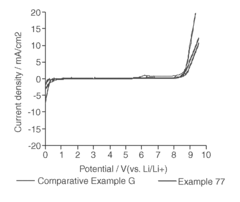
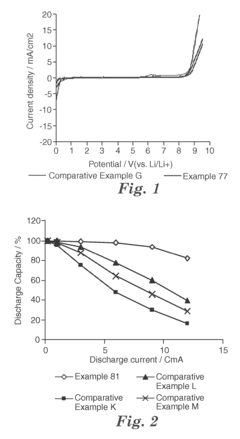
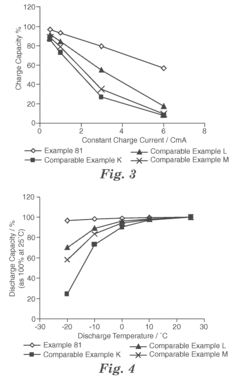
Solvent Engineering in High Performance Flow Battery Electrolytes
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flow Battery Electrolyte Development History and Objectives
Flow batteries emerged in the 1970s as a promising energy storage technology, with NASA's early development of iron-chromium systems marking a significant milestone. The 1980s witnessed the introduction of the vanadium redox flow battery (VRFB) by Maria Skyllas-Kazacos at the University of New South Wales, revolutionizing the field with its single-element chemistry that minimized cross-contamination issues. Throughout the 1990s and early 2000s, research expanded to include various chemistries such as zinc-bromine, polysulfide-bromine, and organic-based systems.
Electrolyte development has been central to flow battery advancement, evolving from simple aqueous solutions to sophisticated engineered solvents. Early systems primarily utilized water-based electrolytes due to their safety, low cost, and high ionic conductivity. However, these systems faced limitations in energy density and operating temperature ranges. The introduction of mixed solvent systems in the late 2000s represented a significant breakthrough, combining water with organic co-solvents to enhance solubility and electrochemical stability.
The past decade has seen accelerated innovation in solvent engineering, with researchers exploring deep eutectic solvents, ionic liquids, and non-aqueous systems to overcome the inherent limitations of purely aqueous electrolytes. These advanced solvent systems aim to expand the electrochemical window, increase active material solubility, and improve temperature resilience—all critical factors for enhancing energy density and overall performance.
Current research objectives in flow battery electrolyte development focus on several key areas. Increasing energy density remains paramount, with targets exceeding 40 Wh/L to compete with lithium-ion technologies. Enhancing cycle life through stable electrolyte formulations that minimize capacity fade is essential for long-term economic viability. Researchers also aim to develop electrolytes with broader temperature operating ranges (-20°C to 60°C) to reduce system complexity and costs associated with thermal management.
Cost reduction represents another critical objective, with efforts to move away from expensive vanadium-based chemistries toward abundant organic materials and earth-abundant metals. Environmental considerations have gained prominence, driving research toward non-toxic, biodegradable electrolyte components that minimize ecological impact throughout the battery lifecycle.
The ultimate goal of current solvent engineering efforts is to develop "designer electrolytes" with precisely tuned properties for specific applications, whether for grid-scale storage, renewable integration, or emerging markets like electric vehicle charging infrastructure. This tailored approach recognizes that no single electrolyte formulation will meet all requirements across diverse deployment scenarios.
Electrolyte development has been central to flow battery advancement, evolving from simple aqueous solutions to sophisticated engineered solvents. Early systems primarily utilized water-based electrolytes due to their safety, low cost, and high ionic conductivity. However, these systems faced limitations in energy density and operating temperature ranges. The introduction of mixed solvent systems in the late 2000s represented a significant breakthrough, combining water with organic co-solvents to enhance solubility and electrochemical stability.
The past decade has seen accelerated innovation in solvent engineering, with researchers exploring deep eutectic solvents, ionic liquids, and non-aqueous systems to overcome the inherent limitations of purely aqueous electrolytes. These advanced solvent systems aim to expand the electrochemical window, increase active material solubility, and improve temperature resilience—all critical factors for enhancing energy density and overall performance.
Current research objectives in flow battery electrolyte development focus on several key areas. Increasing energy density remains paramount, with targets exceeding 40 Wh/L to compete with lithium-ion technologies. Enhancing cycle life through stable electrolyte formulations that minimize capacity fade is essential for long-term economic viability. Researchers also aim to develop electrolytes with broader temperature operating ranges (-20°C to 60°C) to reduce system complexity and costs associated with thermal management.
Cost reduction represents another critical objective, with efforts to move away from expensive vanadium-based chemistries toward abundant organic materials and earth-abundant metals. Environmental considerations have gained prominence, driving research toward non-toxic, biodegradable electrolyte components that minimize ecological impact throughout the battery lifecycle.
The ultimate goal of current solvent engineering efforts is to develop "designer electrolytes" with precisely tuned properties for specific applications, whether for grid-scale storage, renewable integration, or emerging markets like electric vehicle charging infrastructure. This tailored approach recognizes that no single electrolyte formulation will meet all requirements across diverse deployment scenarios.
Market Analysis for Advanced Flow Battery Technologies
The global flow battery market is experiencing significant growth, projected to reach $1.11 billion by 2027, with a compound annual growth rate of 28.7% from 2020. This expansion is primarily driven by increasing demand for long-duration energy storage solutions that can effectively support renewable energy integration into power grids. Flow batteries, with their scalable capacity and long cycle life, are particularly well-positioned to address the intermittency challenges of renewable energy sources.
The market segmentation reveals distinct application sectors, with utility-scale storage representing the largest market share at approximately 60%. Commercial and industrial applications follow at 25%, while emerging applications in microgrids and remote power systems account for the remaining 15%. Geographically, Asia-Pacific leads the market with 40% share, followed by North America (30%) and Europe (20%), with the rest of the world comprising 10%.
Key market drivers include the declining costs of renewable energy generation, stringent environmental regulations promoting clean energy adoption, and increasing grid stability concerns. The levelized cost of storage for flow batteries has decreased by 35% over the past five years, enhancing their competitive position against lithium-ion alternatives for long-duration applications exceeding four hours.
Market barriers remain significant, including high upfront capital costs, limited commercial deployment history, and competition from established battery technologies. The average installation cost for vanadium redox flow batteries currently ranges between $400-700/kWh, still higher than lithium-ion systems at $200-300/kWh, though this gap is narrowing for applications requiring longer discharge durations.
Customer requirements are evolving toward systems with higher energy density, improved round-trip efficiency, and reduced maintenance needs. End-users increasingly demand electrolyte formulations that can operate across wider temperature ranges (-20°C to 60°C) without performance degradation, presenting both challenges and opportunities for solvent engineering innovations.
The competitive landscape features established players like Sumitomo Electric, VRB Energy, and RedT Energy, alongside emerging companies such as ESS Inc., Primus Power, and Invinity Energy Systems. Strategic partnerships between electrolyte developers and system integrators are becoming increasingly common, creating new market dynamics and accelerating commercialization pathways for advanced electrolyte formulations.
The market segmentation reveals distinct application sectors, with utility-scale storage representing the largest market share at approximately 60%. Commercial and industrial applications follow at 25%, while emerging applications in microgrids and remote power systems account for the remaining 15%. Geographically, Asia-Pacific leads the market with 40% share, followed by North America (30%) and Europe (20%), with the rest of the world comprising 10%.
Key market drivers include the declining costs of renewable energy generation, stringent environmental regulations promoting clean energy adoption, and increasing grid stability concerns. The levelized cost of storage for flow batteries has decreased by 35% over the past five years, enhancing their competitive position against lithium-ion alternatives for long-duration applications exceeding four hours.
Market barriers remain significant, including high upfront capital costs, limited commercial deployment history, and competition from established battery technologies. The average installation cost for vanadium redox flow batteries currently ranges between $400-700/kWh, still higher than lithium-ion systems at $200-300/kWh, though this gap is narrowing for applications requiring longer discharge durations.
Customer requirements are evolving toward systems with higher energy density, improved round-trip efficiency, and reduced maintenance needs. End-users increasingly demand electrolyte formulations that can operate across wider temperature ranges (-20°C to 60°C) without performance degradation, presenting both challenges and opportunities for solvent engineering innovations.
The competitive landscape features established players like Sumitomo Electric, VRB Energy, and RedT Energy, alongside emerging companies such as ESS Inc., Primus Power, and Invinity Energy Systems. Strategic partnerships between electrolyte developers and system integrators are becoming increasingly common, creating new market dynamics and accelerating commercialization pathways for advanced electrolyte formulations.
Current Solvent Engineering Challenges in Flow Batteries
Flow battery technology faces several critical solvent engineering challenges that currently limit its widespread commercial adoption. The primary issue revolves around the solubility limitations of active materials in conventional aqueous electrolytes. Most redox-active species exhibit restricted solubility in water, which directly constrains the energy density of flow batteries. This fundamental limitation has prompted researchers to explore alternative solvent systems, yet each alternative introduces its own set of complications.
Non-aqueous solvents, while offering improved solubility for many organic active materials, typically suffer from higher viscosity compared to water-based systems. This increased viscosity translates directly to higher pumping energy requirements, reducing overall system efficiency and increasing operational costs. Additionally, many organic solvents demonstrate lower ionic conductivity than aqueous systems, resulting in higher internal resistance and consequent power density limitations.
Chemical stability presents another significant challenge in solvent engineering. The electrolyte environment in flow batteries is highly reactive, with constant exposure to charged species, potential side reactions, and varying states of charge. Many promising solvents degrade over time through mechanisms such as oxidation, reduction, or chemical interactions with cell components. This degradation not only reduces battery lifetime but can also generate byproducts that further compromise performance.
Temperature sensitivity constitutes a substantial hurdle for practical applications. Ideal flow battery systems should operate across a wide temperature range without significant performance variations. However, many solvent systems exhibit dramatic changes in viscosity, conductivity, and active material solubility with temperature fluctuations. Some promising non-aqueous systems even face freezing or boiling concerns within operational temperature ranges, necessitating complex thermal management systems.
Cost and environmental considerations represent increasingly important challenges. Many high-performance solvents that address the technical limitations remain prohibitively expensive for large-scale energy storage applications. Furthermore, environmental regulations increasingly restrict the use of toxic or environmentally persistent solvents, narrowing the field of viable candidates. The ideal solvent must balance performance requirements with cost-effectiveness and environmental sustainability.
Crossover of active materials between positive and negative electrolytes through the membrane represents another persistent challenge. Different solvents interact uniquely with membrane materials, affecting selectivity and permeability. Finding solvent systems that minimize active material crossover while maintaining ionic conductivity remains difficult, as these properties often present contradictory optimization directions.
Non-aqueous solvents, while offering improved solubility for many organic active materials, typically suffer from higher viscosity compared to water-based systems. This increased viscosity translates directly to higher pumping energy requirements, reducing overall system efficiency and increasing operational costs. Additionally, many organic solvents demonstrate lower ionic conductivity than aqueous systems, resulting in higher internal resistance and consequent power density limitations.
Chemical stability presents another significant challenge in solvent engineering. The electrolyte environment in flow batteries is highly reactive, with constant exposure to charged species, potential side reactions, and varying states of charge. Many promising solvents degrade over time through mechanisms such as oxidation, reduction, or chemical interactions with cell components. This degradation not only reduces battery lifetime but can also generate byproducts that further compromise performance.
Temperature sensitivity constitutes a substantial hurdle for practical applications. Ideal flow battery systems should operate across a wide temperature range without significant performance variations. However, many solvent systems exhibit dramatic changes in viscosity, conductivity, and active material solubility with temperature fluctuations. Some promising non-aqueous systems even face freezing or boiling concerns within operational temperature ranges, necessitating complex thermal management systems.
Cost and environmental considerations represent increasingly important challenges. Many high-performance solvents that address the technical limitations remain prohibitively expensive for large-scale energy storage applications. Furthermore, environmental regulations increasingly restrict the use of toxic or environmentally persistent solvents, narrowing the field of viable candidates. The ideal solvent must balance performance requirements with cost-effectiveness and environmental sustainability.
Crossover of active materials between positive and negative electrolytes through the membrane represents another persistent challenge. Different solvents interact uniquely with membrane materials, affecting selectivity and permeability. Finding solvent systems that minimize active material crossover while maintaining ionic conductivity remains difficult, as these properties often present contradictory optimization directions.
State-of-the-Art Solvent Engineering Approaches
01 Electrolyte composition for improved performance
The composition of electrolytes significantly impacts flow battery performance. Various additives and compounds can be incorporated to enhance conductivity, stability, and energy density. These may include specific salts, acids, or organic compounds that optimize ionic transport while minimizing side reactions. Carefully formulated electrolyte compositions can lead to higher efficiency, longer cycle life, and improved overall battery performance.- Electrolyte composition for improved performance: The composition of electrolytes significantly impacts flow battery performance. Various additives and compounds can be incorporated to enhance conductivity, stability, and energy density. These may include specific salts, organic compounds, or ionic liquids that optimize the electrochemical properties of the electrolyte solution. Properly formulated electrolytes can reduce internal resistance, improve charge transfer kinetics, and extend the operational lifetime of flow batteries.
- Redox-active materials for enhanced energy density: Specialized redox-active materials can be incorporated into flow battery electrolytes to increase energy density and power output. These materials undergo efficient and reversible redox reactions, allowing for greater energy storage capacity. By selecting appropriate redox couples with favorable electrochemical properties, the performance of flow batteries can be significantly improved, enabling higher voltage outputs and better cycling stability.
- Temperature stabilization techniques: Flow battery electrolyte performance is highly dependent on operating temperature. Various approaches can be employed to maintain optimal temperature ranges and improve thermal stability of the electrolyte solutions. These include specialized additives that expand the operational temperature window, thermal management systems, and electrolyte formulations that resist degradation under temperature fluctuations. These techniques help maintain consistent performance across varying environmental conditions.
- Membrane and separator optimization: The interface between electrolytes and membranes plays a crucial role in flow battery performance. Specialized membrane materials and coatings can reduce crossover of active species between electrolyte compartments, improving coulombic efficiency and cycle life. Advanced separator designs that enhance ion selectivity while minimizing resistance contribute to overall system efficiency and prevent capacity fade over extended cycling.
- Electrolyte circulation and flow dynamics: The physical flow characteristics of electrolytes significantly impact battery performance. Optimizing flow rates, channel designs, and pumping systems can reduce concentration polarization and improve mass transport. Advanced flow field designs ensure uniform distribution of electrolytes across electrode surfaces, while precise control of flow parameters helps balance system efficiency with parasitic power losses from pumping. These improvements in flow dynamics lead to higher power density and more consistent performance.
02 Redox-active materials for enhanced energy density
Specific redox-active materials can be incorporated into flow battery electrolytes to increase energy density and power output. These materials undergo reversible oxidation and reduction reactions, storing and releasing energy efficiently. By selecting appropriate redox couples with favorable electrochemical properties, the performance of flow batteries can be significantly improved, enabling higher capacity and better charge-discharge characteristics.Expand Specific Solutions03 Temperature stability and operating range optimization
Electrolyte formulations can be designed to maintain performance across a wide temperature range. Additives that prevent freezing at low temperatures or degradation at high temperatures extend the operational window of flow batteries. Temperature-stable electrolytes ensure consistent ionic conductivity and electrochemical performance under varying environmental conditions, making the batteries more reliable for diverse applications and climates.Expand Specific Solutions04 Membrane compatibility and ion selectivity
Electrolyte formulations must be compatible with membrane materials to prevent crossover and maintain separation between positive and negative electrolytes. Specialized additives can improve ion selectivity while reducing unwanted transfer across membranes. This optimization enhances coulombic efficiency, reduces self-discharge, and extends the operational lifetime of flow batteries by preventing capacity fade due to cross-contamination of electrolytes.Expand Specific Solutions05 Electrolyte stability and cycling performance
Long-term stability of electrolytes is crucial for maintaining flow battery performance over thousands of cycles. Stabilizing additives can prevent degradation mechanisms such as precipitation, side reactions, and decomposition. These formulations maintain consistent electrochemical properties over extended periods, reducing capacity fade and ensuring reliable energy storage capabilities throughout the battery's operational lifetime.Expand Specific Solutions
Leading Companies and Research Institutions in Flow Battery Sector
The flow battery electrolyte market is in a growth phase, with increasing demand driven by renewable energy integration and grid storage needs. The global market size is projected to reach significant expansion in the coming years as energy storage solutions become critical infrastructure components. Technologically, solvent engineering for high-performance flow batteries is advancing rapidly, with key players demonstrating varying levels of maturity. Companies like Lockheed Martin Advanced Energy Storage, 3M Innovative Properties, and SAMSUNG SDI are leading with established research capabilities, while academic institutions such as MIT and University of Science & Technology of China contribute fundamental innovations. Emerging players like Wildcat Discovery Technologies and BroadBit Batteries are introducing disruptive approaches. Traditional chemical manufacturers including Asahi Kasei, Idemitsu Kosan, and DuPont provide essential material expertise, creating a competitive landscape balanced between established corporations and innovative newcomers.
Lockheed Martin Advanced Energy Storage LLC
Technical Solution: Lockheed Martin has developed proprietary solvent engineering technology for flow batteries focused on coordination chemistry approaches. Their patented Flow-Assisted Batteries (FAB) utilize carefully engineered solvent systems that combine ionic liquids with coordinating additives to achieve unprecedented active material solubility (>2.5M) while maintaining low viscosity. Their approach leverages metal-ligand coordination chemistry to stabilize reactive species in solution, enabling the use of high-energy-density redox couples that would otherwise be unstable. Lockheed's solvent systems incorporate fluorinated solvents with specialized additives that form protective coordination spheres around redox-active centers, preventing unwanted side reactions while maintaining fast electron transfer kinetics. Their research demonstrates that these engineered electrolytes can operate across wider temperature ranges (-20°C to 60°C) than conventional systems, with minimal capacity degradation over thousands of cycles. Recent developments include self-healing solvent formulations that can recover from contamination events.
Strengths: Exceptional energy density through higher concentration and voltage; wide operating temperature range increases deployment flexibility; self-healing properties improve system robustness and lifetime. Weaknesses: Higher complexity increases manufacturing challenges; some specialized additives may have higher costs; potential environmental concerns with certain fluorinated components.
Gelion Technologies Pty Ltd.
Technical Solution: Gelion has pioneered innovative gel-based electrolyte systems for flow batteries that fundamentally reimagine solvent engineering approaches. Their patented Endure platform utilizes ionogel technology - a hybrid between conventional liquid electrolytes and solid-state systems - where carefully engineered solvent systems are immobilized within a three-dimensional polymer network. This approach maintains high ionic conductivity (>10 mS/cm) while dramatically reducing crossover issues. Gelion's solvent engineering focuses on creating non-flammable, high-stability formulations that incorporate zinc-bromide chemistry with proprietary additives that prevent bromine volatilization. Their research demonstrates that these gel electrolytes can achieve energy densities exceeding 50 Wh/L while maintaining stable performance over thousands of cycles. Recent innovations include self-healing gel formulations that can recover from mechanical damage, and temperature-stable variants that maintain consistent performance from -5°C to 45°C without external thermal management. Their approach eliminates many of the mechanical complexities of traditional flow batteries while preserving the fundamental electrochemical advantages.
Strengths: Reduced mechanical complexity compared to traditional flow systems; excellent safety profile with non-flammable formulations; significantly reduced active material crossover improves efficiency. Weaknesses: Potentially lower power density compared to fully liquid systems; more complex manufacturing processes for gel-based systems; possible challenges with heat dissipation during rapid charge/discharge.
Key Patents and Breakthroughs in Electrolyte Formulation
Electrolyte composition
PatentInactiveUS7790312B2
Innovation
- The use of partially-fluorinated ether compounds, specifically hydrofluoroether compounds with terminal fluoroalkyl groups and an intervening oxymethylene group, which form stable electrolyte compositions with reduced volatility, flammability, and combustibility, while maintaining adequate ionic conductivity and wetting capabilities.
Electrolyte composition, lithium-containing electrochemical cell, battery pack, and device including the same
PatentInactiveUS20100028784A1
Innovation
- A non-ignitable electrolyte composition comprising a solvent mixture of at least one cyclic carbonate and one highly fluorinated compound, with lithium salts dissolved, where flammable constituents are present in less than 5% by weight, achieving low viscosity and high electrolyte solubility, and utilizing lithium salts like lithium bis(nonafluorobutanesulfonyl)imide or LiPF6 for enhanced safety and performance.
Environmental Impact and Sustainability Considerations
The environmental impact of flow battery electrolytes represents a critical consideration in the advancement of sustainable energy storage technologies. Traditional flow battery systems often rely on electrolytes containing heavy metals such as vanadium or toxic organic compounds, which pose significant environmental risks throughout their lifecycle. Solvent engineering offers promising pathways to develop more environmentally benign electrolyte formulations while maintaining or enhancing performance characteristics.
Water-based electrolytes present the most environmentally favorable option, eliminating concerns related to volatile organic compound emissions and reducing fire hazards. However, their limited electrochemical stability window necessitates innovative solvent engineering approaches to expand their practical application. Recent advances in aqueous organic redox flow batteries demonstrate that careful selection of organic active materials combined with optimized water-based solvents can achieve competitive energy densities while minimizing environmental footprint.
Life cycle assessment (LCA) studies reveal that solvent selection significantly influences the overall environmental impact of flow battery systems. Deep eutectic solvents and ionic liquids, while more expensive than conventional organic solvents, offer substantially reduced volatility and improved safety profiles. Their biodegradability characteristics and potential for recycling further enhance their sustainability credentials, though scale-up challenges remain to be addressed.
The recyclability of electrolyte components represents another crucial sustainability factor. Engineered solvent systems that facilitate efficient separation and recovery of active materials can dramatically reduce waste generation and resource consumption. Research indicates that certain designer solvents can enable selective precipitation of redox-active species, allowing for straightforward regeneration of degraded electrolytes rather than complete replacement.
Energy and resource requirements for electrolyte production must also be considered in sustainability evaluations. Bio-derived solvents synthesized from renewable feedstocks offer promising alternatives to petroleum-based options, potentially reducing carbon footprint by 30-60% according to recent studies. However, land use implications and competition with food production necessitate careful assessment of these alternatives within broader sustainability frameworks.
Regulatory considerations increasingly influence solvent selection in commercial flow battery applications. Global chemical regulations such as REACH in Europe and similar frameworks in other regions are progressively restricting the use of environmentally persistent and bioaccumulative substances. Forward-looking solvent engineering strategies must anticipate these regulatory trends to ensure long-term viability of developed technologies.
Water-based electrolytes present the most environmentally favorable option, eliminating concerns related to volatile organic compound emissions and reducing fire hazards. However, their limited electrochemical stability window necessitates innovative solvent engineering approaches to expand their practical application. Recent advances in aqueous organic redox flow batteries demonstrate that careful selection of organic active materials combined with optimized water-based solvents can achieve competitive energy densities while minimizing environmental footprint.
Life cycle assessment (LCA) studies reveal that solvent selection significantly influences the overall environmental impact of flow battery systems. Deep eutectic solvents and ionic liquids, while more expensive than conventional organic solvents, offer substantially reduced volatility and improved safety profiles. Their biodegradability characteristics and potential for recycling further enhance their sustainability credentials, though scale-up challenges remain to be addressed.
The recyclability of electrolyte components represents another crucial sustainability factor. Engineered solvent systems that facilitate efficient separation and recovery of active materials can dramatically reduce waste generation and resource consumption. Research indicates that certain designer solvents can enable selective precipitation of redox-active species, allowing for straightforward regeneration of degraded electrolytes rather than complete replacement.
Energy and resource requirements for electrolyte production must also be considered in sustainability evaluations. Bio-derived solvents synthesized from renewable feedstocks offer promising alternatives to petroleum-based options, potentially reducing carbon footprint by 30-60% according to recent studies. However, land use implications and competition with food production necessitate careful assessment of these alternatives within broader sustainability frameworks.
Regulatory considerations increasingly influence solvent selection in commercial flow battery applications. Global chemical regulations such as REACH in Europe and similar frameworks in other regions are progressively restricting the use of environmentally persistent and bioaccumulative substances. Forward-looking solvent engineering strategies must anticipate these regulatory trends to ensure long-term viability of developed technologies.
Scalability and Cost Analysis of Advanced Electrolyte Solutions
The economic viability of flow battery systems heavily depends on the scalability and cost-effectiveness of their electrolyte solutions. Current market analysis indicates that electrolyte costs can constitute 30-40% of the total system cost, making it a critical factor in commercial deployment. Advanced solvent engineering approaches must therefore balance performance improvements with economic considerations to achieve market penetration.
Material costs represent a significant portion of electrolyte expenses, particularly for organic solvents used in non-aqueous systems. While acetonitrile offers excellent electrochemical stability and conductivity properties, its cost at industrial scale ($3-5/kg) presents challenges for large-scale implementation. Alternative solvents such as propylene carbonate ($2-3/kg) and dimethyl sulfoxide ($1.5-2.5/kg) offer more favorable economics but may require performance trade-offs.
Production scaling follows distinct cost curves depending on manufacturing volume. Laboratory-scale electrolyte preparation typically costs $200-500/L, while pilot-scale production can reduce costs to $50-100/L. Full industrial-scale manufacturing potentially brings costs down to $5-15/L, contingent upon solvent selection and active material concentration. This represents a critical threshold for commercial viability in grid-scale applications.
Recycling and circular economy approaches present significant opportunities for cost reduction. Advanced separation techniques enable up to 85-95% recovery of organic solvents and active materials from spent electrolytes. Implementation of closed-loop recycling systems could potentially reduce lifetime electrolyte costs by 40-60%, dramatically improving the economic proposition of flow battery technologies.
Supply chain considerations reveal potential bottlenecks in scaling advanced electrolyte solutions. Specialty solvents often have limited production capacity and may face supply constraints with increased demand. Geographic distribution of manufacturing capabilities also presents challenges, with over 70% of advanced organic solvent production concentrated in East Asia, creating potential vulnerabilities in global supply chains.
Comparative economic analysis against competing energy storage technologies indicates that flow batteries with optimized electrolytes can achieve levelized costs of storage (LCOS) of $0.10-0.15/kWh for long-duration applications (8+ hours), competitive with pumped hydro but without geographical constraints. However, lithium-ion systems maintain cost advantages for shorter duration applications, highlighting the importance of targeting appropriate market segments.
Material costs represent a significant portion of electrolyte expenses, particularly for organic solvents used in non-aqueous systems. While acetonitrile offers excellent electrochemical stability and conductivity properties, its cost at industrial scale ($3-5/kg) presents challenges for large-scale implementation. Alternative solvents such as propylene carbonate ($2-3/kg) and dimethyl sulfoxide ($1.5-2.5/kg) offer more favorable economics but may require performance trade-offs.
Production scaling follows distinct cost curves depending on manufacturing volume. Laboratory-scale electrolyte preparation typically costs $200-500/L, while pilot-scale production can reduce costs to $50-100/L. Full industrial-scale manufacturing potentially brings costs down to $5-15/L, contingent upon solvent selection and active material concentration. This represents a critical threshold for commercial viability in grid-scale applications.
Recycling and circular economy approaches present significant opportunities for cost reduction. Advanced separation techniques enable up to 85-95% recovery of organic solvents and active materials from spent electrolytes. Implementation of closed-loop recycling systems could potentially reduce lifetime electrolyte costs by 40-60%, dramatically improving the economic proposition of flow battery technologies.
Supply chain considerations reveal potential bottlenecks in scaling advanced electrolyte solutions. Specialty solvents often have limited production capacity and may face supply constraints with increased demand. Geographic distribution of manufacturing capabilities also presents challenges, with over 70% of advanced organic solvent production concentrated in East Asia, creating potential vulnerabilities in global supply chains.
Comparative economic analysis against competing energy storage technologies indicates that flow batteries with optimized electrolytes can achieve levelized costs of storage (LCOS) of $0.10-0.15/kWh for long-duration applications (8+ hours), competitive with pumped hydro but without geographical constraints. However, lithium-ion systems maintain cost advantages for shorter duration applications, highlighting the importance of targeting appropriate market segments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!