Redox Flow Systems Using Metal Organic Complexes
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Organic Redox Flow Battery Background and Objectives
The evolution of energy storage technologies has witnessed significant advancements over the past decades, with redox flow batteries (RFBs) emerging as promising candidates for grid-scale energy storage applications. Metal organic complexes in redox flow systems represent a cutting-edge approach that combines the versatility of coordination chemistry with the scalability of flow battery architecture. This technological domain has evolved from traditional vanadium-based systems toward more sustainable and cost-effective alternatives utilizing metal organic complexes.
The development trajectory of metal organic redox flow batteries can be traced back to the early 2000s, when researchers began exploring alternatives to conventional inorganic redox couples. The field gained momentum around 2010-2015 with breakthrough publications demonstrating the feasibility of metal coordination complexes as active materials in flow batteries. Recent years have witnessed accelerated research interest, driven by the urgent need for renewable energy integration and grid stabilization solutions.
Metal organic complexes offer distinct advantages in redox flow systems, including tunable redox potentials through ligand modification, enhanced solubility in various electrolytes, and potentially lower material costs compared to precious metal alternatives. The molecular engineering approach enables precise control over electrochemical properties, potentially addressing key limitations of traditional RFB technologies such as energy density constraints and crossover issues.
The primary technical objectives in this field include achieving higher energy densities (>40 Wh/L), extending cycle life (>5000 cycles), reducing system costs (<$150/kWh), and developing environmentally benign electrolyte formulations. Researchers aim to design metal organic complexes with multi-electron transfer capabilities, rapid kinetics, and exceptional stability under charging-discharging conditions. Additionally, there is growing interest in developing bipolar metal organic complexes that can function in single-electrolyte systems.
Current research trends indicate a shift toward earth-abundant metals (iron, manganese, zinc) coordinated with carefully designed organic ligands. This approach aligns with sustainability goals while potentially reducing material costs. Parallel efforts focus on developing compatible membranes and electrode materials optimized for metal organic redox chemistry, as well as advanced computational methods to predict and design novel complexes with desired properties.
The convergence of coordination chemistry, electrochemistry, and materials science in this field presents unique opportunities for interdisciplinary innovation. As renewable energy deployment accelerates globally, the development of efficient, cost-effective energy storage solutions becomes increasingly critical, positioning metal organic redox flow batteries as a strategically important technology for enabling the clean energy transition.
The development trajectory of metal organic redox flow batteries can be traced back to the early 2000s, when researchers began exploring alternatives to conventional inorganic redox couples. The field gained momentum around 2010-2015 with breakthrough publications demonstrating the feasibility of metal coordination complexes as active materials in flow batteries. Recent years have witnessed accelerated research interest, driven by the urgent need for renewable energy integration and grid stabilization solutions.
Metal organic complexes offer distinct advantages in redox flow systems, including tunable redox potentials through ligand modification, enhanced solubility in various electrolytes, and potentially lower material costs compared to precious metal alternatives. The molecular engineering approach enables precise control over electrochemical properties, potentially addressing key limitations of traditional RFB technologies such as energy density constraints and crossover issues.
The primary technical objectives in this field include achieving higher energy densities (>40 Wh/L), extending cycle life (>5000 cycles), reducing system costs (<$150/kWh), and developing environmentally benign electrolyte formulations. Researchers aim to design metal organic complexes with multi-electron transfer capabilities, rapid kinetics, and exceptional stability under charging-discharging conditions. Additionally, there is growing interest in developing bipolar metal organic complexes that can function in single-electrolyte systems.
Current research trends indicate a shift toward earth-abundant metals (iron, manganese, zinc) coordinated with carefully designed organic ligands. This approach aligns with sustainability goals while potentially reducing material costs. Parallel efforts focus on developing compatible membranes and electrode materials optimized for metal organic redox chemistry, as well as advanced computational methods to predict and design novel complexes with desired properties.
The convergence of coordination chemistry, electrochemistry, and materials science in this field presents unique opportunities for interdisciplinary innovation. As renewable energy deployment accelerates globally, the development of efficient, cost-effective energy storage solutions becomes increasingly critical, positioning metal organic redox flow batteries as a strategically important technology for enabling the clean energy transition.
Market Analysis for Metal Organic Complex Energy Storage
The global market for energy storage solutions is experiencing unprecedented growth, with redox flow batteries (RFBs) emerging as a promising technology for grid-scale applications. Metal organic complexes represent a significant innovation within this sector, offering potential advantages over traditional vanadium-based systems. Current market valuations place the global flow battery market at approximately $290 million as of 2022, with projections indicating growth to reach $1.1 billion by 2027, representing a compound annual growth rate (CAGR) of 30.4%.
Metal organic complex-based flow batteries are positioned to capture a growing share of this expanding market due to their enhanced energy density, reduced material costs, and improved environmental profile compared to conventional systems. Market research indicates that the cost reduction potential of these systems could be as high as 60% compared to vanadium-based alternatives, primarily through the replacement of expensive metal components with organic or metal-organic alternatives.
Demand drivers for metal organic complex energy storage include the accelerating global transition to renewable energy sources, increasing grid instability issues, and the need for long-duration energy storage solutions. Particularly strong market potential exists in regions with high renewable penetration such as Europe, California, and parts of Asia-Pacific, where grid stabilization requirements are creating urgent demand for flexible storage solutions.
Industry analysts have identified several key market segments for metal organic complex flow batteries: utility-scale storage (representing the largest potential market share at 45%), commercial and industrial applications (30%), microgrids (15%), and emerging applications including electric vehicle charging infrastructure (10%). The utility segment shows particular promise due to the technology's scalability and long discharge duration capabilities.
Regulatory environments are increasingly favorable for advanced energy storage technologies, with policies in major markets including investment tax credits, capacity payments, and renewable portfolio standards that directly benefit flow battery deployment. The European Union's Green Deal and similar initiatives in North America and Asia are creating substantial market pull for innovative storage solutions.
Customer adoption barriers remain significant, including concerns about technology maturity, system reliability, and operational complexity. Market research indicates that early commercial deployments demonstrating 10+ year operational lifetimes with minimal maintenance requirements will be critical for widespread market acceptance. Price sensitivity varies by application, with utility customers focused primarily on levelized cost of storage (LCOS), while commercial customers often prioritize total cost of ownership including installation and maintenance considerations.
Metal organic complex-based flow batteries are positioned to capture a growing share of this expanding market due to their enhanced energy density, reduced material costs, and improved environmental profile compared to conventional systems. Market research indicates that the cost reduction potential of these systems could be as high as 60% compared to vanadium-based alternatives, primarily through the replacement of expensive metal components with organic or metal-organic alternatives.
Demand drivers for metal organic complex energy storage include the accelerating global transition to renewable energy sources, increasing grid instability issues, and the need for long-duration energy storage solutions. Particularly strong market potential exists in regions with high renewable penetration such as Europe, California, and parts of Asia-Pacific, where grid stabilization requirements are creating urgent demand for flexible storage solutions.
Industry analysts have identified several key market segments for metal organic complex flow batteries: utility-scale storage (representing the largest potential market share at 45%), commercial and industrial applications (30%), microgrids (15%), and emerging applications including electric vehicle charging infrastructure (10%). The utility segment shows particular promise due to the technology's scalability and long discharge duration capabilities.
Regulatory environments are increasingly favorable for advanced energy storage technologies, with policies in major markets including investment tax credits, capacity payments, and renewable portfolio standards that directly benefit flow battery deployment. The European Union's Green Deal and similar initiatives in North America and Asia are creating substantial market pull for innovative storage solutions.
Customer adoption barriers remain significant, including concerns about technology maturity, system reliability, and operational complexity. Market research indicates that early commercial deployments demonstrating 10+ year operational lifetimes with minimal maintenance requirements will be critical for widespread market acceptance. Price sensitivity varies by application, with utility customers focused primarily on levelized cost of storage (LCOS), while commercial customers often prioritize total cost of ownership including installation and maintenance considerations.
Technical Challenges in Metal Organic Redox Flow Systems
Metal organic complexes in redox flow batteries (RFBs) face several significant technical challenges that impede their widespread commercial adoption. The primary obstacle lies in the stability of these complexes during extended charge-discharge cycles. Metal organic compounds often undergo degradation through various mechanisms including ligand dissociation, metal center oxidation state changes beyond reversible limits, and unwanted side reactions with electrolyte components or trace impurities like oxygen and water.
Solubility limitations represent another major challenge. While high solubility is essential for achieving competitive energy densities, many promising metal organic complexes exhibit insufficient solubility in conventional electrolyte systems. This constraint directly impacts the practical energy density achievable in these systems, making them less competitive against conventional energy storage technologies.
Crossover of active species through the membrane separator presents a persistent technical hurdle. The relatively small size of many metal organic complexes compared to polymeric redox active materials facilitates their migration across membranes, leading to capacity fade and efficiency losses. This phenomenon necessitates the development of specialized membranes or larger molecular architectures to mitigate crossover effects.
Electrochemical kinetics pose additional challenges. Some metal organic complexes exhibit slow electron transfer rates, resulting in high overpotentials during operation. This inefficiency manifests as energy losses and reduced round-trip efficiency, a critical parameter for economic viability of flow battery systems.
The cost factor remains a significant barrier to commercialization. Many high-performance metal organic complexes rely on precious metals or complex synthetic pathways, driving up system costs. The synthesis scalability of these compounds often involves multi-step processes with low overall yields, further exacerbating economic challenges.
Environmental and safety concerns must also be addressed. Certain metal complexes may present toxicity risks or environmental hazards if released, necessitating robust containment systems and end-of-life management strategies. The long-term environmental impact of these materials remains inadequately characterized in many cases.
Temperature sensitivity affects operational flexibility, as many metal organic complexes show significant performance variations across temperature ranges. This characteristic limits deployment in regions with extreme climates or necessitates additional temperature control systems, increasing system complexity and cost.
Solubility limitations represent another major challenge. While high solubility is essential for achieving competitive energy densities, many promising metal organic complexes exhibit insufficient solubility in conventional electrolyte systems. This constraint directly impacts the practical energy density achievable in these systems, making them less competitive against conventional energy storage technologies.
Crossover of active species through the membrane separator presents a persistent technical hurdle. The relatively small size of many metal organic complexes compared to polymeric redox active materials facilitates their migration across membranes, leading to capacity fade and efficiency losses. This phenomenon necessitates the development of specialized membranes or larger molecular architectures to mitigate crossover effects.
Electrochemical kinetics pose additional challenges. Some metal organic complexes exhibit slow electron transfer rates, resulting in high overpotentials during operation. This inefficiency manifests as energy losses and reduced round-trip efficiency, a critical parameter for economic viability of flow battery systems.
The cost factor remains a significant barrier to commercialization. Many high-performance metal organic complexes rely on precious metals or complex synthetic pathways, driving up system costs. The synthesis scalability of these compounds often involves multi-step processes with low overall yields, further exacerbating economic challenges.
Environmental and safety concerns must also be addressed. Certain metal complexes may present toxicity risks or environmental hazards if released, necessitating robust containment systems and end-of-life management strategies. The long-term environmental impact of these materials remains inadequately characterized in many cases.
Temperature sensitivity affects operational flexibility, as many metal organic complexes show significant performance variations across temperature ranges. This characteristic limits deployment in regions with extreme climates or necessitates additional temperature control systems, increasing system complexity and cost.
Current Metal Organic Complex Electrolyte Solutions
01 Metal organic complexes as active materials in redox flow batteries
Metal organic complexes can be used as active materials in redox flow batteries due to their tunable redox properties and stability. These complexes typically consist of metal centers coordinated with organic ligands, providing multiple oxidation states suitable for energy storage applications. The complexes can be dissolved in appropriate electrolytes to create anolytes and catholytes with high energy density and good electrochemical reversibility, enhancing the overall performance of redox flow systems.- Metal organic complexes as active materials in redox flow batteries: Metal organic complexes can be used as active materials in redox flow batteries due to their tunable redox properties and stability. These complexes typically consist of metal centers coordinated with organic ligands, providing multiple oxidation states suitable for energy storage applications. The use of these complexes can enhance the energy density and cycling stability of redox flow systems compared to traditional inorganic materials.
- Electrolyte compositions containing metal organic complexes: Specialized electrolyte compositions containing metal organic complexes can be formulated for redox flow systems. These electrolytes typically include supporting salts, solvents, and additives that enhance the solubility and stability of the metal organic complexes. The composition can be optimized to improve ionic conductivity, reduce membrane crossover, and enhance the overall performance of the redox flow battery system.
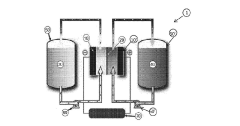
- Cell design and system architecture for metal organic complex-based flow batteries: Specific cell designs and system architectures have been developed to accommodate metal organic complex-based flow batteries. These designs address unique challenges such as electrolyte circulation, electrode materials compatible with metal organic complexes, and membrane selection to prevent crossover. The system architecture may include specialized pumps, tanks, and thermal management systems optimized for the chemical properties of metal organic complexes.
- Control systems and methods for redox flow batteries using metal organic complexes: Advanced control systems and methods have been developed specifically for redox flow batteries utilizing metal organic complexes. These systems monitor and regulate parameters such as flow rate, temperature, state of charge, and system pressure to optimize performance and prevent degradation of the metal organic complexes. The control methods may include predictive algorithms that adjust operating conditions based on the unique electrochemical behavior of these complexes.
- Novel metal organic complex structures for enhanced performance: Innovative metal organic complex structures have been designed specifically for redox flow battery applications. These structures feature modified ligand designs, alternative metal centers, or hybrid structures that combine multiple functional groups. The novel complexes aim to address challenges such as solubility limitations, stability during cycling, and redox potential tuning to achieve higher energy density and longer cycle life in flow battery systems.
02 Electrolyte compositions for metal organic complex-based flow batteries
Specialized electrolyte compositions are essential for optimizing the performance of metal organic complex-based flow batteries. These electrolytes typically contain supporting salts, solvents, and additives that enhance the solubility and stability of the metal organic complexes. The composition can be tailored to improve ionic conductivity, reduce membrane crossover, and extend the operational lifetime of the battery. Proper electrolyte formulation also helps to mitigate side reactions and improve the overall energy efficiency of the redox flow system.Expand Specific Solutions03 Cell design and system architecture for metal organic redox flow batteries
Innovative cell designs and system architectures are crucial for maximizing the performance of metal organic redox flow batteries. These designs focus on optimizing flow distribution, minimizing pressure drop, and enhancing mass transfer at the electrode surfaces. Advanced system architectures incorporate features such as integrated sensors for state-of-charge monitoring, thermal management systems, and automated electrolyte balancing mechanisms. Proper cell and system design can significantly improve power density, energy efficiency, and operational reliability of metal organic complex-based redox flow systems.Expand Specific Solutions04 Membrane technologies for metal organic redox flow systems
Specialized membrane technologies are developed for metal organic redox flow systems to prevent crossover of active species while maintaining high ionic conductivity. These membranes are designed to be chemically stable in the presence of metal organic complexes and their oxidized/reduced forms. Advanced membrane materials include modified perfluorosulfonic acid polymers, hydrocarbon-based ionomers, and composite structures with tailored pore sizes. The membrane properties significantly impact the coulombic efficiency, voltage efficiency, and cycle life of metal organic complex-based redox flow batteries.Expand Specific Solutions05 Integration and control systems for metal organic redox flow batteries
Advanced integration and control systems are essential for the practical implementation of metal organic redox flow batteries in grid-scale energy storage applications. These systems include power electronics for AC/DC conversion, battery management systems for monitoring state-of-charge and state-of-health, and control algorithms for optimizing charging/discharging protocols. Sophisticated integration approaches enable the coordination of multiple flow battery modules and their integration with renewable energy sources. Effective control systems can extend battery lifetime, improve safety, and enhance the economic viability of metal organic complex-based redox flow energy storage solutions.Expand Specific Solutions
Leading Research Groups and Industrial Players
The redox flow systems using metal organic complexes market is in an early growth phase, characterized by significant research activity but limited commercial deployment. The market size is projected to expand substantially as energy storage demands increase, with an estimated CAGR of 15-20% over the next five years. Technologically, the field shows varying maturity levels across different applications. Leading academic institutions like MIT, University of California, and ETH Zurich are driving fundamental research, while companies such as Sumitomo Electric, Lockheed Martin Advanced Energy Storage, and KEMIWATT are advancing commercial applications. Emerging players like Storagenergy Technologies and Sudoc LLC are introducing innovative approaches, focusing on sustainability and performance improvements. The competitive landscape reflects a blend of established industrial conglomerates and specialized startups working to overcome cost and scalability challenges.
The Regents of the University of California
Technical Solution: The University of California has developed significant innovations in metal organic complex-based redox flow systems through their research on metallo-organic coordination polymers. Their approach utilizes transition metal centers (primarily Fe, Cu, and Mn) coordinated with organic ligands to create highly soluble and electrochemically active species. UC researchers have demonstrated aqueous systems achieving concentrations of 1.2M with voltage windows exceeding 2.0V through careful pH control and supporting electrolyte optimization. Their technology incorporates novel membrane materials based on sulfonated aromatic polymers that demonstrate exceptional selectivity while maintaining conductivity above 100 mS/cm. The UC system achieves power densities of 200-250 mW/cm² and energy efficiencies approaching 80% under practical operating conditions. Their research has also pioneered computational screening methods that have identified over 50 promising metal-ligand combinations with optimized redox properties and solubility characteristics for next-generation flow battery applications.
Strengths: Utilizes earth-abundant materials; operates in aqueous electrolytes enhancing safety; demonstrates good power performance and efficiency. Weaknesses: Some metal-organic complexes show degradation pathways during extended cycling; membrane technology requires further development to reduce costs; system complexity increases with supporting electrolyte requirements.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered innovative redox flow systems using metal organic complexes through their development of the "metallocene flow battery" technology. Their research utilizes ferrocene and viologen-based complexes as active materials in non-aqueous electrolytes, achieving theoretical energy densities up to 50 Wh/L. MIT's approach incorporates tailored ligand designs that enhance solubility limits (exceeding 1.0M concentration) while maintaining electrochemical reversibility. Their system employs specialized size-exclusion membranes that effectively prevent active material crossover while facilitating rapid ion transport. MIT researchers have demonstrated prototype systems with round-trip efficiencies of 85-90% and minimal capacity fade (<0.01% per cycle) over extended cycling. The technology incorporates computational modeling to optimize molecular structures for specific electrochemical properties, enabling precise tuning of redox potentials and reaction kinetics for different application requirements.
Strengths: Exceptional energy density potential; highly tunable redox properties through molecular engineering; demonstrated long-term cycling stability. Weaknesses: Non-aqueous electrolytes may present safety concerns; higher material costs compared to traditional flow battery chemistries; technology still primarily at laboratory scale rather than commercial deployment.
Key Patents and Scientific Breakthroughs
Asymmetric metal complex as an active material of a flow battery
PatentActiveUS20220093927A1
Innovation
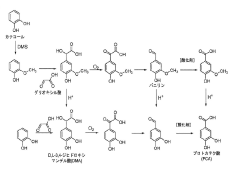
- The use of asymmetrical metal complexes coordinated with two to six hydrophilic ligands, including specific metal centers like iron and bipyridine ligands, to enhance solubility and modulate electrochemical properties, thereby increasing redox potential and stability.
Metal complexe of substituted catecholate and redox flow battery containing the same
PatentActiveJP2021064608A
Innovation
- The use of substituted catecholate ligands in coordination compounds to enhance the solubility and stability of active materials in flow battery electrolytes, preventing cross-contamination and improving energy density.
Environmental Impact and Sustainability Assessment
The environmental impact of redox flow systems using metal organic complexes represents a critical dimension in evaluating their viability as sustainable energy storage solutions. These systems offer significant environmental advantages compared to conventional battery technologies, particularly in terms of reduced reliance on rare earth metals and environmentally harmful materials. Metal organic complexes typically utilize more abundant transition metals such as iron, vanadium, and chromium, which can be sourced with lower environmental footprints than lithium or cobalt used in conventional batteries.
Life cycle assessment (LCA) studies indicate that redox flow batteries with metal organic complexes demonstrate lower carbon emissions during manufacturing processes compared to lithium-ion alternatives. The primary environmental benefit stems from the separation of power and energy components, allowing for longer operational lifespans and reduced material replacement requirements. Most metal organic complexes used in these systems demonstrate degradation patterns that produce fewer toxic byproducts than conventional battery chemistries.
Water usage represents a significant environmental consideration for these systems, as most redox flow batteries require aqueous electrolytes. Recent innovations in metal organic complex formulations have focused on reducing water requirements through higher energy density solutions and more efficient electrolyte compositions. Additionally, research indicates that properly designed systems can implement closed-loop water recycling mechanisms, substantially reducing the overall water footprint during operation.
End-of-life management presents both challenges and opportunities for metal organic complex-based flow systems. The recyclability of these materials exceeds that of conventional battery technologies, with recovery rates for transition metals potentially reaching 85-95% with appropriate processing techniques. The organic components can be designed for biodegradability or thermal recovery, further enhancing the sustainability profile of these systems.
Energy return on investment (EROI) analyses demonstrate favorable outcomes for metal organic complex flow batteries, particularly in grid-scale applications where their long cycle life (often exceeding 10,000 cycles) compensates for the initial energy investment in manufacturing. This contrasts favorably with lithium-ion technologies, which typically require replacement after 1,000-3,000 cycles in similar applications.
Regulatory frameworks are evolving to address the specific environmental considerations of these emerging technologies. The European Union's Battery Directive and similar regulations in North America are beginning to incorporate provisions specifically addressing flow battery technologies, with particular emphasis on recyclability requirements and restrictions on hazardous materials. These regulatory developments will significantly influence the environmental compliance landscape for metal organic complex flow systems in coming years.
Life cycle assessment (LCA) studies indicate that redox flow batteries with metal organic complexes demonstrate lower carbon emissions during manufacturing processes compared to lithium-ion alternatives. The primary environmental benefit stems from the separation of power and energy components, allowing for longer operational lifespans and reduced material replacement requirements. Most metal organic complexes used in these systems demonstrate degradation patterns that produce fewer toxic byproducts than conventional battery chemistries.
Water usage represents a significant environmental consideration for these systems, as most redox flow batteries require aqueous electrolytes. Recent innovations in metal organic complex formulations have focused on reducing water requirements through higher energy density solutions and more efficient electrolyte compositions. Additionally, research indicates that properly designed systems can implement closed-loop water recycling mechanisms, substantially reducing the overall water footprint during operation.
End-of-life management presents both challenges and opportunities for metal organic complex-based flow systems. The recyclability of these materials exceeds that of conventional battery technologies, with recovery rates for transition metals potentially reaching 85-95% with appropriate processing techniques. The organic components can be designed for biodegradability or thermal recovery, further enhancing the sustainability profile of these systems.
Energy return on investment (EROI) analyses demonstrate favorable outcomes for metal organic complex flow batteries, particularly in grid-scale applications where their long cycle life (often exceeding 10,000 cycles) compensates for the initial energy investment in manufacturing. This contrasts favorably with lithium-ion technologies, which typically require replacement after 1,000-3,000 cycles in similar applications.
Regulatory frameworks are evolving to address the specific environmental considerations of these emerging technologies. The European Union's Battery Directive and similar regulations in North America are beginning to incorporate provisions specifically addressing flow battery technologies, with particular emphasis on recyclability requirements and restrictions on hazardous materials. These regulatory developments will significantly influence the environmental compliance landscape for metal organic complex flow systems in coming years.
Scale-up and Commercialization Roadmap
The commercialization pathway for metal organic complex-based redox flow batteries (MOC-RFBs) requires strategic planning across multiple dimensions. Initial scale-up efforts must focus on optimizing electrolyte formulations that maintain stability and performance at industrial volumes. Laboratory-scale synthesis protocols need systematic adaptation to accommodate ton-scale production while maintaining precise quality control parameters and reducing production costs.
Manufacturing infrastructure represents a critical investment phase, requiring specialized equipment for metal complex synthesis, membrane fabrication, and system assembly. Strategic partnerships with chemical manufacturers can accelerate this transition by leveraging existing production capabilities and supply chains. Early commercial deployments should target niche applications where the unique advantages of MOC-RFBs—such as potentially higher energy density and lower toxicity compared to vanadium systems—provide compelling value propositions.
Cost reduction trajectories must be clearly mapped, identifying key materials and components driving expenses. Current projections indicate that metal organic complex electrolytes could achieve cost parity with traditional systems within 5-7 years through economies of scale and continued materials innovation. Standardization efforts will be essential for market acceptance, requiring industry collaboration to establish performance metrics, safety protocols, and interoperability standards.
Regulatory pathways present both challenges and opportunities. Environmental impact assessments must demonstrate the sustainability advantages of MOC-RFBs, particularly regarding reduced toxicity and improved recyclability compared to conventional battery technologies. Certification processes for grid-connected energy storage applications vary significantly by region, necessitating tailored compliance strategies for different markets.
Market entry strategies should follow a phased approach, beginning with specialized applications in microgrids, telecommunications backup, and remote power systems before expanding to utility-scale implementations. Strategic partnerships with established energy storage integrators can provide access to existing distribution channels and customer relationships, accelerating commercial adoption.
Investment requirements follow a staged funding model, with initial R&D phases requiring $5-10 million, pilot manufacturing facilities needing $20-50 million, and full commercial scale-up potentially requiring $100+ million. Return on investment timelines typically project 3-5 years for early commercial applications and 7-10 years for achieving significant market penetration in grid-scale storage markets.
Manufacturing infrastructure represents a critical investment phase, requiring specialized equipment for metal complex synthesis, membrane fabrication, and system assembly. Strategic partnerships with chemical manufacturers can accelerate this transition by leveraging existing production capabilities and supply chains. Early commercial deployments should target niche applications where the unique advantages of MOC-RFBs—such as potentially higher energy density and lower toxicity compared to vanadium systems—provide compelling value propositions.
Cost reduction trajectories must be clearly mapped, identifying key materials and components driving expenses. Current projections indicate that metal organic complex electrolytes could achieve cost parity with traditional systems within 5-7 years through economies of scale and continued materials innovation. Standardization efforts will be essential for market acceptance, requiring industry collaboration to establish performance metrics, safety protocols, and interoperability standards.
Regulatory pathways present both challenges and opportunities. Environmental impact assessments must demonstrate the sustainability advantages of MOC-RFBs, particularly regarding reduced toxicity and improved recyclability compared to conventional battery technologies. Certification processes for grid-connected energy storage applications vary significantly by region, necessitating tailored compliance strategies for different markets.
Market entry strategies should follow a phased approach, beginning with specialized applications in microgrids, telecommunications backup, and remote power systems before expanding to utility-scale implementations. Strategic partnerships with established energy storage integrators can provide access to existing distribution channels and customer relationships, accelerating commercial adoption.
Investment requirements follow a staged funding model, with initial R&D phases requiring $5-10 million, pilot manufacturing facilities needing $20-50 million, and full commercial scale-up potentially requiring $100+ million. Return on investment timelines typically project 3-5 years for early commercial applications and 7-10 years for achieving significant market penetration in grid-scale storage markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!