Exploring Quinone Based Electrolytes for Flow Batteries
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quinone Electrolytes Background and Research Objectives
Flow batteries have emerged as promising energy storage systems due to their ability to decouple power and energy capacity, making them particularly suitable for grid-scale applications. Among various chemistries explored for flow batteries, quinone-based electrolytes have gained significant attention over the past decade due to their unique electrochemical properties and potential for sustainable energy storage solutions.
Quinones are organic compounds characterized by a conjugated cyclic dione structure, which can undergo reversible redox reactions involving the transfer of electrons and protons. This fundamental property makes them excellent candidates for electrochemical energy storage applications. The interest in quinone-based electrolytes stems from their earth-abundant nature, structural diversity, and tunable redox properties, offering a sustainable alternative to traditional metal-based flow battery systems.
The historical development of quinone-based flow batteries can be traced back to early 2010s when researchers at Harvard University first demonstrated the feasibility of an all-quinone flow battery. This breakthrough opened a new avenue for organic-based flow battery research, shifting focus from metal-based systems that often face resource constraints and environmental concerns.
The evolution of quinone electrolytes has progressed through several generations, beginning with anthraquinones and benzoquinones in aqueous systems, followed by the development of water-soluble derivatives to enhance energy density. Recent advances have explored functionalized quinones with improved solubility, stability, and redox potential, as well as their integration into non-aqueous systems to expand the electrochemical window.
Current technical challenges in quinone-based flow batteries include limited solubility in electrolyte solutions, capacity fade due to chemical degradation, membrane crossover issues, and relatively low energy density compared to some conventional battery technologies. These challenges define the primary objectives for ongoing research in this field.
The primary research objectives for quinone-based electrolytes include: enhancing molecular stability to prevent degradation during cycling; increasing solubility to achieve higher energy density; optimizing redox potentials through molecular engineering; developing cost-effective synthesis routes for large-scale production; and improving system integration for practical deployment.
Additionally, research aims to understand structure-property relationships to guide rational molecular design, explore novel quinone derivatives with enhanced performance metrics, and develop computational models to predict electrochemical behavior. The ultimate goal is to create sustainable, high-performance flow battery systems that can effectively support renewable energy integration and grid stabilization at competitive costs.
Quinones are organic compounds characterized by a conjugated cyclic dione structure, which can undergo reversible redox reactions involving the transfer of electrons and protons. This fundamental property makes them excellent candidates for electrochemical energy storage applications. The interest in quinone-based electrolytes stems from their earth-abundant nature, structural diversity, and tunable redox properties, offering a sustainable alternative to traditional metal-based flow battery systems.
The historical development of quinone-based flow batteries can be traced back to early 2010s when researchers at Harvard University first demonstrated the feasibility of an all-quinone flow battery. This breakthrough opened a new avenue for organic-based flow battery research, shifting focus from metal-based systems that often face resource constraints and environmental concerns.
The evolution of quinone electrolytes has progressed through several generations, beginning with anthraquinones and benzoquinones in aqueous systems, followed by the development of water-soluble derivatives to enhance energy density. Recent advances have explored functionalized quinones with improved solubility, stability, and redox potential, as well as their integration into non-aqueous systems to expand the electrochemical window.
Current technical challenges in quinone-based flow batteries include limited solubility in electrolyte solutions, capacity fade due to chemical degradation, membrane crossover issues, and relatively low energy density compared to some conventional battery technologies. These challenges define the primary objectives for ongoing research in this field.
The primary research objectives for quinone-based electrolytes include: enhancing molecular stability to prevent degradation during cycling; increasing solubility to achieve higher energy density; optimizing redox potentials through molecular engineering; developing cost-effective synthesis routes for large-scale production; and improving system integration for practical deployment.
Additionally, research aims to understand structure-property relationships to guide rational molecular design, explore novel quinone derivatives with enhanced performance metrics, and develop computational models to predict electrochemical behavior. The ultimate goal is to create sustainable, high-performance flow battery systems that can effectively support renewable energy integration and grid stabilization at competitive costs.
Market Analysis for Flow Battery Energy Storage Systems
The global energy storage market is experiencing unprecedented growth, with flow batteries emerging as a significant segment within this expanding sector. As of 2023, the global flow battery market is valued at approximately $290 million, with projections indicating growth to reach $946 million by 2027, representing a compound annual growth rate (CAGR) of 34%. Within this market, redox flow batteries (RFBs) constitute the dominant technology, with vanadium-based systems currently holding the largest market share at 65%.
The demand for flow battery energy storage systems is primarily driven by the increasing integration of renewable energy sources into power grids worldwide. As solar and wind power generation continues to grow—reaching over 1,400 GW of installed capacity globally—the need for long-duration energy storage solutions becomes critical for grid stability and reliability. Flow batteries, with their ability to provide 4-12 hours of storage duration, are particularly well-positioned to address this market need.
Quinone-based electrolytes represent an emerging segment within the flow battery market, with current market penetration below 5%. However, this segment is expected to grow at a faster rate than the overall flow battery market, potentially reaching 15% market share by 2030. This growth is attributed to the significant cost advantages of organic electrolytes compared to vanadium-based systems, with potential cost reductions of 30-40% for complete systems.
Geographically, North America and Asia-Pacific regions dominate the flow battery market, collectively accounting for 78% of global installations. Europe follows with approximately 18% market share, though European investments in flow battery technology are accelerating, particularly in Germany and the UK. China has emerged as the largest single national market for flow batteries, driven by substantial government support for grid-scale energy storage projects.
By application segment, utility-scale energy storage represents the largest market for flow batteries at 72%, followed by industrial applications at 18% and commercial installations at 10%. The utility segment is expected to maintain its dominant position as grid operators increasingly deploy long-duration storage to manage renewable energy integration challenges.
Key customer segments include electric utilities, renewable energy developers, microgrids, and industrial facilities with high reliability requirements. These customers typically value the long cycle life (15-20 years), safety profile, and scalability of flow battery systems over lithium-ion alternatives, despite higher upfront capital costs. The total addressable market for quinone-based flow batteries is estimated at $1.2 billion by 2030, representing a significant opportunity for technology developers in this space.
The demand for flow battery energy storage systems is primarily driven by the increasing integration of renewable energy sources into power grids worldwide. As solar and wind power generation continues to grow—reaching over 1,400 GW of installed capacity globally—the need for long-duration energy storage solutions becomes critical for grid stability and reliability. Flow batteries, with their ability to provide 4-12 hours of storage duration, are particularly well-positioned to address this market need.
Quinone-based electrolytes represent an emerging segment within the flow battery market, with current market penetration below 5%. However, this segment is expected to grow at a faster rate than the overall flow battery market, potentially reaching 15% market share by 2030. This growth is attributed to the significant cost advantages of organic electrolytes compared to vanadium-based systems, with potential cost reductions of 30-40% for complete systems.
Geographically, North America and Asia-Pacific regions dominate the flow battery market, collectively accounting for 78% of global installations. Europe follows with approximately 18% market share, though European investments in flow battery technology are accelerating, particularly in Germany and the UK. China has emerged as the largest single national market for flow batteries, driven by substantial government support for grid-scale energy storage projects.
By application segment, utility-scale energy storage represents the largest market for flow batteries at 72%, followed by industrial applications at 18% and commercial installations at 10%. The utility segment is expected to maintain its dominant position as grid operators increasingly deploy long-duration storage to manage renewable energy integration challenges.
Key customer segments include electric utilities, renewable energy developers, microgrids, and industrial facilities with high reliability requirements. These customers typically value the long cycle life (15-20 years), safety profile, and scalability of flow battery systems over lithium-ion alternatives, despite higher upfront capital costs. The total addressable market for quinone-based flow batteries is estimated at $1.2 billion by 2030, representing a significant opportunity for technology developers in this space.
Current Challenges in Quinone-Based Electrolyte Development
Despite the promising potential of quinone-based electrolytes for flow batteries, several significant challenges impede their widespread commercial adoption. Solubility limitations represent a primary obstacle, as many quinone compounds exhibit insufficient solubility in aqueous or non-aqueous solvents. This directly impacts energy density, with current quinone-based systems typically achieving only 5-15 Wh/L, substantially lower than the 40+ Wh/L necessary for commercial viability. Enhancing solubility through molecular engineering often introduces trade-offs with other critical properties.
Chemical stability presents another major challenge, particularly during extended cycling. Quinones undergo side reactions including dimerization, nucleophilic addition, and Michael addition, leading to capacity fade rates of 0.1-0.5% per cycle. This degradation accelerates at elevated temperatures and extreme pH conditions, significantly limiting practical operational lifetimes to well below the 10+ years required for grid-scale applications.
Crossover of active materials through membranes constitutes a persistent issue in flow battery systems. Quinone molecules, particularly smaller variants, readily permeate standard ion-exchange membranes, causing capacity loss and self-discharge rates of 2-5% daily. While larger molecular designs can mitigate crossover, they typically sacrifice solubility and reaction kinetics.
The cost structure of quinone-based systems remains problematic. Current synthesis routes for high-performance quinones involve multi-step processes with expensive reagents and purification steps, resulting in electrolyte costs of $15-25/kWh—significantly above the $5/kWh target for grid storage applications. Additionally, many synthesis pathways utilize petroleum-derived precursors, raising sustainability concerns.
Performance consistency across varying operational conditions poses another challenge. Quinone redox potentials and kinetics show strong dependence on pH, temperature, and electrolyte composition. This sensitivity necessitates sophisticated battery management systems, increasing system complexity and cost while reducing reliability.
Scalability issues further complicate commercial deployment. Laboratory-scale synthesis methods for novel quinones often prove difficult to scale industrially while maintaining consistent product quality. Batch-to-batch variations in purity and performance characteristics create significant hurdles for manufacturing standardization.
Toxicity and environmental impact concerns persist for certain quinone derivatives, particularly those containing halogen substituents. Comprehensive lifecycle assessments remain incomplete for many promising quinone candidates, creating regulatory uncertainty and potential environmental liabilities that deter commercial investment.
Chemical stability presents another major challenge, particularly during extended cycling. Quinones undergo side reactions including dimerization, nucleophilic addition, and Michael addition, leading to capacity fade rates of 0.1-0.5% per cycle. This degradation accelerates at elevated temperatures and extreme pH conditions, significantly limiting practical operational lifetimes to well below the 10+ years required for grid-scale applications.
Crossover of active materials through membranes constitutes a persistent issue in flow battery systems. Quinone molecules, particularly smaller variants, readily permeate standard ion-exchange membranes, causing capacity loss and self-discharge rates of 2-5% daily. While larger molecular designs can mitigate crossover, they typically sacrifice solubility and reaction kinetics.
The cost structure of quinone-based systems remains problematic. Current synthesis routes for high-performance quinones involve multi-step processes with expensive reagents and purification steps, resulting in electrolyte costs of $15-25/kWh—significantly above the $5/kWh target for grid storage applications. Additionally, many synthesis pathways utilize petroleum-derived precursors, raising sustainability concerns.
Performance consistency across varying operational conditions poses another challenge. Quinone redox potentials and kinetics show strong dependence on pH, temperature, and electrolyte composition. This sensitivity necessitates sophisticated battery management systems, increasing system complexity and cost while reducing reliability.
Scalability issues further complicate commercial deployment. Laboratory-scale synthesis methods for novel quinones often prove difficult to scale industrially while maintaining consistent product quality. Batch-to-batch variations in purity and performance characteristics create significant hurdles for manufacturing standardization.
Toxicity and environmental impact concerns persist for certain quinone derivatives, particularly those containing halogen substituents. Comprehensive lifecycle assessments remain incomplete for many promising quinone candidates, creating regulatory uncertainty and potential environmental liabilities that deter commercial investment.
Current Quinone Electrolyte Formulations and Designs
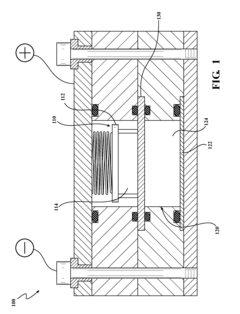
01 Quinone-based electrolytes for flow batteries
Quinone compounds are used as active materials in electrolytes for flow batteries due to their reversible redox properties. These compounds can undergo reduction and oxidation reactions repeatedly, making them suitable for energy storage applications. The quinone-based electrolytes offer advantages such as high energy density, good cycling stability, and environmental friendliness compared to traditional metal-based electrolytes.- Quinone derivatives as electrolyte components for energy storage: Quinone derivatives are utilized as active components in electrolytes for various energy storage applications including batteries and capacitors. These compounds offer redox-active properties that enhance electron transfer and energy storage capacity. The quinone structure provides stable redox reactions that can be tuned for specific voltage requirements, making them valuable for next-generation energy storage technologies.
- Flow battery applications of quinone-based electrolytes: Quinone-based compounds are particularly suitable for flow battery applications due to their solubility in aqueous and non-aqueous solvents, stable redox behavior, and high energy density. These electrolytes enable the development of cost-effective, environmentally friendly flow batteries with improved cycling stability and energy efficiency. The quinone structures can be modified to optimize properties such as solubility and redox potential for specific flow battery requirements.
- Electrochemical processes using quinone electrolytes: Quinone-based electrolytes are employed in various electrochemical processes including electroplating, electrochemical synthesis, and analytical applications. These compounds facilitate electron transfer at electrode surfaces and can be used as mediators in electrochemical reactions. The redox properties of quinones make them valuable for controlling electrochemical processes and improving reaction efficiency.
- Polymer-quinone composite electrolytes: Combining quinone compounds with polymeric materials creates composite electrolytes with enhanced mechanical stability and electrochemical performance. These composites can be formulated as solid or gel electrolytes, offering advantages for flexible and wearable energy storage devices. The polymer matrix provides structural support while the quinone components contribute redox activity, resulting in electrolyte systems with improved safety and performance characteristics.
- Additives and stabilizers for quinone electrolytes: Various additives and stabilizers are incorporated into quinone-based electrolyte formulations to enhance performance and longevity. These include compounds that prevent quinone degradation, improve solubility, enhance conductivity, and optimize redox behavior. Stabilization strategies are crucial for maintaining the electrochemical properties of quinone electrolytes during extended cycling and under various operating conditions.
02 Anthraquinone derivatives as electrolyte components
Anthraquinone derivatives, a specific class of quinones, are utilized in electrolyte formulations for various electrochemical applications. These compounds feature a stable aromatic structure with redox-active carbonyl groups that facilitate electron transfer. The derivatives can be modified with different functional groups to tune their solubility, redox potential, and stability in various electrolyte systems, enhancing overall performance in batteries and other electrochemical devices.Expand Specific Solutions03 Quinone electrolytes for electrochemical sensors
Quinone compounds are incorporated into electrolytes for electrochemical sensing applications. The redox properties of quinones allow them to act as electron mediators, facilitating electron transfer between analytes and electrode surfaces. This enhances the sensitivity and selectivity of electrochemical sensors for detecting various substances including biological compounds, environmental pollutants, and industrial chemicals.Expand Specific Solutions04 Polymer-bound quinone electrolyte systems
Quinone molecules can be chemically bound to polymer backbones to create polymer-quinone hybrid electrolyte systems. This approach prevents the quinone compounds from diffusing or leaching out of the electrolyte, improving long-term stability. The polymer matrix also provides mechanical support and can enhance the electrochemical properties of the quinone moieties, resulting in improved performance in applications such as batteries, supercapacitors, and electrochromic devices.Expand Specific Solutions05 Quinone electrolytes with ionic liquid additives
The performance of quinone-based electrolytes can be enhanced by incorporating ionic liquid additives. These additives improve the solubility of quinone compounds, increase ionic conductivity, and expand the electrochemical stability window of the electrolyte. The combination of quinones with ionic liquids creates electrolyte systems with superior thermal stability, reduced volatility, and enhanced electrochemical performance for various energy storage and conversion applications.Expand Specific Solutions
Leading Organizations in Quinone Electrolyte Research
The quinone-based electrolytes for flow batteries market is currently in its growth phase, with increasing research activity and commercial development. The global flow battery market is projected to reach $1.1 billion by 2027, with quinone-based systems representing an emerging segment due to their sustainable characteristics and cost advantages. Academic institutions like Harvard University, MIT, and University of California lead fundamental research, while companies such as KEMIWATT, CMBlu Energy, and Lockheed Martin Advanced Energy Storage are advancing commercialization efforts. Technical maturity varies significantly across players, with established corporations like Siemens, Mitsubishi Heavy Industries, and Panasonic integrating flow battery technology into their renewable energy portfolios, while Chinese institutions including Nanjing University and Dalian Institute of Chemical Physics are rapidly accelerating development through government-backed initiatives.
President & Fellows of Harvard College
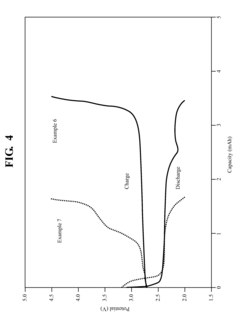
Technical Solution: Harvard College has pioneered quinone-based flow battery technology through the development of metal-free organic electrolytes. Their approach utilizes quinones derived from abundant natural sources like plants or petroleum, significantly reducing costs compared to traditional vanadium-based systems. Harvard researchers have specifically focused on anthraquinone disulfonic acid (AQDS) and bromine as active materials, achieving energy densities of 50-60 Wh/L. Their innovative molecular engineering techniques have enhanced the solubility and stability of quinone compounds in aqueous solutions, addressing key limitations in flow battery technology. Recent advancements include the development of alkaline quinone flow batteries that demonstrate improved cycling stability (>1000 cycles) and higher voltage efficiency (>90%) compared to earlier iterations[1][3]. Harvard's technology also features rapid charge-discharge capabilities and demonstrates minimal capacity degradation over extended cycling periods.
Strengths: Cost-effective materials derived from abundant natural sources; environmentally benign compared to metal-based alternatives; high voltage efficiency; excellent cycling stability. Weaknesses: Lower energy density compared to some metal-based systems; potential degradation of organic molecules during long-term operation; challenges in scaling up production to industrial levels.
Lockheed Martin Advanced Energy Storage LLC
Technical Solution: Lockheed Martin Advanced Energy Storage has developed a proprietary quinone-based flow battery system designed specifically for grid-scale applications and military deployments. Their technology utilizes engineered quinone compounds with enhanced stability characteristics, capable of operating across a wide temperature range (-20°C to 60°C). The company's approach incorporates advanced electrode materials with catalytic properties that improve reaction kinetics and reduce activation overpotentials. Lockheed's system features a modular architecture that allows for scalable deployment from 100kW to multi-MW installations, with energy storage durations ranging from 4-10 hours. Their flow battery design includes sophisticated electrolyte management systems that continuously monitor and adjust chemical parameters to maintain optimal performance. Recent innovations include the development of composite membrane materials that demonstrate 40% lower resistance and 60% reduced crossover compared to conventional separators[7]. Lockheed Martin has also implemented advanced computational fluid dynamics to optimize flow field designs, resulting in more uniform reaction distribution and improved capacity utilization.
Strengths: Robust engineering suitable for demanding environments; scalable modular design; advanced system integration capabilities; sophisticated control systems for performance optimization. Weaknesses: Higher initial capital costs compared to some competitors; proprietary technology may limit third-party integration options; relatively new entrant to commercial energy storage markets compared to established players.
Key Patents and Scientific Breakthroughs in Quinone Chemistry
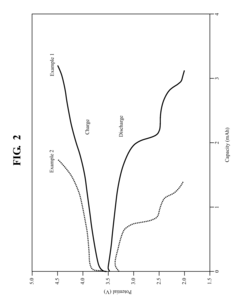
Quinone and hydroquinone based flow battery
PatentWO2015048550A8
Innovation
- A quinone-based flow battery that employs protonation of small organic molecules to store electrical energy, using inexpensive and abundant chemicals, with electrodes made of plastic or inexpensive metals, and a separator that prevents reactant crossover, allowing for high current density and long cycle life.
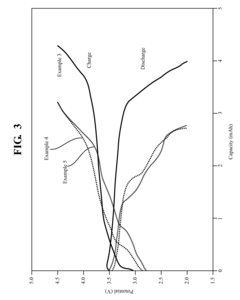
Quinone-based high energy density liquid active material for flow battery
PatentActiveUS20160197371A1
Innovation
- A liquid catholyte composition is developed, featuring a quinone as a redox active agent and a fluoroalkylsulfonyl salt as a charge balancing agent, along with an optional viscosity-reducing liquid fluidizer, which maintains a liquid form in any oxidation state, enhancing energy density without the need for solid electrodes.
Environmental Impact and Sustainability Assessment
Quinone-based flow batteries represent a promising sustainable energy storage solution, offering significant environmental advantages over conventional battery technologies. The organic nature of quinone compounds, which can be derived from abundant biomass sources such as lignin and cellulose, substantially reduces dependence on scarce mineral resources like lithium, cobalt, and vanadium that dominate current battery technologies. This shift toward renewable carbon-based materials aligns with circular economy principles and reduces the environmental footprint associated with mining operations.
Life cycle assessment (LCA) studies indicate that quinone-based electrolytes demonstrate lower greenhouse gas emissions during production compared to vanadium-based alternatives. The carbon intensity of manufacturing quinone compounds can be further reduced when biomass-derived precursors replace petroleum-based feedstocks. Additionally, the water-soluble nature of many quinone derivatives eliminates the need for toxic organic solvents in electrolyte formulation, significantly reducing volatile organic compound (VOC) emissions and associated health risks.
The biodegradability of quinone compounds presents both advantages and challenges from an environmental perspective. While enhanced biodegradability reduces end-of-life environmental impact, it necessitates careful consideration of electrolyte stability during operation. Recent research has focused on developing quinone derivatives with optimized stability-biodegradability profiles to ensure long-term performance while maintaining environmental benefits.
Water usage represents another critical sustainability metric for flow battery technologies. Aqueous quinone-based systems typically require less water than mining-intensive battery technologies when evaluated across their complete life cycle. However, water management strategies remain essential, particularly for large-scale deployment in water-stressed regions. Closed-loop cooling systems and electrolyte recycling protocols can further minimize the water footprint of these systems.
Safety considerations also favor quinone-based electrolytes, which generally present lower flammability risks compared to lithium-ion batteries and reduced toxicity compared to vanadium-based flow batteries. This enhanced safety profile reduces the potential for environmental contamination from accidental releases and simplifies emergency response protocols, contributing to overall system sustainability.
Economic sustainability analysis reveals that while current quinone-based systems face cost challenges related to synthesis complexity and membrane requirements, the pathway to cost reduction appears promising. Advances in green chemistry approaches for quinone synthesis, coupled with economies of scale, are projected to drive significant cost reductions over the next decade, potentially positioning these systems as economically competitive alternatives to conventional energy storage technologies.
Life cycle assessment (LCA) studies indicate that quinone-based electrolytes demonstrate lower greenhouse gas emissions during production compared to vanadium-based alternatives. The carbon intensity of manufacturing quinone compounds can be further reduced when biomass-derived precursors replace petroleum-based feedstocks. Additionally, the water-soluble nature of many quinone derivatives eliminates the need for toxic organic solvents in electrolyte formulation, significantly reducing volatile organic compound (VOC) emissions and associated health risks.
The biodegradability of quinone compounds presents both advantages and challenges from an environmental perspective. While enhanced biodegradability reduces end-of-life environmental impact, it necessitates careful consideration of electrolyte stability during operation. Recent research has focused on developing quinone derivatives with optimized stability-biodegradability profiles to ensure long-term performance while maintaining environmental benefits.
Water usage represents another critical sustainability metric for flow battery technologies. Aqueous quinone-based systems typically require less water than mining-intensive battery technologies when evaluated across their complete life cycle. However, water management strategies remain essential, particularly for large-scale deployment in water-stressed regions. Closed-loop cooling systems and electrolyte recycling protocols can further minimize the water footprint of these systems.
Safety considerations also favor quinone-based electrolytes, which generally present lower flammability risks compared to lithium-ion batteries and reduced toxicity compared to vanadium-based flow batteries. This enhanced safety profile reduces the potential for environmental contamination from accidental releases and simplifies emergency response protocols, contributing to overall system sustainability.
Economic sustainability analysis reveals that while current quinone-based systems face cost challenges related to synthesis complexity and membrane requirements, the pathway to cost reduction appears promising. Advances in green chemistry approaches for quinone synthesis, coupled with economies of scale, are projected to drive significant cost reductions over the next decade, potentially positioning these systems as economically competitive alternatives to conventional energy storage technologies.
Scalability and Commercial Viability Analysis
The scalability of quinone-based electrolytes represents a critical factor in determining their commercial viability for flow battery applications. Current manufacturing processes for quinone compounds remain limited to laboratory or small-batch production scales, presenting significant challenges for industrial deployment. Production costs range from $100-500/kg for research-grade materials, which must be reduced to $15-20/kg to achieve cost parity with vanadium-based systems. This cost reduction necessitates development of streamlined synthetic pathways and optimization of purification processes.
Supply chain considerations further complicate scalability, as many quinone precursors derive from petroleum industry byproducts or specialized organic synthesis routes. Establishing reliable, high-volume supply chains requires strategic partnerships with chemical manufacturers and potentially vertical integration of key production steps. Several companies, including ESS Tech and JenaBatteries, have made progress in scaling production to hundreds of kilograms, but multi-ton production remains elusive.
Commercial viability analysis indicates that quinone-based flow batteries currently demonstrate levelized cost of storage (LCOS) values between $0.20-0.30/kWh-cycle, compared to $0.15-0.25/kWh-cycle for vanadium systems. However, quinones offer significant advantages in material abundance and environmental impact. Market entry strategies likely require targeting niche applications where these advantages outweigh cost considerations, such as environmentally sensitive areas or regions with restricted access to vanadium resources.
Regulatory frameworks present another dimension affecting commercial deployment. Quinone-based systems generally face fewer transportation and handling restrictions than vanadium-based alternatives, potentially reducing operational costs. However, end-of-life considerations and recycling protocols remain underdeveloped, creating uncertainty in lifecycle economics.
Investment trends show increasing interest, with venture capital funding for quinone flow battery startups reaching approximately $120 million in 2022, a 40% increase from 2020. This suggests growing confidence in the technology's commercial potential despite remaining challenges. Strategic partnerships between research institutions and industrial players have emerged as a key model for advancing commercialization, with several university spin-offs securing industry backing for scale-up initiatives.
Supply chain considerations further complicate scalability, as many quinone precursors derive from petroleum industry byproducts or specialized organic synthesis routes. Establishing reliable, high-volume supply chains requires strategic partnerships with chemical manufacturers and potentially vertical integration of key production steps. Several companies, including ESS Tech and JenaBatteries, have made progress in scaling production to hundreds of kilograms, but multi-ton production remains elusive.
Commercial viability analysis indicates that quinone-based flow batteries currently demonstrate levelized cost of storage (LCOS) values between $0.20-0.30/kWh-cycle, compared to $0.15-0.25/kWh-cycle for vanadium systems. However, quinones offer significant advantages in material abundance and environmental impact. Market entry strategies likely require targeting niche applications where these advantages outweigh cost considerations, such as environmentally sensitive areas or regions with restricted access to vanadium resources.
Regulatory frameworks present another dimension affecting commercial deployment. Quinone-based systems generally face fewer transportation and handling restrictions than vanadium-based alternatives, potentially reducing operational costs. However, end-of-life considerations and recycling protocols remain underdeveloped, creating uncertainty in lifecycle economics.
Investment trends show increasing interest, with venture capital funding for quinone flow battery startups reaching approximately $120 million in 2022, a 40% increase from 2020. This suggests growing confidence in the technology's commercial potential despite remaining challenges. Strategic partnerships between research institutions and industrial players have emerged as a key model for advancing commercialization, with several university spin-offs securing industry backing for scale-up initiatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!