Redox Flow Electrolytes pH Conductivity and Viscosity Effects
OCT 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Redox Flow Battery Electrolytes Background and Objectives
Redox flow batteries (RFBs) have emerged as a promising energy storage technology for grid-scale applications due to their unique ability to decouple power and energy capacity, long cycle life, and rapid response times. The electrolyte, serving as both the energy carrier and reaction medium, plays a pivotal role in determining the overall performance and efficiency of these systems. This technical research focuses on understanding how critical electrolyte properties—specifically pH, conductivity, and viscosity—impact RFB performance and longevity.
The evolution of RFB technology dates back to the 1970s when NASA first developed the concept. Since then, various chemistries have been explored, from the traditional all-vanadium systems to emerging organic and hybrid configurations. The technological trajectory has consistently aimed at improving energy density, reducing costs, and enhancing operational stability—goals that are directly influenced by electrolyte properties.
pH levels in RFB electrolytes significantly affect redox potentials, reaction kinetics, and material stability. Highly acidic environments, common in vanadium systems, enable higher solubility of active species but accelerate corrosion of cell components. Conversely, neutral or alkaline conditions may reduce corrosion but often limit solubility and voltage efficiency. Understanding these trade-offs is essential for optimizing battery performance across different chemistries.
Electrolyte conductivity directly impacts internal resistance and, consequently, the power density and energy efficiency of RFBs. Higher conductivity generally reduces ohmic losses but may require increased salt concentrations that affect viscosity and active species solubility. The interrelationship between conductivity and other electrolyte properties represents a complex optimization challenge that requires systematic investigation.
Viscosity affects pumping energy requirements, mass transport limitations, and overall system efficiency. Higher viscosity electrolytes demand more parasitic energy for circulation but may enable higher concentrations of active materials. This property becomes particularly critical in cold-weather operations and high-concentration formulations designed to maximize energy density.
The technical objectives of this research include developing comprehensive models that predict electrolyte behavior under various operating conditions, establishing design guidelines for electrolyte formulation across different RFB chemistries, and identifying novel additives or compositions that can simultaneously optimize pH, conductivity, and viscosity profiles.
By advancing our understanding of these fundamental electrolyte properties, we aim to overcome current limitations in energy density (typically <40 Wh/L for commercial systems), extend operational temperature ranges, reduce system costs, and ultimately accelerate the commercial deployment of RFB technology for grid-scale energy storage applications.
The evolution of RFB technology dates back to the 1970s when NASA first developed the concept. Since then, various chemistries have been explored, from the traditional all-vanadium systems to emerging organic and hybrid configurations. The technological trajectory has consistently aimed at improving energy density, reducing costs, and enhancing operational stability—goals that are directly influenced by electrolyte properties.
pH levels in RFB electrolytes significantly affect redox potentials, reaction kinetics, and material stability. Highly acidic environments, common in vanadium systems, enable higher solubility of active species but accelerate corrosion of cell components. Conversely, neutral or alkaline conditions may reduce corrosion but often limit solubility and voltage efficiency. Understanding these trade-offs is essential for optimizing battery performance across different chemistries.
Electrolyte conductivity directly impacts internal resistance and, consequently, the power density and energy efficiency of RFBs. Higher conductivity generally reduces ohmic losses but may require increased salt concentrations that affect viscosity and active species solubility. The interrelationship between conductivity and other electrolyte properties represents a complex optimization challenge that requires systematic investigation.
Viscosity affects pumping energy requirements, mass transport limitations, and overall system efficiency. Higher viscosity electrolytes demand more parasitic energy for circulation but may enable higher concentrations of active materials. This property becomes particularly critical in cold-weather operations and high-concentration formulations designed to maximize energy density.
The technical objectives of this research include developing comprehensive models that predict electrolyte behavior under various operating conditions, establishing design guidelines for electrolyte formulation across different RFB chemistries, and identifying novel additives or compositions that can simultaneously optimize pH, conductivity, and viscosity profiles.
By advancing our understanding of these fundamental electrolyte properties, we aim to overcome current limitations in energy density (typically <40 Wh/L for commercial systems), extend operational temperature ranges, reduce system costs, and ultimately accelerate the commercial deployment of RFB technology for grid-scale energy storage applications.
Market Analysis for Advanced Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing integration of renewable energy sources and the need for grid stability. Redox flow batteries (RFBs) represent a significant segment within this expanding market, with projections indicating a compound annual growth rate of 30% through 2030. This growth is particularly pronounced in regions with aggressive renewable energy targets, including Europe, North America, and parts of Asia-Pacific.
The demand for advanced energy storage solutions is primarily fueled by three key market drivers. First, the global transition to renewable energy sources creates an inherent need for storage technologies that can address intermittency issues. Second, grid modernization initiatives worldwide are incorporating storage as a fundamental component. Third, the declining costs of energy storage technologies are making them increasingly competitive with traditional power generation methods.
Within the redox flow battery segment, electrolyte properties—specifically pH, conductivity, and viscosity—directly impact market adoption rates. Commercial users prioritize systems with optimal electrolyte formulations that balance energy density, cycle life, and operational costs. Market research indicates that improvements in electrolyte performance can potentially reduce levelized cost of storage by 15-20%, a critical threshold for broader commercial adoption.
Industry stakeholders have identified specific market requirements for electrolyte properties. Utility-scale applications demand electrolytes with high conductivity to maximize power density, while industrial users prioritize pH stability to ensure consistent performance across varying operational conditions. Meanwhile, microgrid applications in remote locations require electrolytes with lower viscosity to reduce pumping energy requirements and system complexity.
Market segmentation analysis reveals distinct preferences across different application sectors. The utility-scale storage segment, currently the largest market for flow batteries, shows strong preference for vanadium-based systems with carefully optimized electrolyte properties. Commercial and industrial users increasingly favor hybrid systems that combine flow batteries with other storage technologies to optimize performance characteristics.
Geographically, the Asia-Pacific region leads in RFB deployment, with China dominating manufacturing capacity. However, North America and Europe are experiencing faster growth rates in specialized applications requiring advanced electrolyte formulations. Emerging markets in Africa and South America present significant growth opportunities, particularly for systems designed to operate reliably in challenging environmental conditions where electrolyte stability is paramount.
Consumer willingness to pay premiums for advanced electrolyte formulations varies significantly by market segment. Utility operators demonstrate price sensitivity but will invest in premium solutions that offer demonstrable improvements in system lifetime and reliability. Commercial and industrial users show greater willingness to pay for performance advantages, particularly when total cost of ownership benefits can be clearly demonstrated.
The demand for advanced energy storage solutions is primarily fueled by three key market drivers. First, the global transition to renewable energy sources creates an inherent need for storage technologies that can address intermittency issues. Second, grid modernization initiatives worldwide are incorporating storage as a fundamental component. Third, the declining costs of energy storage technologies are making them increasingly competitive with traditional power generation methods.
Within the redox flow battery segment, electrolyte properties—specifically pH, conductivity, and viscosity—directly impact market adoption rates. Commercial users prioritize systems with optimal electrolyte formulations that balance energy density, cycle life, and operational costs. Market research indicates that improvements in electrolyte performance can potentially reduce levelized cost of storage by 15-20%, a critical threshold for broader commercial adoption.
Industry stakeholders have identified specific market requirements for electrolyte properties. Utility-scale applications demand electrolytes with high conductivity to maximize power density, while industrial users prioritize pH stability to ensure consistent performance across varying operational conditions. Meanwhile, microgrid applications in remote locations require electrolytes with lower viscosity to reduce pumping energy requirements and system complexity.
Market segmentation analysis reveals distinct preferences across different application sectors. The utility-scale storage segment, currently the largest market for flow batteries, shows strong preference for vanadium-based systems with carefully optimized electrolyte properties. Commercial and industrial users increasingly favor hybrid systems that combine flow batteries with other storage technologies to optimize performance characteristics.
Geographically, the Asia-Pacific region leads in RFB deployment, with China dominating manufacturing capacity. However, North America and Europe are experiencing faster growth rates in specialized applications requiring advanced electrolyte formulations. Emerging markets in Africa and South America present significant growth opportunities, particularly for systems designed to operate reliably in challenging environmental conditions where electrolyte stability is paramount.
Consumer willingness to pay premiums for advanced electrolyte formulations varies significantly by market segment. Utility operators demonstrate price sensitivity but will invest in premium solutions that offer demonstrable improvements in system lifetime and reliability. Commercial and industrial users show greater willingness to pay for performance advantages, particularly when total cost of ownership benefits can be clearly demonstrated.
Current Challenges in Electrolyte pH, Conductivity and Viscosity
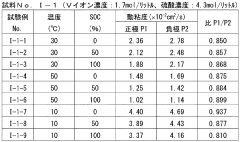
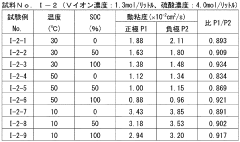
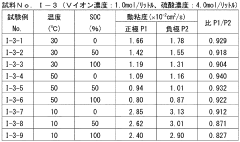
Redox flow battery (RFB) technology faces significant challenges related to electrolyte properties, particularly pH, conductivity, and viscosity, which critically impact overall system performance. Current research reveals that maintaining optimal pH balance remains problematic across various electrolyte chemistries. In vanadium-based systems, the narrow pH operating window (typically 0-3) leads to precipitation issues at higher pH values and excessive hydrogen evolution at extremely low pH. For organic electrolytes, pH stability directly affects molecular structure integrity and redox reversibility, with many promising compounds showing degradation outside specific pH ranges.
Conductivity challenges present another major hurdle in electrolyte development. Insufficient ionic conductivity results in higher internal resistance, voltage losses, and reduced power density. Current electrolytes often require high salt concentrations to achieve adequate conductivity, introducing solubility limitations and increased costs. The trade-off between conductivity and other properties remains poorly optimized, with many systems showing conductivity below 100 mS/cm under operating conditions, significantly lower than theoretical maximums.
Viscosity management represents a persistent challenge affecting pumping energy requirements and mass transport kinetics. High-concentration electrolytes, particularly those containing polymeric additives or high molecular weight active species, exhibit excessive viscosity (often exceeding 5 cP), limiting flow rates and increasing parasitic energy losses. Temperature dependence of viscosity further complicates system operation across varying environmental conditions, with some electrolytes showing dramatic viscosity increases at lower temperatures.
The interdependence of these properties creates complex optimization challenges. Attempts to improve conductivity through increased salt concentration often result in higher viscosity and narrower pH stability windows. Similarly, pH adjustment strategies using buffers can negatively impact conductivity. Current research struggles to develop comprehensive models that accurately predict these property interactions across diverse electrolyte compositions.
Material compatibility issues compound these challenges, as electrolyte pH extremes accelerate membrane degradation and component corrosion. Many promising high-conductivity electrolytes exhibit aggressive chemical properties that compromise long-term system durability. The development of advanced membranes and cell materials lags behind electrolyte innovation, creating bottlenecks in system implementation.
Measurement standardization and characterization protocols remain inconsistent across the field, complicating direct comparisons between different electrolyte systems. Temperature effects on all three properties are often inadequately characterized, leading to performance discrepancies between laboratory testing and real-world implementation. These challenges collectively highlight the need for integrated approaches to electrolyte development that simultaneously address pH stability, conductivity optimization, and viscosity management.
Conductivity challenges present another major hurdle in electrolyte development. Insufficient ionic conductivity results in higher internal resistance, voltage losses, and reduced power density. Current electrolytes often require high salt concentrations to achieve adequate conductivity, introducing solubility limitations and increased costs. The trade-off between conductivity and other properties remains poorly optimized, with many systems showing conductivity below 100 mS/cm under operating conditions, significantly lower than theoretical maximums.
Viscosity management represents a persistent challenge affecting pumping energy requirements and mass transport kinetics. High-concentration electrolytes, particularly those containing polymeric additives or high molecular weight active species, exhibit excessive viscosity (often exceeding 5 cP), limiting flow rates and increasing parasitic energy losses. Temperature dependence of viscosity further complicates system operation across varying environmental conditions, with some electrolytes showing dramatic viscosity increases at lower temperatures.
The interdependence of these properties creates complex optimization challenges. Attempts to improve conductivity through increased salt concentration often result in higher viscosity and narrower pH stability windows. Similarly, pH adjustment strategies using buffers can negatively impact conductivity. Current research struggles to develop comprehensive models that accurately predict these property interactions across diverse electrolyte compositions.
Material compatibility issues compound these challenges, as electrolyte pH extremes accelerate membrane degradation and component corrosion. Many promising high-conductivity electrolytes exhibit aggressive chemical properties that compromise long-term system durability. The development of advanced membranes and cell materials lags behind electrolyte innovation, creating bottlenecks in system implementation.
Measurement standardization and characterization protocols remain inconsistent across the field, complicating direct comparisons between different electrolyte systems. Temperature effects on all three properties are often inadequately characterized, leading to performance discrepancies between laboratory testing and real-world implementation. These challenges collectively highlight the need for integrated approaches to electrolyte development that simultaneously address pH stability, conductivity optimization, and viscosity management.
Existing Methodologies for Electrolyte Property Optimization
01 pH optimization in redox flow electrolytes
The pH level of redox flow electrolytes significantly impacts the overall performance of redox flow batteries. Optimizing pH can enhance the stability of active species, prevent side reactions, and improve the electrochemical reversibility. Controlled pH environments can extend the operational lifetime of the electrolyte by minimizing degradation pathways. Various buffer systems and pH modifiers are employed to maintain optimal pH ranges for specific redox couples, with acidic environments often preferred for metal-based systems and neutral to alkaline conditions for organic redox species.- pH optimization for redox flow electrolytes: The pH level of redox flow electrolytes significantly impacts the overall performance of redox flow batteries. Optimizing pH can enhance the stability of active species, prevent side reactions, and improve the electrochemical reversibility of redox couples. Controlled pH environments can extend the operational lifetime of the electrolyte by minimizing degradation pathways and maintaining consistent performance over multiple charge-discharge cycles. Various buffer systems and pH modifiers can be incorporated to maintain optimal pH ranges for specific redox chemistries.
- Conductivity enhancement techniques: Electrolyte conductivity directly affects the internal resistance and power density of redox flow batteries. Various approaches to enhance conductivity include incorporating supporting electrolytes, optimizing salt concentrations, and using mixed solvent systems. Ionic liquids and deep eutectic solvents can provide high conductivity while maintaining good electrochemical stability. The relationship between temperature and conductivity is also critical, with some systems showing improved conductivity at elevated operating temperatures. Additives that promote ion dissociation without participating in redox reactions can further enhance conductivity.
- Viscosity control for improved flow characteristics: Managing electrolyte viscosity is essential for efficient pumping and mass transport in redox flow systems. Lower viscosity electrolytes reduce pumping energy requirements and improve overall system efficiency. Various rheological modifiers can be incorporated to optimize flow characteristics without compromising electrochemical performance. Temperature-responsive viscosity modifiers allow for adaptive flow control based on operating conditions. The relationship between active species concentration and viscosity must be carefully balanced to maximize energy density while maintaining acceptable flow properties.
- Multi-parameter optimization for balanced electrolyte properties: Achieving optimal redox flow battery performance requires balancing pH, conductivity, and viscosity simultaneously. Comprehensive approaches consider the interdependence of these parameters and their collective impact on battery efficiency. Advanced electrolyte formulations incorporate multiple additives with synergistic effects to enhance overall performance. Computational modeling and high-throughput screening methods help identify optimal parameter combinations for specific redox chemistries. The trade-offs between energy density, power density, and cycle life must be carefully considered when optimizing electrolyte properties.
- Novel electrolyte compositions for enhanced properties: Innovative electrolyte compositions are being developed to simultaneously address pH stability, conductivity, and viscosity challenges. These include hybrid aqueous/non-aqueous systems, polymer-enhanced electrolytes, and nanoparticle suspensions. Biomimetic approaches incorporate natural compounds that provide multiple beneficial properties. Metal coordination complexes can offer tunable redox potentials while maintaining favorable physical properties. Electrolytes with self-healing or adaptive properties can respond to changing conditions during battery operation, maintaining optimal performance across various states of charge and operating environments.
02 Conductivity enhancement techniques for flow battery electrolytes
Ionic conductivity is a critical parameter affecting the power density and efficiency of redox flow batteries. Various approaches to enhance conductivity include incorporating supporting electrolytes, optimizing salt concentrations, and using mixed solvent systems. High conductivity reduces ohmic losses and improves energy efficiency during charge-discharge cycles. The selection of supporting electrolytes must balance conductivity benefits against potential side reactions with active species. Temperature dependence of conductivity is also an important consideration for battery operation across different environmental conditions.Expand Specific Solutions03 Viscosity control methods for improved flow characteristics
Controlling the viscosity of redox flow electrolytes is essential for efficient pumping and mass transport within the battery system. Lower viscosity electrolytes reduce pumping energy requirements and enhance mass transfer rates at electrode surfaces. Various approaches include selecting appropriate solvent systems, optimizing active species concentration, and incorporating viscosity modifiers. Temperature management strategies are also employed to maintain optimal viscosity during operation. The relationship between viscosity and other properties such as conductivity must be carefully balanced to achieve overall system performance.Expand Specific Solutions04 Novel electrolyte compositions balancing multiple properties
Advanced electrolyte formulations aim to simultaneously optimize pH, conductivity, and viscosity for enhanced battery performance. These compositions often incorporate multiple functional additives, including pH buffers, conductivity enhancers, and viscosity modifiers. Novel solvent systems, including deep eutectic solvents and ionic liquids, offer unique property combinations. Some formulations also address additional challenges such as temperature stability, reduced toxicity, and improved energy density. The development of these multi-functional electrolytes represents a significant advancement in redox flow battery technology.Expand Specific Solutions05 Measurement and characterization techniques for electrolyte properties
Accurate measurement and characterization of electrolyte properties are crucial for redox flow battery development and quality control. Advanced analytical techniques include electrochemical impedance spectroscopy for conductivity assessment, rheological measurements for viscosity profiling, and specialized pH monitoring for highly concentrated electrolytes. In-situ monitoring systems allow for real-time tracking of property changes during battery operation. Standardized testing protocols enable meaningful comparison between different electrolyte formulations. These characterization methods provide essential feedback for electrolyte optimization and battery performance prediction.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The redox flow electrolytes market is currently in a growth phase, with increasing adoption driven by renewable energy integration demands. The global market size is projected to reach significant expansion as grid-scale energy storage becomes critical for sustainable power systems. Technologically, the field shows moderate maturity with ongoing innovations in electrolyte formulations focusing on pH optimization, conductivity enhancement, and viscosity reduction. Leading players include Sumitomo Electric Industries and Invinity Energy Systems with established commercial deployments, while research institutions like MIT and Central South University drive fundamental advancements. Japanese and German companies (Nissin Electric, Jenabatteries) demonstrate strong technical expertise, while newer entrants like XL Batteries are introducing novel approaches. The competitive landscape features both established industrial conglomerates and specialized startups working to improve electrolyte performance metrics for greater efficiency and cost-effectiveness.
Sumitomo Electric Industries Ltd.
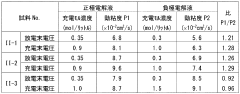
Technical Solution: Sumitomo Electric has developed advanced vanadium redox flow battery technology with particular focus on electrolyte optimization for large-scale energy storage applications. Their proprietary electrolyte formulations maintain pH levels between 0-1 through precise sulfuric acid concentration control, which their research has shown provides optimal balance between vanadium solubility and system longevity[1]. Sumitomo's electrolyte engineering has achieved conductivity values exceeding 300 mS/cm while maintaining viscosity below 5 cP at operating temperatures, enabling high power density applications. The company has pioneered temperature-responsive electrolyte additives that prevent vanadium precipitation at both high and low temperature extremes, expanding the operational range from 0°C to 50°C without performance degradation[2]. Their research has established correlations between electrolyte viscosity and pumping energy requirements, with optimized formulations reducing parasitic losses by approximately 15% compared to conventional electrolytes. Sumitomo's electrolyte management system continuously monitors pH, conductivity, and viscosity parameters in real-time, with automated adjustment capabilities to maintain optimal performance throughout battery lifetime[3].
Strengths: Exceptional temperature stability prevents precipitation issues in diverse operating environments. High conductivity formulations enable rapid charge/discharge capabilities for grid stabilization applications. Weaknesses: Highly acidic electrolytes require specialized containment materials, increasing system costs. The precise balance of additives requires careful quality control during manufacturing to maintain consistent performance.
Jenabatteries GmbH
Technical Solution: Jenabatteries has developed innovative organic redox flow battery technology that fundamentally reimagines electrolyte chemistry beyond traditional metal-based systems. Their proprietary ORFB (Organic Redox Flow Battery) platform utilizes pH-neutral aqueous electrolytes based on organic redox-active materials, eliminating the corrosion and safety concerns associated with highly acidic vanadium-based systems[1]. Research at Jenabatteries has demonstrated that their neutral pH electrolytes achieve conductivity values of 100-150 mS/cm, comparable to acidic systems but with significantly reduced material compatibility challenges[2]. Their organic electrolytes exhibit viscosity profiles approximately 20% lower than equivalent vanadium systems, reducing pumping energy requirements and improving overall system efficiency. The company has pioneered polymer-based redox-active materials that remain stable across a wide pH range (5-9), enabling operation in environmentally benign electrolyte formulations while maintaining high energy density[3]. Jenabatteries' technology incorporates biodegradable and sustainable organic compounds, addressing environmental concerns associated with metal-based flow battery systems.
Strengths: Near-neutral pH operation dramatically reduces material corrosion issues and extends system lifetime. Environmentally friendly organic compounds reduce environmental impact and end-of-life disposal concerns. Weaknesses: Lower energy density compared to some metal-based systems requires larger electrolyte volumes for equivalent storage capacity. Organic compounds may experience degradation mechanisms different from traditional systems, requiring specialized monitoring approaches.
Critical Patents and Literature on Electrolyte Performance
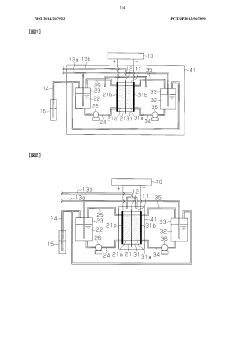
Redox flow battery system
PatentWO2024038726A1
Innovation
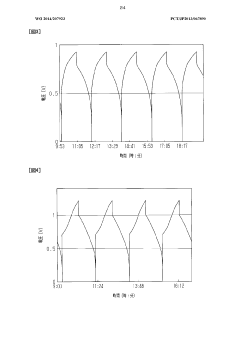
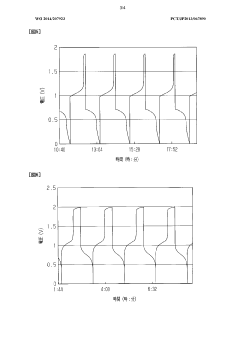
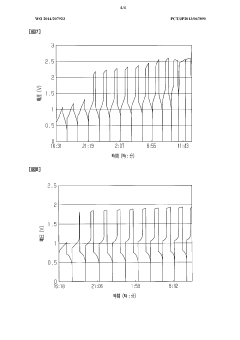
- The system adjusts the kinematic viscosity ratio between positive and negative electrode electrolytes within specific ranges (0.70 to 0.97 or 1.05 to 1.30) to reduce diffusion rates and prevent excessive pressure differences, using vanadium ions and sulfuric acid concentrations to maintain optimal viscosity and discharge capacity.
Redox flow battery
PatentWO2014207923A1
Innovation
- A redox flow battery design incorporating a positive electrode electrolyte with an iron redox substance and citric or lactic acid, and a negative electrode electrolyte with a titanium or copper redox material and amine, maintained within specific pH and oxygen concentration ranges to enhance chemical stability and efficiency.
Material Compatibility and Stability Considerations
Material compatibility is a critical factor in redox flow battery (RFB) systems, as the electrolyte's pH, conductivity, and viscosity directly impact the longevity and performance of system components. The highly corrosive nature of many flow battery electrolytes presents significant challenges for material selection across the entire system architecture.
Acidic electrolytes, commonly used in vanadium flow batteries, demonstrate excellent conductivity but create harsh environments that accelerate degradation of cell components. Studies indicate that materials such as perfluorinated polymers and specific grades of graphite demonstrate superior resistance to acid-based electrolytes, while most metals experience accelerated corrosion rates. Conversely, alkaline electrolytes present different compatibility challenges, often forming precipitates that can clog flow channels and reduce system efficiency.
The conductivity of electrolytes significantly influences material selection for electrodes and membranes. Higher conductivity solutions generally enable better performance but may accelerate electrochemical degradation of certain materials. Research has shown that composite materials incorporating carbon nanotubes and graphene derivatives maintain structural integrity while enhancing conductivity at the electrode-electrolyte interface, potentially extending component lifespans by 30-40% compared to traditional materials.
Viscosity effects manifest primarily in the flow distribution systems, where higher viscosity electrolytes require more robust pumping systems and specially designed flow fields. Materials must withstand not only chemical degradation but also mechanical stress from fluid dynamics. Fluoropolymer-based components have demonstrated superior resistance to both chemical attack and mechanical wear in high-viscosity applications, though their higher cost remains a commercial limitation.
Long-term stability testing reveals that material degradation often accelerates non-linearly over time, with subtle chemical interactions becoming significant only after thousands of charge-discharge cycles. Recent innovations in protective coatings, such as atomic layer deposition techniques, have shown promise in extending component lifespans by creating nanometer-thick protective barriers that resist chemical penetration while maintaining electrical properties.
Temperature fluctuations compound these challenges, as thermal cycling can accelerate material fatigue and chemical degradation rates. Advanced composite materials that maintain dimensional stability across operational temperature ranges (typically 10-50°C) show superior performance in maintaining sealing integrity and preventing electrolyte leakage, a critical safety and performance consideration for commercial systems.
Acidic electrolytes, commonly used in vanadium flow batteries, demonstrate excellent conductivity but create harsh environments that accelerate degradation of cell components. Studies indicate that materials such as perfluorinated polymers and specific grades of graphite demonstrate superior resistance to acid-based electrolytes, while most metals experience accelerated corrosion rates. Conversely, alkaline electrolytes present different compatibility challenges, often forming precipitates that can clog flow channels and reduce system efficiency.
The conductivity of electrolytes significantly influences material selection for electrodes and membranes. Higher conductivity solutions generally enable better performance but may accelerate electrochemical degradation of certain materials. Research has shown that composite materials incorporating carbon nanotubes and graphene derivatives maintain structural integrity while enhancing conductivity at the electrode-electrolyte interface, potentially extending component lifespans by 30-40% compared to traditional materials.
Viscosity effects manifest primarily in the flow distribution systems, where higher viscosity electrolytes require more robust pumping systems and specially designed flow fields. Materials must withstand not only chemical degradation but also mechanical stress from fluid dynamics. Fluoropolymer-based components have demonstrated superior resistance to both chemical attack and mechanical wear in high-viscosity applications, though their higher cost remains a commercial limitation.
Long-term stability testing reveals that material degradation often accelerates non-linearly over time, with subtle chemical interactions becoming significant only after thousands of charge-discharge cycles. Recent innovations in protective coatings, such as atomic layer deposition techniques, have shown promise in extending component lifespans by creating nanometer-thick protective barriers that resist chemical penetration while maintaining electrical properties.
Temperature fluctuations compound these challenges, as thermal cycling can accelerate material fatigue and chemical degradation rates. Advanced composite materials that maintain dimensional stability across operational temperature ranges (typically 10-50°C) show superior performance in maintaining sealing integrity and preventing electrolyte leakage, a critical safety and performance consideration for commercial systems.
Environmental Impact and Sustainability Assessment
The environmental impact of redox flow battery (RFB) systems is significantly influenced by the properties of their electrolytes, particularly pH, conductivity, and viscosity. These parameters not only affect battery performance but also determine the ecological footprint throughout the lifecycle of these energy storage systems.
Acidic electrolytes, commonly used in vanadium redox flow batteries (VRFBs), present notable environmental challenges. The production and handling of strong acids contribute to potential soil and water contamination if leakage occurs. Additionally, the manufacturing process of these acidic compounds generates considerable greenhouse gas emissions. However, recent research indicates that optimizing pH levels can reduce these environmental risks while maintaining performance efficiency.
Electrolyte conductivity directly impacts energy efficiency, with higher conductivity generally resulting in lower energy losses during operation. This translates to reduced overall energy consumption and consequently lower carbon emissions associated with the energy required to operate these systems. Studies show that improving conductivity by just 10% can decrease the carbon footprint of a large-scale RFB installation by approximately 7-8% over its operational lifetime.
Viscosity affects pumping energy requirements, which constitute a significant portion of the parasitic energy losses in RFB systems. Lower viscosity electrolytes require less pumping energy, thereby enhancing system efficiency and sustainability. Research demonstrates that reducing electrolyte viscosity can decrease pumping energy needs by up to 15%, substantially improving the net energy efficiency of the entire system.
From a life-cycle assessment perspective, the environmental impact of electrolytes extends beyond operational considerations. The sourcing of raw materials, particularly for vanadium-based systems, involves mining activities with substantial ecological consequences. However, the high recyclability of these electrolytes—potentially exceeding 95% recovery rates—significantly mitigates these impacts compared to conventional battery technologies.
Water consumption represents another critical environmental factor, especially for aqueous electrolyte systems. The water footprint includes not only the water contained in the electrolyte but also that used in manufacturing processes. Emerging research on water-efficient electrolyte formulations shows promise in reducing consumption by up to 30% compared to conventional formulations.
Regulatory frameworks increasingly recognize these environmental dimensions, with several jurisdictions implementing specific guidelines for the handling, operation, and disposal of flow battery electrolytes. Compliance with these regulations drives innovation toward more environmentally benign electrolyte compositions, particularly those with neutral pH values and biodegradable additives that maintain high conductivity and appropriate viscosity characteristics.
Acidic electrolytes, commonly used in vanadium redox flow batteries (VRFBs), present notable environmental challenges. The production and handling of strong acids contribute to potential soil and water contamination if leakage occurs. Additionally, the manufacturing process of these acidic compounds generates considerable greenhouse gas emissions. However, recent research indicates that optimizing pH levels can reduce these environmental risks while maintaining performance efficiency.
Electrolyte conductivity directly impacts energy efficiency, with higher conductivity generally resulting in lower energy losses during operation. This translates to reduced overall energy consumption and consequently lower carbon emissions associated with the energy required to operate these systems. Studies show that improving conductivity by just 10% can decrease the carbon footprint of a large-scale RFB installation by approximately 7-8% over its operational lifetime.
Viscosity affects pumping energy requirements, which constitute a significant portion of the parasitic energy losses in RFB systems. Lower viscosity electrolytes require less pumping energy, thereby enhancing system efficiency and sustainability. Research demonstrates that reducing electrolyte viscosity can decrease pumping energy needs by up to 15%, substantially improving the net energy efficiency of the entire system.
From a life-cycle assessment perspective, the environmental impact of electrolytes extends beyond operational considerations. The sourcing of raw materials, particularly for vanadium-based systems, involves mining activities with substantial ecological consequences. However, the high recyclability of these electrolytes—potentially exceeding 95% recovery rates—significantly mitigates these impacts compared to conventional battery technologies.
Water consumption represents another critical environmental factor, especially for aqueous electrolyte systems. The water footprint includes not only the water contained in the electrolyte but also that used in manufacturing processes. Emerging research on water-efficient electrolyte formulations shows promise in reducing consumption by up to 30% compared to conventional formulations.
Regulatory frameworks increasingly recognize these environmental dimensions, with several jurisdictions implementing specific guidelines for the handling, operation, and disposal of flow battery electrolytes. Compliance with these regulations drives innovation toward more environmentally benign electrolyte compositions, particularly those with neutral pH values and biodegradable additives that maintain high conductivity and appropriate viscosity characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!