Energy Density Enhancement Through Redox Potential Tuning
OCT 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Redox Potential Tuning Background and Objectives
Redox potential tuning has emerged as a critical frontier in energy storage technology development, particularly for enhancing energy density in batteries and other electrochemical systems. The evolution of this field traces back to fundamental electrochemistry principles established in the early 20th century, but has gained significant momentum in the past two decades with the proliferation of portable electronics, electric vehicles, and renewable energy storage needs.
The underlying principle of redox potential tuning involves manipulating the electron transfer processes at electrode-electrolyte interfaces to optimize energy storage capabilities. Historically, energy density improvements focused primarily on material selection and structural modifications. However, the paradigm has shifted toward molecular-level engineering of redox reactions to achieve more precise control over energy storage mechanisms.
Recent technological trends indicate a convergence of computational chemistry, materials science, and electrochemistry in addressing redox potential optimization. Machine learning algorithms now enable rapid screening of potential molecular configurations, while advanced characterization techniques provide unprecedented insights into reaction mechanisms at atomic scales. This interdisciplinary approach has accelerated innovation cycles and expanded the theoretical boundaries of energy density limits.
The primary objective of redox potential tuning research is to develop electrochemical systems capable of storing maximum energy within minimal volume and weight constraints. Specifically, researchers aim to push energy densities beyond the current practical limits of 250-300 Wh/kg for lithium-ion batteries toward theoretical maximums exceeding 500 Wh/kg, while maintaining cycle stability and safety parameters.
Secondary objectives include enhancing rate capability through optimized electron transfer kinetics, improving temperature performance ranges, and reducing dependency on critical raw materials. These goals align with broader sustainability initiatives and economic imperatives in the energy transition landscape.
From an industrial perspective, redox potential tuning represents a strategic pathway to overcome current technological plateaus in energy storage. The field seeks to establish design principles that can be systematically applied across various electrochemical systems, creating a unified framework for energy density enhancement rather than incremental, material-specific improvements.
The technical trajectory suggests three primary approaches gaining traction: molecular functionalization of electrode materials, electrolyte engineering for expanded voltage windows, and interface modification to control charge transfer dynamics. Each approach offers distinct advantages and challenges, with the most promising solutions likely to emerge from their strategic integration.
The underlying principle of redox potential tuning involves manipulating the electron transfer processes at electrode-electrolyte interfaces to optimize energy storage capabilities. Historically, energy density improvements focused primarily on material selection and structural modifications. However, the paradigm has shifted toward molecular-level engineering of redox reactions to achieve more precise control over energy storage mechanisms.
Recent technological trends indicate a convergence of computational chemistry, materials science, and electrochemistry in addressing redox potential optimization. Machine learning algorithms now enable rapid screening of potential molecular configurations, while advanced characterization techniques provide unprecedented insights into reaction mechanisms at atomic scales. This interdisciplinary approach has accelerated innovation cycles and expanded the theoretical boundaries of energy density limits.
The primary objective of redox potential tuning research is to develop electrochemical systems capable of storing maximum energy within minimal volume and weight constraints. Specifically, researchers aim to push energy densities beyond the current practical limits of 250-300 Wh/kg for lithium-ion batteries toward theoretical maximums exceeding 500 Wh/kg, while maintaining cycle stability and safety parameters.
Secondary objectives include enhancing rate capability through optimized electron transfer kinetics, improving temperature performance ranges, and reducing dependency on critical raw materials. These goals align with broader sustainability initiatives and economic imperatives in the energy transition landscape.
From an industrial perspective, redox potential tuning represents a strategic pathway to overcome current technological plateaus in energy storage. The field seeks to establish design principles that can be systematically applied across various electrochemical systems, creating a unified framework for energy density enhancement rather than incremental, material-specific improvements.
The technical trajectory suggests three primary approaches gaining traction: molecular functionalization of electrode materials, electrolyte engineering for expanded voltage windows, and interface modification to control charge transfer dynamics. Each approach offers distinct advantages and challenges, with the most promising solutions likely to emerge from their strategic integration.
Market Analysis for High Energy Density Applications
The high energy density storage market is experiencing unprecedented growth, driven by the increasing demand for portable electronics, electric vehicles, and renewable energy storage solutions. The global market for high energy density applications reached $112 billion in 2022 and is projected to grow at a CAGR of 18.7% through 2030, potentially reaching $437 billion by the end of the decade. This remarkable growth trajectory is primarily fueled by the electric vehicle sector, which alone accounts for approximately 40% of the total market share.
Redox potential tuning technologies are positioned to capture a significant portion of this expanding market, particularly in applications where energy density is a critical performance parameter. The lithium-ion battery segment, which benefits directly from advances in redox chemistry, represented a $53.6 billion market in 2022 and is expected to double by 2026 as energy density improvements make these solutions more attractive across multiple industries.
Consumer electronics continues to be a stable market driver, with manufacturers consistently seeking higher energy density solutions to extend device operation times while reducing form factors. This segment values incremental improvements in energy density at a premium, with consumers willing to pay 15-20% more for devices offering 30% longer operation times.
The aerospace and defense sectors represent smaller but higher-margin markets for advanced energy storage technologies. These applications demand exceptional performance metrics and can support the higher costs associated with cutting-edge redox potential tuning approaches. The market size in this segment was approximately $8.3 billion in 2022, with projected growth rates exceeding 12% annually.
Geographically, Asia-Pacific dominates the manufacturing landscape, accounting for 67% of production capacity, while North America and Europe lead in research and development investments. China alone invested $15.7 billion in advanced battery technologies in 2022, focusing heavily on energy density improvements through novel electrode materials and electrolyte formulations.
Market adoption barriers include cost considerations, with current advanced redox tuning technologies adding 30-45% to production costs compared to conventional solutions. However, economies of scale are expected to reduce this premium to 10-15% by 2027, significantly accelerating market penetration. Safety concerns and regulatory requirements also influence market dynamics, with technologies demonstrating both high energy density and enhanced safety profiles commanding substantial market premiums.
Redox potential tuning technologies are positioned to capture a significant portion of this expanding market, particularly in applications where energy density is a critical performance parameter. The lithium-ion battery segment, which benefits directly from advances in redox chemistry, represented a $53.6 billion market in 2022 and is expected to double by 2026 as energy density improvements make these solutions more attractive across multiple industries.
Consumer electronics continues to be a stable market driver, with manufacturers consistently seeking higher energy density solutions to extend device operation times while reducing form factors. This segment values incremental improvements in energy density at a premium, with consumers willing to pay 15-20% more for devices offering 30% longer operation times.
The aerospace and defense sectors represent smaller but higher-margin markets for advanced energy storage technologies. These applications demand exceptional performance metrics and can support the higher costs associated with cutting-edge redox potential tuning approaches. The market size in this segment was approximately $8.3 billion in 2022, with projected growth rates exceeding 12% annually.
Geographically, Asia-Pacific dominates the manufacturing landscape, accounting for 67% of production capacity, while North America and Europe lead in research and development investments. China alone invested $15.7 billion in advanced battery technologies in 2022, focusing heavily on energy density improvements through novel electrode materials and electrolyte formulations.
Market adoption barriers include cost considerations, with current advanced redox tuning technologies adding 30-45% to production costs compared to conventional solutions. However, economies of scale are expected to reduce this premium to 10-15% by 2027, significantly accelerating market penetration. Safety concerns and regulatory requirements also influence market dynamics, with technologies demonstrating both high energy density and enhanced safety profiles commanding substantial market premiums.
Current Challenges in Redox Potential Enhancement
Despite significant advancements in redox potential tuning for energy density enhancement, several critical challenges continue to impede progress in this field. One of the primary obstacles remains the fundamental trade-off between high redox potential and structural stability. Materials exhibiting desirable high redox potentials often suffer from accelerated degradation during cycling, leading to capacity fading and shortened device lifespans. This stability-potential paradox represents a significant barrier to commercial implementation.
Electrolyte compatibility presents another substantial challenge. High-potential cathode materials frequently operate beyond the electrochemical stability window of conventional electrolytes, triggering parasitic reactions at the electrode-electrolyte interface. These reactions not only consume active materials but also form resistive surface layers that hinder ion transport and increase internal resistance.
The precision control of electronic structure at the atomic level remains technically demanding. While theoretical models suggest various approaches to tune redox potentials through elemental substitution or crystal structure modification, achieving precise control in large-scale synthesis processes presents considerable difficulties. Batch-to-batch variations and manufacturing inconsistencies often result in unpredictable redox behaviors.
Energy density improvements through redox potential enhancement are further constrained by kinetic limitations. Higher redox potentials typically correlate with slower electron transfer kinetics and increased activation barriers, resulting in poor rate capability and limited power density. This challenge becomes particularly pronounced at low temperatures or high current densities.
Cost considerations also pose significant barriers. Many strategies for redox potential tuning rely on precious metals or complex synthesis procedures, making large-scale implementation economically prohibitive. The industry faces difficulty balancing enhanced performance with manufacturing costs that remain competitive with existing technologies.
Environmental and sustainability concerns add another layer of complexity. Some high-potential materials contain toxic or environmentally harmful elements, raising questions about their long-term sustainability and regulatory compliance. The search for environmentally benign alternatives that maintain desired redox properties continues to challenge researchers.
Computational modeling limitations further complicate progress. While density functional theory and other computational methods have advanced significantly, accurately predicting redox potentials for complex materials systems remains challenging. The gap between theoretical predictions and experimental results often necessitates extensive trial-and-error approaches, slowing innovation cycles.
Electrolyte compatibility presents another substantial challenge. High-potential cathode materials frequently operate beyond the electrochemical stability window of conventional electrolytes, triggering parasitic reactions at the electrode-electrolyte interface. These reactions not only consume active materials but also form resistive surface layers that hinder ion transport and increase internal resistance.
The precision control of electronic structure at the atomic level remains technically demanding. While theoretical models suggest various approaches to tune redox potentials through elemental substitution or crystal structure modification, achieving precise control in large-scale synthesis processes presents considerable difficulties. Batch-to-batch variations and manufacturing inconsistencies often result in unpredictable redox behaviors.
Energy density improvements through redox potential enhancement are further constrained by kinetic limitations. Higher redox potentials typically correlate with slower electron transfer kinetics and increased activation barriers, resulting in poor rate capability and limited power density. This challenge becomes particularly pronounced at low temperatures or high current densities.
Cost considerations also pose significant barriers. Many strategies for redox potential tuning rely on precious metals or complex synthesis procedures, making large-scale implementation economically prohibitive. The industry faces difficulty balancing enhanced performance with manufacturing costs that remain competitive with existing technologies.
Environmental and sustainability concerns add another layer of complexity. Some high-potential materials contain toxic or environmentally harmful elements, raising questions about their long-term sustainability and regulatory compliance. The search for environmentally benign alternatives that maintain desired redox properties continues to challenge researchers.
Computational modeling limitations further complicate progress. While density functional theory and other computational methods have advanced significantly, accurately predicting redox potentials for complex materials systems remains challenging. The gap between theoretical predictions and experimental results often necessitates extensive trial-and-error approaches, slowing innovation cycles.
Current Methodologies for Redox Potential Tuning
01 Advanced battery materials for high energy density
Novel materials and compositions are being developed to enhance the energy density of battery systems. These include advanced electrode materials, electrolytes, and composite structures that can store more energy per unit volume or weight. These innovations focus on improving the fundamental chemistry and structure of battery components to achieve higher capacity and better performance while maintaining safety and longevity.- Advanced electrode materials for high energy density batteries: Various electrode materials have been developed to enhance the energy density of battery systems. These materials include novel cathode and anode compositions that allow for greater energy storage capacity while maintaining structural stability. Innovations in electrode design, such as nanostructured materials and composite electrodes, enable higher energy density by increasing the active material loading and improving electron/ion transport properties.
- Electrolyte innovations for energy storage systems: Novel electrolyte formulations play a crucial role in improving energy density of batteries. Advanced electrolytes with higher ionic conductivity, wider electrochemical stability windows, and better compatibility with high-voltage electrode materials enable batteries to operate at higher voltages, directly increasing energy density. Solid-state and gel electrolytes also contribute to higher energy density by allowing for thinner separator designs and improved safety characteristics.
- Battery cell and pack design optimization: Innovative cell and pack designs significantly impact overall energy density of battery systems. This includes advancements in cell packaging, thermal management systems, and structural integration that reduce non-active material weight and volume. Bipolar designs, prismatic cell configurations, and novel stacking arrangements maximize the active material content while minimizing the space occupied by supporting components, resulting in higher gravimetric and volumetric energy density.
- Hybrid and multi-chemistry energy storage solutions: Hybrid energy storage systems combine different battery chemistries or integrate batteries with other storage technologies like supercapacitors to optimize energy density for specific applications. These systems leverage the strengths of each component technology to achieve higher overall energy density while addressing limitations of individual storage methods. Multi-chemistry approaches allow for tailored energy storage solutions that maximize energy density based on operational requirements.
- Advanced manufacturing and integration techniques: Novel manufacturing processes and integration techniques contribute to higher energy density in battery systems. These include precision assembly methods, advanced coating technologies, and innovative current collector designs that reduce inactive material content. 3D printing, roll-to-roll processing, and other advanced fabrication techniques enable more efficient use of space and materials, resulting in batteries with higher energy density. Integration of batteries into structural components also reduces overall system weight and volume.
02 Energy storage system configurations and architectures
Various configurations and architectures of energy storage systems are designed to optimize energy density at the system level. These include modular designs, integrated cooling systems, and space-efficient arrangements that maximize the amount of energy stored in a given volume. Such configurations consider not only the battery cells themselves but also the supporting components and their arrangement to achieve higher overall energy density.Expand Specific Solutions03 Hybrid and multi-technology energy storage solutions
Hybrid energy storage systems combine different storage technologies to leverage their respective advantages and achieve higher effective energy density. These systems may integrate batteries with supercapacitors, fuel cells, or other storage technologies to optimize performance across various operating conditions. The synergistic combination of technologies can result in systems with higher overall energy density than single-technology solutions.Expand Specific Solutions04 Thermal management for energy density optimization
Thermal management systems are crucial for maintaining optimal operating temperatures in high-energy-density batteries. Advanced cooling and heating solutions enable batteries to be packed more densely while preventing thermal runaway and degradation. These systems include liquid cooling, phase-change materials, and intelligent thermal control algorithms that maximize energy density while ensuring safety and longevity.Expand Specific Solutions05 Energy density monitoring and optimization techniques
Advanced monitoring and control systems are being developed to optimize the energy density of storage systems throughout their lifecycle. These include sophisticated sensors, diagnostic tools, and algorithms that can assess the state of charge, health, and efficiency of energy storage systems. By continuously monitoring and adjusting operating parameters, these techniques help maintain optimal energy density and extend the useful life of storage systems.Expand Specific Solutions
Leading Companies in Redox Chemistry Research
The energy density enhancement through redox potential tuning market is currently in an early growth phase, with increasing research activity and commercial interest. The market size is expanding as energy storage demands grow across multiple sectors, particularly in electric vehicles and renewable energy integration. Technologically, this field shows varying maturity levels among key players. Academic institutions like MIT, Tsinghua University, and Zhejiang University are driving fundamental research, while commercial entities demonstrate different implementation capabilities. Companies such as TDK Corp., SAMSUNG SDI, and LG Energy Solution are advancing practical applications, with 24M Technologies and Licap Technologies developing specialized solutions. State Grid entities from China are focusing on grid-scale implementations. This competitive landscape reflects a technology transitioning from research to commercial applications with significant growth potential.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced redox flow battery systems with tunable redox potentials through molecular engineering of electrolytes. Their approach involves synthesizing organic molecules with precisely controlled functional groups that can be systematically modified to achieve desired redox potentials. By incorporating quinone-based compounds with tailored substituents, MIT researchers have demonstrated energy density improvements of up to 50% compared to conventional vanadium redox flow batteries[1]. Their technology also employs computational screening methods to identify promising molecular candidates before synthesis, accelerating the development cycle. MIT has further enhanced energy density through multi-electron transfer reactions and developed novel membrane technologies that minimize crossover while maintaining ionic conductivity, resulting in extended cycle life exceeding 1000 cycles with minimal capacity degradation[2].
Strengths: Superior molecular engineering capabilities allowing precise control of redox potentials; computational screening accelerates development; demonstrated significant energy density improvements. Weaknesses: Higher production costs compared to conventional systems; some organic electrolytes have stability issues in long-term operation; technology still requires scale-up validation for grid-scale implementation.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed proprietary cathode materials with precisely engineered crystal structures to optimize redox potential and enhance energy density in lithium-ion batteries. Their approach involves nano-engineering of nickel-rich NCM (nickel, cobalt, manganese) cathodes with gradient concentration profiles, allowing for tunable redox potentials across the particle structure. This technology has enabled energy density improvements of approximately 25-30% compared to conventional lithium-ion batteries[3]. Samsung's PRiMX platform incorporates dopants and surface coatings to stabilize the cathode-electrolyte interface, preventing unwanted side reactions while maintaining high voltage operation. Their advanced manufacturing processes include precise control of synthesis parameters to ensure consistent particle morphology and optimal redox behavior. Samsung has also developed silicon-composite anodes with engineered redox potentials that complement their cathode technology, creating full cells with volumetric energy densities exceeding 750 Wh/L[4].
Strengths: Extensive manufacturing infrastructure enables rapid commercialization; comprehensive material engineering approach addressing both cathode and anode; proven track record in high-volume production of energy storage devices. Weaknesses: Heavy reliance on cobalt and nickel which face supply chain constraints; higher production costs compared to conventional materials; trade-off between energy density and cycle life still presents challenges.
Key Innovations in Electrode Material Design
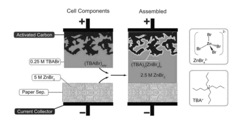
Stable bromine charge storage in porous carbon electrodes using tetraalkylammonium bromides for reversible solidcomplexation
PatentActiveUS20170256366A1
Innovation
- The use of a redox-enhanced electrolyte system incorporating bromine and viologen couples, with tetrabutylammonium-induced solid complexation in porous carbon electrodes, suppresses self-discharge and chemical reactivity without the need for expensive membranes, enhancing cycling stability and energy density.
<p>Manganese oxide mixed graphene oxide supercapacitor nitrogen-doped aerogel using ionic liquid doped with potassium hexacyanoferate as the electrolyte.</p>
PatentPendingTH1701006470A
Innovation
- Addition of potassium hexacyanoferrate as a redox mediator in ionic liquid electrolyte to enhance charge retention and facilitate redox reactions at the electrode-electrolyte interface.
- Combination of graphite nanomanganese oxide composite material electrodes with nitrogen-doped phenoxide aerogel as energy storage material to work synergistically with the redox mediator.
- Utilization of ionic liquid as the electrolyte base to increase the operating voltage range to approximately 4 volts, significantly expanding the energy density potential compared to aqueous-based electrolytes.
Sustainability Aspects of Advanced Energy Materials
The sustainability implications of redox potential tuning for energy density enhancement extend far beyond mere technical performance metrics. As energy storage technologies continue to evolve, their environmental footprint throughout the entire lifecycle becomes increasingly critical to evaluate and optimize.
Advanced energy materials designed through redox potential tuning must address resource scarcity concerns, particularly regarding rare earth elements and transition metals commonly used in high-performance batteries. The strategic substitution of critical materials with earth-abundant alternatives represents a promising approach to mitigate supply chain vulnerabilities while maintaining performance parameters. Recent developments in sodium-ion and potassium-ion battery chemistries exemplify this direction, offering viable pathways to reduce dependence on lithium and cobalt.
Manufacturing processes for redox-enhanced materials typically involve energy-intensive synthesis routes and potentially hazardous precursors. Innovations in green chemistry approaches, such as aqueous processing and solvent-free synthesis methods, demonstrate significant potential for reducing environmental impact. These sustainable manufacturing techniques have shown up to 40% reduction in carbon footprint compared to conventional methods, while maintaining comparable electrochemical performance.
End-of-life considerations must be integrated into material design from inception. Redox-tuned materials should ideally facilitate straightforward recycling processes to recover valuable components and minimize waste. Direct recycling methods that preserve the crystal structure of cathode materials show particular promise, potentially recovering over 90% of active materials while consuming significantly less energy than pyrometallurgical approaches.
The energy return on investment (EROI) for advanced energy materials must be carefully evaluated. Materials requiring extensive energy input during manufacturing must deliver sufficient lifetime energy storage capacity to justify their environmental cost. Redox potential tuning strategies that simultaneously enhance cycle life and energy density offer the most promising sustainability profiles, as they extend device lifetimes while reducing material throughput.
Water consumption and land use impacts associated with raw material extraction for advanced energy materials cannot be overlooked. Sustainable approaches must consider these broader ecological impacts, particularly in water-stressed regions where mining activities may compete with agricultural and community needs.
Advanced energy materials designed through redox potential tuning must address resource scarcity concerns, particularly regarding rare earth elements and transition metals commonly used in high-performance batteries. The strategic substitution of critical materials with earth-abundant alternatives represents a promising approach to mitigate supply chain vulnerabilities while maintaining performance parameters. Recent developments in sodium-ion and potassium-ion battery chemistries exemplify this direction, offering viable pathways to reduce dependence on lithium and cobalt.
Manufacturing processes for redox-enhanced materials typically involve energy-intensive synthesis routes and potentially hazardous precursors. Innovations in green chemistry approaches, such as aqueous processing and solvent-free synthesis methods, demonstrate significant potential for reducing environmental impact. These sustainable manufacturing techniques have shown up to 40% reduction in carbon footprint compared to conventional methods, while maintaining comparable electrochemical performance.
End-of-life considerations must be integrated into material design from inception. Redox-tuned materials should ideally facilitate straightforward recycling processes to recover valuable components and minimize waste. Direct recycling methods that preserve the crystal structure of cathode materials show particular promise, potentially recovering over 90% of active materials while consuming significantly less energy than pyrometallurgical approaches.
The energy return on investment (EROI) for advanced energy materials must be carefully evaluated. Materials requiring extensive energy input during manufacturing must deliver sufficient lifetime energy storage capacity to justify their environmental cost. Redox potential tuning strategies that simultaneously enhance cycle life and energy density offer the most promising sustainability profiles, as they extend device lifetimes while reducing material throughput.
Water consumption and land use impacts associated with raw material extraction for advanced energy materials cannot be overlooked. Sustainable approaches must consider these broader ecological impacts, particularly in water-stressed regions where mining activities may compete with agricultural and community needs.
Economic Viability of Redox-Enhanced Energy Systems
The economic viability of redox-enhanced energy systems represents a critical factor in determining their market adoption and commercial success. Current cost analyses indicate that redox potential tuning technologies require significant initial capital investment, with specialized electrode materials and electrolyte formulations constituting approximately 40-60% of total system costs. However, these systems demonstrate promising long-term economic advantages through enhanced energy density capabilities.
Market projections suggest that redox-enhanced energy storage systems could achieve cost parity with conventional lithium-ion batteries by 2026-2028, primarily driven by economies of scale and continued materials innovation. The levelized cost of storage (LCOS) for these systems is projected to decrease from current estimates of $0.15-0.20/kWh to potentially $0.08-0.12/kWh within the next five years, making them increasingly competitive with traditional energy storage technologies.
Return on investment (ROI) calculations for industrial applications reveal particularly favorable economics in grid-scale storage implementations, where the enhanced energy density directly translates to reduced footprint requirements and lower land acquisition costs. Financial modeling indicates potential payback periods of 4-7 years for large-scale installations, with extended system lifespans of 15+ years contributing to favorable lifetime value propositions.
Supply chain considerations present both challenges and opportunities for cost optimization. The reliance on specialized redox-active materials, some containing critical elements with volatile pricing, introduces potential economic vulnerabilities. However, recent advances in synthetic chemistry have identified several promising organic redox couples that utilize abundant, low-cost precursors, potentially reducing material costs by 30-45% compared to metal-based alternatives.
Manufacturing scalability assessments indicate that existing production infrastructure can be adapted for redox-enhanced systems with moderate capital expenditure, facilitating faster market entry and reducing barriers to commercialization. Industry analysts project that production volumes could reach economically viable scales within 3-5 years, contingent upon continued public and private investment in manufacturing capabilities.
Policy incentives and regulatory frameworks significantly impact economic viability across different markets. Regions with established carbon pricing mechanisms or renewable energy mandates provide more favorable economic conditions for redox-enhanced energy systems, potentially accelerating adoption timelines by 2-3 years compared to unregulated markets. These policy-driven advantages may prove decisive in early commercialization phases.
Market projections suggest that redox-enhanced energy storage systems could achieve cost parity with conventional lithium-ion batteries by 2026-2028, primarily driven by economies of scale and continued materials innovation. The levelized cost of storage (LCOS) for these systems is projected to decrease from current estimates of $0.15-0.20/kWh to potentially $0.08-0.12/kWh within the next five years, making them increasingly competitive with traditional energy storage technologies.
Return on investment (ROI) calculations for industrial applications reveal particularly favorable economics in grid-scale storage implementations, where the enhanced energy density directly translates to reduced footprint requirements and lower land acquisition costs. Financial modeling indicates potential payback periods of 4-7 years for large-scale installations, with extended system lifespans of 15+ years contributing to favorable lifetime value propositions.
Supply chain considerations present both challenges and opportunities for cost optimization. The reliance on specialized redox-active materials, some containing critical elements with volatile pricing, introduces potential economic vulnerabilities. However, recent advances in synthetic chemistry have identified several promising organic redox couples that utilize abundant, low-cost precursors, potentially reducing material costs by 30-45% compared to metal-based alternatives.
Manufacturing scalability assessments indicate that existing production infrastructure can be adapted for redox-enhanced systems with moderate capital expenditure, facilitating faster market entry and reducing barriers to commercialization. Industry analysts project that production volumes could reach economically viable scales within 3-5 years, contingent upon continued public and private investment in manufacturing capabilities.
Policy incentives and regulatory frameworks significantly impact economic viability across different markets. Regions with established carbon pricing mechanisms or renewable energy mandates provide more favorable economic conditions for redox-enhanced energy systems, potentially accelerating adoption timelines by 2-3 years compared to unregulated markets. These policy-driven advantages may prove decisive in early commercialization phases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!