China and Russia. Price volatility has been substantial, with vanadium pentoxide prices fluctuating between $5-16 per pound over the past five years, creating significant cost uncertainties for manufacturers and end-users.
The market for alternative electrolytes is segmented by chemistry type, with organic compounds, iron-based solutions, and zinc-bromine systems emerging as the most commercially promising alternatives. Organic electrolytes, particularly quinone-based systems, are attracting substantial investment with funding exceeding $150 million in the past three years. These alternatives offer potential cost reductions of 30-50% compared to vanadium systems.
End-user demand is primarily driven by utility-scale applications, which account for 68% of the current market. Commercial and industrial applications represent 24%, with the remaining 8% distributed across microgrids and specialized applications. Geographically, China leads adoption with 42% market share, followed by North America (27%), Europe (21%), and other regions (10%).
Key market drivers include declining renewable energy costs, with solar PV and wind energy now cheaper than fossil fuel alternatives in many markets. This has created increased demand for storage solutions that can address intermittency issues. Additionally, policy support through initiatives like the U.S. Inflation Reduction Act, which provides investment tax credits for energy storage, and the EU's Green Deal are accelerating market growth.
Market barriers include technology readiness levels, with most alternative electrolytes at TRL 5-7 compared to vanadium's TRL 9. Scaling challenges and limited field demonstration data create adoption hesitancy among conservative utility customers. Furthermore, the lack of standardized testing protocols for new electrolyte chemistries complicates performance comparisons and risk assessments.
Customer requirements are evolving toward longer duration storage (8+ hours), improved round-trip efficiency (target >80%), and reduced levelized cost of storage (target <$0.05/kWh-cycle). These market demands are creating specific opportunities for alternative electrolytes that can deliver on these metrics while avoiding vanadium's supply chain and cost challenges.
Current Challenges in Non-Vanadium Electrolyte Technologies
Despite the promising attributes of non-vanadium electrolytes for redox flow batteries (RFBs), several significant challenges impede their widespread commercial adoption. The primary obstacle remains the lower energy density compared to vanadium-based systems, with most alternatives struggling to exceed 25-30 Wh/L, whereas vanadium systems can achieve 35-40 Wh/L. This limitation directly impacts the economic viability of large-scale energy storage applications.
Stability issues present another critical challenge, as many non-vanadium electrolytes suffer from accelerated capacity fade during cycling. Iron-chromium systems, for instance, experience significant capacity degradation after 200-300 cycles, whereas commercial viability typically requires 10,000+ cycles. This degradation often stems from side reactions, crossover of active species through membranes, and precipitation of active materials.
Membrane compatibility poses a substantial hurdle for organic and organometallic electrolytes. These systems frequently exhibit higher crossover rates through conventional ion-exchange membranes, leading to irreversible capacity loss and reduced coulombic efficiency. While specialized membranes can mitigate these issues, they significantly increase system costs, offsetting the potential cost advantages of non-vanadium chemistries.
Cost-performance balance remains elusive for many alternatives. While materials like iron, zinc, and organic compounds offer lower raw material costs than vanadium, the additional system components required to address their inherent limitations often negate these savings. For example, zinc-bromine systems require complex flow management to prevent zinc dendrite formation, adding complexity and cost.
Scale-up challenges persist across most non-vanadium technologies. Laboratory-scale demonstrations often fail to translate successfully to industrial-scale implementations due to unforeseen chemical and engineering complications. Issues such as shunt currents, flow distribution, and thermal management become increasingly problematic at larger scales.
Environmental and safety concerns also limit certain promising chemistries. Bromine-based systems require sophisticated containment strategies due to bromine's toxicity and volatility. Similarly, some organic electrolytes utilize environmentally persistent compounds or require toxic solvents for dissolution, raising end-of-life disposal concerns.
Standardization and regulatory frameworks lag behind technological development. Unlike the relatively mature vanadium technology, alternative chemistries lack established performance metrics, safety standards, and regulatory guidelines, creating market entry barriers and increasing investment risk for commercial deployment.
Stability issues present another critical challenge, as many non-vanadium electrolytes suffer from accelerated capacity fade during cycling. Iron-chromium systems, for instance, experience significant capacity degradation after 200-300 cycles, whereas commercial viability typically requires 10,000+ cycles. This degradation often stems from side reactions, crossover of active species through membranes, and precipitation of active materials.
Membrane compatibility poses a substantial hurdle for organic and organometallic electrolytes. These systems frequently exhibit higher crossover rates through conventional ion-exchange membranes, leading to irreversible capacity loss and reduced coulombic efficiency. While specialized membranes can mitigate these issues, they significantly increase system costs, offsetting the potential cost advantages of non-vanadium chemistries.
Cost-performance balance remains elusive for many alternatives. While materials like iron, zinc, and organic compounds offer lower raw material costs than vanadium, the additional system components required to address their inherent limitations often negate these savings. For example, zinc-bromine systems require complex flow management to prevent zinc dendrite formation, adding complexity and cost.
Scale-up challenges persist across most non-vanadium technologies. Laboratory-scale demonstrations often fail to translate successfully to industrial-scale implementations due to unforeseen chemical and engineering complications. Issues such as shunt currents, flow distribution, and thermal management become increasingly problematic at larger scales.
Environmental and safety concerns also limit certain promising chemistries. Bromine-based systems require sophisticated containment strategies due to bromine's toxicity and volatility. Similarly, some organic electrolytes utilize environmentally persistent compounds or require toxic solvents for dissolution, raising end-of-life disposal concerns.
Standardization and regulatory frameworks lag behind technological development. Unlike the relatively mature vanadium technology, alternative chemistries lack established performance metrics, safety standards, and regulatory guidelines, creating market entry barriers and increasing investment risk for commercial deployment.
Current Non-Vanadium Electrolyte Solutions and Approaches
01 Iron-based alternatives to vanadium electrolytes
Iron-based compounds can serve as effective alternatives to vanadium in redox flow batteries. These electrolytes typically utilize iron in different oxidation states (Fe²⁺/Fe³⁺) to store and release energy. Iron-based systems offer advantages including lower cost, reduced toxicity, and abundant material availability compared to vanadium. These systems can be formulated with various supporting electrolytes to enhance stability and electrochemical performance.- Iron-based alternatives to vanadium electrolytes: Iron-based compounds can serve as effective alternatives to vanadium in redox flow batteries. These electrolytes typically utilize iron in different oxidation states (Fe²⁺/Fe³⁺) to store and release energy. Iron-based systems offer advantages including lower cost, reduced toxicity, and abundant material availability compared to vanadium. These systems can be formulated with various supporting electrolytes to enhance stability and electrochemical performance.
- Organic redox active materials as vanadium alternatives: Organic compounds can be used as redox active materials in flow batteries, providing alternatives to metal-based systems. These organic electrolytes utilize reversible redox reactions of organic molecules to store and release energy. Benefits include tunability of molecular structures, potentially lower environmental impact, and the possibility of using sustainable precursors. Various organic compounds including quinones, viologens, and TEMPO derivatives have shown promise as redox active materials.
- Metal complexes and coordination compounds: Metal complexes and coordination compounds offer another approach to replacing vanadium in redox flow batteries. These systems typically involve transition metals coordinated with specific ligands to achieve desired electrochemical properties. The coordination environment can be engineered to tune redox potentials, solubility, and stability. These systems can provide higher energy density and wider operating temperature ranges compared to traditional vanadium systems.
- Zinc-based redox flow electrolytes: Zinc-based systems represent a promising alternative to vanadium redox flow batteries. These systems typically utilize zinc metal deposition/dissolution at one electrode coupled with another redox couple. Zinc-based electrolytes offer advantages including high energy density, relatively low cost, and good electrochemical reversibility. Various formulations have been developed to address challenges such as dendrite formation and limited cycle life.
- Hybrid and multi-element electrolyte systems: Hybrid and multi-element electrolyte systems combine different redox active species to leverage the advantages of each component. These systems may incorporate combinations of different metals, organic compounds, or both to achieve improved performance characteristics. By carefully selecting complementary redox couples, these hybrid systems can achieve higher voltage, improved stability, or enhanced power density compared to single-element systems. These approaches often focus on optimizing the electrolyte composition to balance cost, performance, and durability.
02 Organic compound electrolytes for redox flow batteries
Organic compounds can be used as active materials in redox flow battery electrolytes, offering alternatives to metal-based systems like vanadium. These organic electrolytes utilize redox-active organic molecules that can undergo reversible oxidation and reduction. Benefits include tunable properties through molecular design, potentially lower environmental impact, and the possibility of using sustainable or bio-derived precursors. Various organic structures including quinones, viologens, and TEMPO derivatives have shown promise in flow battery applications.Expand Specific Solutions03 Metal complexes and coordination compounds as electrolytes
Metal complexes and coordination compounds can serve as effective redox-active species in flow battery electrolytes. These systems typically involve transition metals coordinated with ligands that stabilize different oxidation states and tune redox potentials. Examples include complexes of iron, chromium, manganese, or copper with various organic ligands. These electrolytes can offer advantages including high solubility, stable redox behavior, and tunable electrochemical properties through ligand modification.Expand Specific Solutions04 Zinc-based redox flow battery electrolytes
Zinc-based systems represent promising alternatives to vanadium in redox flow batteries. These electrolytes typically utilize zinc metal deposition/dissolution at one electrode coupled with another redox couple. Zinc-based systems offer advantages including high energy density, relatively low cost materials, and good electrochemical reversibility. Various formulations may include additives to prevent dendrite formation and improve cycling efficiency.Expand Specific Solutions05 Electrolyte additives and supporting electrolytes for non-vanadium systems
Various additives and supporting electrolytes can enhance the performance of non-vanadium redox flow battery systems. These components can improve electrolyte stability, conductivity, solubility of active species, and overall electrochemical performance. Examples include specific acids, buffers, chelating agents, and stabilizers that prevent side reactions or degradation. Proper selection of these supporting materials is crucial for achieving competitive performance in alternatives to vanadium-based systems.Expand Specific Solutions
Leading Companies and Research Institutions in Alternative Electrolytes
The vanadium alternatives in redox flow electrolyte market is in a growth phase, with increasing research activity addressing vanadium's cost and supply limitations. The global market is expanding rapidly, projected to reach significant scale as energy storage demands grow. Technologically, alternatives are advancing through various approaches: Resonac Holdings and Sumitomo Electric focus on material innovations; Dalian Bolong and LE System are developing cost-effective vanadium recovery methods; while research institutions like KIER, Fraunhofer, and Chinese Academy of Sciences are exploring novel electrolyte chemistries. Companies like Standard Energy and Imergy Power Systems are commercializing alternative systems, though technical challenges around energy density and stability remain before widespread adoption can occur.
Sumitomo Electric Industries Ltd.
Technical Solution: Sumitomo Electric Industries has developed a zinc-bromine flow battery technology as a viable alternative to vanadium-based systems. Their approach utilizes zinc metal deposition on the negative electrode and bromine/bromide reactions on the positive side, achieving energy densities of 65-75 Wh/L - approximately twice that of conventional vanadium systems. Sumitomo's proprietary technology addresses the historical challenges of zinc-bromine batteries through several innovations. They've developed specialized complexing agents that sequester bromine in the electrolyte, reducing its volatility and corrosivity while maintaining electrochemical accessibility. Their electrode design incorporates carbon-based materials with optimized surface treatments that enhance zinc deposition uniformity and prevent dendrite formation. A key advancement is their membrane technology, which effectively prevents bromine crossover while maintaining high ionic conductivity. Sumitomo has also engineered an integrated electrolyte management system that continuously monitors and adjusts electrolyte composition to maintain optimal performance throughout thousands of cycles. Their commercial systems have demonstrated round-trip efficiencies of 70-75% and operational lifetimes exceeding 3000 cycles with minimal capacity degradation.
Strengths: Higher energy density than vanadium systems at potentially lower cost; utilizes more abundant materials; compact system design reduces installation footprint. Weaknesses: Bromine presents safety and handling challenges despite complexing agents; zinc dendrite formation remains a concern for very long-term operation; system requires more complex management controls than vanadium batteries to maintain optimal performance.
Imergy Power Systems, Inc.
Technical Solution: Imergy Power Systems developed a proprietary vanadium alternative technology centered on iron-chromium (Fe-Cr) chemistry for redox flow batteries. Their approach utilized mixed-metal electrolytes where iron replaces the more expensive V(II)/V(III) couple and chromium substitutes for the V(IV)/V(V) couple. The company engineered a unique electrolyte formulation that addressed the historical challenges of Fe-Cr systems, particularly the slow kinetics of the chromium redox reaction. By incorporating specific catalysts and electrolyte additives, Imergy achieved reaction rates comparable to vanadium systems while maintaining stability across thousands of cycles. Their technology extracted metals from waste streams including mining tailings and fly ash, reducing raw material costs by approximately 40% compared to traditional vanadium sources. The system operated effectively across a wider temperature range (0-50°C) than conventional vanadium flow batteries without precipitation issues, eliminating the need for expensive thermal management systems in many deployment scenarios.
Strengths: Significantly lower material costs than vanadium; utilizes metals recoverable from waste streams; operates across wider temperature ranges without precipitation issues. Weaknesses: Lower energy density than vanadium systems (approximately 15-20 Wh/L vs. 25-35 Wh/L for vanadium); chromium presents environmental concerns if not properly managed; cross-contamination between electrolytes remains a technical challenge requiring specialized membranes.
Key Patents and Scientific Breakthroughs in Alternative Electrolytes
Electrolyte for oxidation-reduction secondary battery comprising vanadium ions, and secondary battery comprising same
PatentWO2025009917A1
Innovation
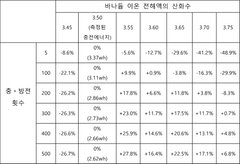
- An electrolyte solution with vanadium ions is developed, where the oxidation number of vanadium is adjusted to be between 3.50 and 4.00, specifically between 3.55 and 3.75, using vanadium oxide and an acidic solution, and an electrochemical method is employed to achieve the desired oxidation state, enhancing the energy density and stability of the battery.
Electrolyte for highly stable vanadium redox flow battery with improved vanadium solubility and Method of preparing the same
PatentActiveKR1020240105675A
Innovation
- A vanadium electrolyte solution is developed by combining vanadium ions with a complex polysaccharide, such as hyaluronic acid, in a supporting electrolyte to increase vanadium ion concentration, using catalyst acids like sulfuric acid to enhance solubility and energy density.
Supply Chain Considerations for Alternative Redox Flow Materials
The global supply chain for redox flow battery materials presents significant challenges and opportunities when considering alternatives to vanadium-based electrolytes. Vanadium's price volatility and geographical concentration of reserves have driven research into alternative materials that can offer more stable supply chains and reduced costs.
Iron-based alternatives represent one of the most promising options from a supply chain perspective. Iron is abundant globally, with major mining operations across all continents, reducing geopolitical supply risks. The annual global iron ore production exceeds 2.2 billion tons, making it approximately 100,000 times more abundant than vanadium. This abundance translates to significantly lower and more stable pricing, with iron compounds typically costing 1/10th to 1/20th of vanadium compounds per unit weight.
Organic redox materials offer another compelling supply chain advantage. These carbon-based compounds can be synthesized from widely available precursors, potentially enabling localized production facilities near battery manufacturing sites. This distributed manufacturing capability reduces dependency on specific mining regions and minimizes transportation costs and associated carbon footprint. However, the supply chain for high-purity organic precursors remains less developed than for metal-based alternatives.
Zinc-bromine systems benefit from established industrial supply chains for both zinc and bromine. Zinc is produced in over 50 countries, with major operations in China, Australia, and Peru. Bromine production, while more concentrated in the Dead Sea region, the United States, and China, still offers greater supply diversity than vanadium. The chemical industry's long-standing use of these elements has created robust logistics networks that flow battery manufacturers can leverage.
Manganese-based electrolytes present another viable alternative with favorable supply chain characteristics. Global manganese reserves are estimated at 1.3 billion tons, with significant production in South Africa, Australia, and Brazil. The established use of manganese in steel production and other industries has created mature extraction and processing infrastructure that could be adapted for battery-grade materials.
Critical considerations for alternative material supply chains include processing requirements, as some alternatives may require more complex or energy-intensive purification than vanadium. Additionally, recycling infrastructure development will be essential for long-term sustainability, as efficient recovery of electrolyte materials at end-of-life could significantly reduce supply pressures and environmental impacts. Regulatory frameworks governing the transportation and handling of alternative electrolytes must also be evaluated, as they can substantially impact logistics costs and operational flexibility.
Iron-based alternatives represent one of the most promising options from a supply chain perspective. Iron is abundant globally, with major mining operations across all continents, reducing geopolitical supply risks. The annual global iron ore production exceeds 2.2 billion tons, making it approximately 100,000 times more abundant than vanadium. This abundance translates to significantly lower and more stable pricing, with iron compounds typically costing 1/10th to 1/20th of vanadium compounds per unit weight.
Organic redox materials offer another compelling supply chain advantage. These carbon-based compounds can be synthesized from widely available precursors, potentially enabling localized production facilities near battery manufacturing sites. This distributed manufacturing capability reduces dependency on specific mining regions and minimizes transportation costs and associated carbon footprint. However, the supply chain for high-purity organic precursors remains less developed than for metal-based alternatives.
Zinc-bromine systems benefit from established industrial supply chains for both zinc and bromine. Zinc is produced in over 50 countries, with major operations in China, Australia, and Peru. Bromine production, while more concentrated in the Dead Sea region, the United States, and China, still offers greater supply diversity than vanadium. The chemical industry's long-standing use of these elements has created robust logistics networks that flow battery manufacturers can leverage.
Manganese-based electrolytes present another viable alternative with favorable supply chain characteristics. Global manganese reserves are estimated at 1.3 billion tons, with significant production in South Africa, Australia, and Brazil. The established use of manganese in steel production and other industries has created mature extraction and processing infrastructure that could be adapted for battery-grade materials.
Critical considerations for alternative material supply chains include processing requirements, as some alternatives may require more complex or energy-intensive purification than vanadium. Additionally, recycling infrastructure development will be essential for long-term sustainability, as efficient recovery of electrolyte materials at end-of-life could significantly reduce supply pressures and environmental impacts. Regulatory frameworks governing the transportation and handling of alternative electrolytes must also be evaluated, as they can substantially impact logistics costs and operational flexibility.
Environmental Impact Assessment of Next-Generation Electrolytes
The environmental impact of next-generation electrolytes for redox flow batteries represents a critical consideration in the transition away from vanadium-based systems. Traditional vanadium electrolytes pose significant environmental concerns due to their toxicity, resource scarcity, and energy-intensive extraction processes. Mining vanadium typically results in substantial land disturbance, habitat destruction, and potential water contamination through acid mine drainage and heavy metal leaching.
Next-generation alternatives such as organic compounds, iron-based solutions, and zinc-bromine systems generally demonstrate improved environmental profiles. Organic electrolytes derived from sustainable sources can significantly reduce the carbon footprint associated with battery production. Life cycle assessments indicate that organic quinone-based electrolytes may reduce greenhouse gas emissions by 30-45% compared to vanadium systems, primarily due to less energy-intensive synthesis pathways.
Iron-based alternatives leverage Earth-abundant materials with well-established recycling infrastructures, minimizing end-of-life environmental impacts. These systems typically require 60-70% less energy during material extraction phases compared to vanadium, translating to reduced carbon emissions throughout the supply chain. Additionally, iron compounds present substantially lower ecotoxicity profiles in aquatic environments, with studies demonstrating 5-10 times lower impact on freshwater ecosystems.
Water consumption represents another critical environmental metric. Advanced membrane technologies paired with next-generation electrolytes can reduce operational water requirements by up to 40% compared to conventional systems. This improvement becomes particularly significant in water-stressed regions where large-scale energy storage deployment is anticipated.
End-of-life management presents both challenges and opportunities. While vanadium systems benefit from established recycling protocols with recovery rates exceeding 90%, emerging electrolyte chemistries require development of specialized recycling processes. Preliminary research indicates that organic electrolytes may be biodegradable under specific conditions, potentially offering novel disposal pathways unavailable to metal-based systems.
Regulatory frameworks worldwide are increasingly incorporating environmental impact assessments into energy storage deployment strategies. The European Union's Battery Directive revision specifically addresses flow battery electrolytes, establishing thresholds for toxic material content and mandating recycling programs. Similar regulatory developments in North America and Asia will likely accelerate the transition toward environmentally superior electrolyte formulations in coming years.
Next-generation alternatives such as organic compounds, iron-based solutions, and zinc-bromine systems generally demonstrate improved environmental profiles. Organic electrolytes derived from sustainable sources can significantly reduce the carbon footprint associated with battery production. Life cycle assessments indicate that organic quinone-based electrolytes may reduce greenhouse gas emissions by 30-45% compared to vanadium systems, primarily due to less energy-intensive synthesis pathways.
Iron-based alternatives leverage Earth-abundant materials with well-established recycling infrastructures, minimizing end-of-life environmental impacts. These systems typically require 60-70% less energy during material extraction phases compared to vanadium, translating to reduced carbon emissions throughout the supply chain. Additionally, iron compounds present substantially lower ecotoxicity profiles in aquatic environments, with studies demonstrating 5-10 times lower impact on freshwater ecosystems.
Water consumption represents another critical environmental metric. Advanced membrane technologies paired with next-generation electrolytes can reduce operational water requirements by up to 40% compared to conventional systems. This improvement becomes particularly significant in water-stressed regions where large-scale energy storage deployment is anticipated.
End-of-life management presents both challenges and opportunities. While vanadium systems benefit from established recycling protocols with recovery rates exceeding 90%, emerging electrolyte chemistries require development of specialized recycling processes. Preliminary research indicates that organic electrolytes may be biodegradable under specific conditions, potentially offering novel disposal pathways unavailable to metal-based systems.
Regulatory frameworks worldwide are increasingly incorporating environmental impact assessments into energy storage deployment strategies. The European Union's Battery Directive revision specifically addresses flow battery electrolytes, establishing thresholds for toxic material content and mandating recycling programs. Similar regulatory developments in North America and Asia will likely accelerate the transition toward environmentally superior electrolyte formulations in coming years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!