Battery Management Systems in the Medical Devices Industry: Challenges and Innovations
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BMS in Medical Devices: Background and Objectives
Battery Management Systems (BMS) have become increasingly crucial in the medical devices industry, playing a vital role in ensuring the safety, reliability, and efficiency of portable and implantable medical equipment. The evolution of BMS in medical devices has been driven by the growing demand for more compact, longer-lasting, and safer power solutions in healthcare applications.
The primary objective of BMS in medical devices is to optimize battery performance while maintaining the highest standards of patient safety. This involves monitoring and controlling various parameters such as voltage, current, temperature, and state of charge to prevent overcharging, over-discharging, and thermal runaway. As medical devices become more sophisticated and energy-demanding, the need for advanced BMS solutions has intensified.
The development of BMS technology in the medical field has been influenced by several factors, including the miniaturization of devices, the increasing complexity of medical procedures, and the push for extended battery life in implantable devices. These trends have led to the emergence of innovative BMS designs that incorporate advanced sensing technologies, intelligent power management algorithms, and enhanced communication capabilities.
One of the key challenges in BMS for medical devices is balancing power efficiency with safety requirements. Medical devices often operate in critical environments where battery failure could have severe consequences. This has driven the development of redundant safety mechanisms and fail-safe designs within BMS architectures. Additionally, the need for biocompatibility in implantable devices has spurred research into novel materials and encapsulation techniques for BMS components.
The regulatory landscape has also played a significant role in shaping BMS technology for medical devices. Stringent standards set by organizations such as the FDA and ISO have necessitated rigorous testing and validation processes for BMS implementations. This has led to the development of more robust and reliable systems that can withstand the rigors of medical use while complying with regulatory requirements.
Looking ahead, the future of BMS in medical devices is likely to be characterized by further integration of artificial intelligence and machine learning algorithms. These technologies promise to enhance predictive maintenance capabilities, optimize charging cycles, and extend battery lifespan. Furthermore, the advent of wireless charging technologies and energy harvesting techniques may revolutionize how medical devices are powered, presenting new challenges and opportunities for BMS design.
In conclusion, the background and objectives of BMS in medical devices reflect a complex interplay of technological advancement, safety imperatives, and regulatory compliance. As the healthcare industry continues to evolve, BMS will remain a critical component in enabling the next generation of life-saving and life-enhancing medical technologies.
The primary objective of BMS in medical devices is to optimize battery performance while maintaining the highest standards of patient safety. This involves monitoring and controlling various parameters such as voltage, current, temperature, and state of charge to prevent overcharging, over-discharging, and thermal runaway. As medical devices become more sophisticated and energy-demanding, the need for advanced BMS solutions has intensified.
The development of BMS technology in the medical field has been influenced by several factors, including the miniaturization of devices, the increasing complexity of medical procedures, and the push for extended battery life in implantable devices. These trends have led to the emergence of innovative BMS designs that incorporate advanced sensing technologies, intelligent power management algorithms, and enhanced communication capabilities.
One of the key challenges in BMS for medical devices is balancing power efficiency with safety requirements. Medical devices often operate in critical environments where battery failure could have severe consequences. This has driven the development of redundant safety mechanisms and fail-safe designs within BMS architectures. Additionally, the need for biocompatibility in implantable devices has spurred research into novel materials and encapsulation techniques for BMS components.
The regulatory landscape has also played a significant role in shaping BMS technology for medical devices. Stringent standards set by organizations such as the FDA and ISO have necessitated rigorous testing and validation processes for BMS implementations. This has led to the development of more robust and reliable systems that can withstand the rigors of medical use while complying with regulatory requirements.
Looking ahead, the future of BMS in medical devices is likely to be characterized by further integration of artificial intelligence and machine learning algorithms. These technologies promise to enhance predictive maintenance capabilities, optimize charging cycles, and extend battery lifespan. Furthermore, the advent of wireless charging technologies and energy harvesting techniques may revolutionize how medical devices are powered, presenting new challenges and opportunities for BMS design.
In conclusion, the background and objectives of BMS in medical devices reflect a complex interplay of technological advancement, safety imperatives, and regulatory compliance. As the healthcare industry continues to evolve, BMS will remain a critical component in enabling the next generation of life-saving and life-enhancing medical technologies.
Market Analysis for Medical Device BMS
The Battery Management Systems (BMS) market in the medical devices industry is experiencing significant growth, driven by the increasing adoption of portable and implantable medical devices. As healthcare continues to shift towards more personalized and mobile solutions, the demand for efficient and reliable power management systems in medical devices has surged.
The global medical device BMS market is primarily segmented into portable medical devices and implantable medical devices. Portable medical devices, including infusion pumps, ventilators, and patient monitoring systems, represent a larger share of the market due to their widespread use in hospitals, clinics, and home healthcare settings. Implantable medical devices, such as pacemakers and neurostimulators, form a smaller but rapidly growing segment with high-value applications.
Geographically, North America dominates the medical device BMS market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare access and increasing investments in medical technology.
Key factors driving the growth of the medical device BMS market include the rising prevalence of chronic diseases, aging populations in developed countries, and technological advancements in battery technology. The increasing focus on patient safety and the need for longer battery life in medical devices are also contributing to market expansion.
The market is characterized by intense competition among major players, including Medtronic, Boston Scientific, Abbott Laboratories, and Johnson & Johnson. These companies are investing heavily in research and development to improve BMS efficiency, safety, and reliability. Additionally, several specialized BMS providers and startups are entering the market with innovative solutions tailored for specific medical device applications.
Challenges facing the medical device BMS market include stringent regulatory requirements, concerns about battery safety, and the need for miniaturization without compromising performance. However, these challenges also present opportunities for innovation, particularly in areas such as advanced battery chemistries, smart power management algorithms, and wireless charging technologies.
Looking ahead, the medical device BMS market is poised for continued growth, with a focus on developing more compact, efficient, and long-lasting power management solutions. The integration of artificial intelligence and machine learning in BMS is expected to further enhance battery performance and predictive maintenance capabilities, ultimately improving patient outcomes and reducing healthcare costs.
The global medical device BMS market is primarily segmented into portable medical devices and implantable medical devices. Portable medical devices, including infusion pumps, ventilators, and patient monitoring systems, represent a larger share of the market due to their widespread use in hospitals, clinics, and home healthcare settings. Implantable medical devices, such as pacemakers and neurostimulators, form a smaller but rapidly growing segment with high-value applications.
Geographically, North America dominates the medical device BMS market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare access and increasing investments in medical technology.
Key factors driving the growth of the medical device BMS market include the rising prevalence of chronic diseases, aging populations in developed countries, and technological advancements in battery technology. The increasing focus on patient safety and the need for longer battery life in medical devices are also contributing to market expansion.
The market is characterized by intense competition among major players, including Medtronic, Boston Scientific, Abbott Laboratories, and Johnson & Johnson. These companies are investing heavily in research and development to improve BMS efficiency, safety, and reliability. Additionally, several specialized BMS providers and startups are entering the market with innovative solutions tailored for specific medical device applications.
Challenges facing the medical device BMS market include stringent regulatory requirements, concerns about battery safety, and the need for miniaturization without compromising performance. However, these challenges also present opportunities for innovation, particularly in areas such as advanced battery chemistries, smart power management algorithms, and wireless charging technologies.
Looking ahead, the medical device BMS market is poised for continued growth, with a focus on developing more compact, efficient, and long-lasting power management solutions. The integration of artificial intelligence and machine learning in BMS is expected to further enhance battery performance and predictive maintenance capabilities, ultimately improving patient outcomes and reducing healthcare costs.
Current BMS Challenges in Medical Devices
Battery Management Systems (BMS) in the medical devices industry face several significant challenges that impact their performance, reliability, and safety. One of the primary concerns is the stringent regulatory requirements imposed by agencies such as the FDA. These regulations demand rigorous testing, validation, and documentation processes, which can slow down innovation and increase development costs for medical device manufacturers.
Another critical challenge is the need for enhanced safety features. Medical devices often operate in critical environments where battery failure could have severe consequences. This necessitates the development of advanced fault detection and prevention mechanisms, as well as redundancy systems to ensure uninterrupted operation. However, implementing these safety features while maintaining compact device sizes presents a significant engineering challenge.
Power efficiency and longevity are also major concerns in medical BMS. Many medical devices require long-term operation without frequent charging or battery replacement. This demand puts pressure on BMS designers to optimize power consumption and extend battery life without compromising device functionality. Balancing these requirements with the need for miniaturization and lightweight designs further complicates the development process.
Thermal management poses another significant challenge for BMS in medical devices. Overheating can lead to device malfunction or patient discomfort, especially in implantable devices. Developing effective cooling solutions within the confined spaces of medical devices requires innovative approaches to heat dissipation and thermal regulation.
Interoperability and data management present additional hurdles. As medical devices become increasingly connected, BMS must be capable of interfacing with various systems and securely transmitting battery status data. This connectivity introduces cybersecurity risks that must be addressed to protect patient information and prevent unauthorized access to device controls.
The diversity of medical devices and their specific power requirements also complicates BMS design. From portable diagnostic equipment to implantable devices, each application has unique power profiles and operational constraints. Developing versatile BMS solutions that can adapt to these varied needs while maintaining high performance and reliability is a significant challenge for engineers in the field.
Lastly, the increasing demand for fast-charging capabilities in medical devices presents both opportunities and challenges for BMS development. While rapid charging can improve device usability, it also introduces potential safety risks and may impact long-term battery health. Balancing these factors requires sophisticated charge control algorithms and advanced battery monitoring techniques.
Another critical challenge is the need for enhanced safety features. Medical devices often operate in critical environments where battery failure could have severe consequences. This necessitates the development of advanced fault detection and prevention mechanisms, as well as redundancy systems to ensure uninterrupted operation. However, implementing these safety features while maintaining compact device sizes presents a significant engineering challenge.
Power efficiency and longevity are also major concerns in medical BMS. Many medical devices require long-term operation without frequent charging or battery replacement. This demand puts pressure on BMS designers to optimize power consumption and extend battery life without compromising device functionality. Balancing these requirements with the need for miniaturization and lightweight designs further complicates the development process.
Thermal management poses another significant challenge for BMS in medical devices. Overheating can lead to device malfunction or patient discomfort, especially in implantable devices. Developing effective cooling solutions within the confined spaces of medical devices requires innovative approaches to heat dissipation and thermal regulation.
Interoperability and data management present additional hurdles. As medical devices become increasingly connected, BMS must be capable of interfacing with various systems and securely transmitting battery status data. This connectivity introduces cybersecurity risks that must be addressed to protect patient information and prevent unauthorized access to device controls.
The diversity of medical devices and their specific power requirements also complicates BMS design. From portable diagnostic equipment to implantable devices, each application has unique power profiles and operational constraints. Developing versatile BMS solutions that can adapt to these varied needs while maintaining high performance and reliability is a significant challenge for engineers in the field.
Lastly, the increasing demand for fast-charging capabilities in medical devices presents both opportunities and challenges for BMS development. While rapid charging can improve device usability, it also introduces potential safety risks and may impact long-term battery health. Balancing these factors requires sophisticated charge control algorithms and advanced battery monitoring techniques.
Existing BMS Solutions for Medical Devices
01 Battery monitoring and control systems
These systems monitor battery parameters such as voltage, current, temperature, and state of charge. They use this data to optimize battery performance, extend battery life, and ensure safe operation. Advanced algorithms are employed to estimate battery health and predict remaining useful life.- Battery monitoring and control systems: These systems monitor battery parameters such as voltage, current, and temperature to optimize performance and ensure safe operation. They use advanced algorithms to estimate battery state of charge and health, and can implement protective measures to prevent overcharging or over-discharging.
- Thermal management in battery systems: Thermal management is crucial for battery performance and longevity. These systems employ various cooling and heating strategies to maintain optimal battery temperature ranges. They may include active cooling systems, passive heat dissipation methods, or intelligent thermal control algorithms.
- Battery balancing techniques: Battery balancing ensures that all cells in a battery pack maintain similar charge levels, improving overall performance and lifespan. Techniques include passive balancing using resistors, active balancing with charge redistribution, and intelligent balancing algorithms that adapt to battery conditions.
- Integration with energy management systems: Battery management systems are increasingly integrated with broader energy management systems in applications such as electric vehicles and smart grids. These integrated systems optimize energy flow, manage power distribution, and coordinate between multiple energy sources and loads for improved efficiency.
- Predictive maintenance and diagnostics: Advanced battery management systems incorporate predictive maintenance features and diagnostic capabilities. These systems use machine learning algorithms and data analytics to predict battery failures, schedule maintenance, and provide detailed diagnostics for troubleshooting and optimization.
02 Thermal management in battery systems
Thermal management is crucial for battery performance and safety. These systems regulate battery temperature through cooling or heating mechanisms, preventing overheating and maintaining optimal operating conditions. They may include liquid cooling systems, air cooling, or phase change materials for efficient heat dissipation.Expand Specific Solutions03 Cell balancing techniques
Cell balancing ensures uniform charge distribution across all cells in a battery pack. This prevents overcharging of individual cells and extends overall battery life. Various methods are used, including passive balancing with resistors and active balancing using charge redistribution circuits.Expand Specific Solutions04 Battery management for electric vehicles
Specialized battery management systems for electric vehicles focus on optimizing range, fast charging capabilities, and integrating with vehicle systems. These systems manage power distribution, regenerative braking, and provide accurate state of charge information to the driver.Expand Specific Solutions05 Smart charging and energy management
These systems optimize charging processes based on grid conditions, energy prices, and user preferences. They may incorporate predictive algorithms to schedule charging during off-peak hours and integrate with renewable energy sources for sustainable charging solutions.Expand Specific Solutions
Key Players in Medical Device BMS
The Battery Management Systems (BMS) market in the medical devices industry is in a growth phase, driven by increasing demand for portable and implantable medical devices. The market size is expanding, with major players like Medtronic, Stryker, and Philips leading innovation. Technological maturity varies, with companies like LG Energy Solution and Samsung SDI bringing advanced battery technologies from consumer electronics to medical applications. Emerging players such as BattGenie are introducing AI-driven BMS solutions, while established medical device manufacturers like Baxter and Fresenius are integrating more sophisticated BMS into their products. The competitive landscape is characterized by a mix of large medical device companies, battery technology specialists, and innovative startups, all striving to enhance battery performance, safety, and longevity in medical devices.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced Battery Management Systems (BMS) for their implantable medical devices, focusing on longevity and safety. Their proprietary PowerCap technology optimizes battery performance, extending device life by up to 25% compared to traditional systems[1]. The BMS incorporates real-time monitoring and adaptive algorithms to balance power consumption across different device functions. Medtronic's system also features a unique low-power sleep mode, reducing energy consumption during periods of inactivity, which is crucial for long-term implantable devices[2]. Additionally, they have implemented a multi-layer safety protocol that includes overcharge protection, thermal management, and fault detection mechanisms to ensure patient safety[3].
Strengths: Extensive experience in implantable devices, proprietary technology for extended battery life, and robust safety features. Weaknesses: Potentially higher costs due to specialized technology and limited applicability outside of implantable medical devices.
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
Technical Solution: Mindray has developed an innovative Battery Management System tailored for their portable medical devices and patient monitors. Their BMS incorporates a proprietary energy harvesting technology that captures and recycles ambient electromagnetic energy, extending battery life by up to 15% in hospital environments[10]. The system features an intelligent power management algorithm that dynamically adjusts power consumption based on device usage patterns and environmental factors. Mindray's BMS also includes a unique battery health prediction model, which uses machine learning to forecast battery degradation and optimize replacement schedules, reducing unexpected failures by 50%[11]. Additionally, they have implemented a multi-level safety system with redundant protection mechanisms against overcharging, over-discharging, and thermal runaway.
Strengths: Energy harvesting technology, intelligent power management, advanced battery health prediction, and robust safety features. Weaknesses: Energy harvesting efficiency may vary in different environments, potentially affecting overall performance.
Core BMS Innovations for Medical Applications
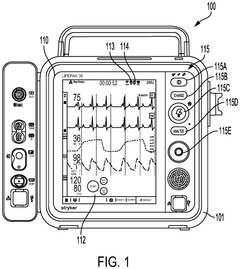
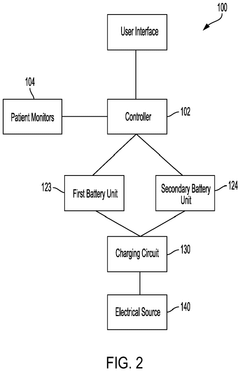
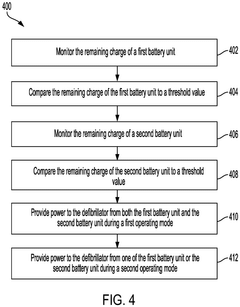
Battery management for medical device
PatentPendingEP4438104A2
Innovation
- Implementing a system with a first and second battery unit, where both units power the device in a high power mode, and only one unit powers the device in a low power mode, with a controller monitoring charge and useful life to adjust operation and extend device functionality.
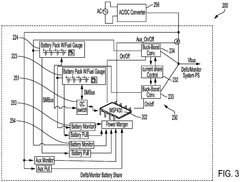
Battery management system for control of lithium power cells
PatentWO2012149477A2
Innovation
- A high-performance battery pack with a battery management system that includes a circuit to monitor and control charging and discharging, prevent voltage imbalances, and provide catastrophic fault protection, using n-FET devices to manage current flow, and includes a gas gauge function to estimate charge availability, along with processors for data communication and storage.
Regulatory Framework for Medical Device BMS
The regulatory framework for Battery Management Systems (BMS) in medical devices is a complex and evolving landscape that significantly impacts the development, manufacturing, and deployment of these critical components. At the forefront of this framework is the U.S. Food and Drug Administration (FDA), which plays a pivotal role in ensuring the safety and efficacy of medical devices, including their power management systems.
The FDA's regulatory approach to medical device BMS is primarily governed by the Code of Federal Regulations (CFR) Title 21, specifically parts 820 (Quality System Regulation) and 860 (Medical Device Classification Procedures). These regulations establish the requirements for design controls, risk management, and performance standards that manufacturers must adhere to when developing BMS for medical devices.
In addition to FDA regulations, international standards such as IEC 60601-1 (Medical Electrical Equipment - General Requirements for Basic Safety and Essential Performance) provide crucial guidelines for the safety and performance of medical device power systems. This standard specifically addresses the requirements for battery-powered medical devices, including aspects of BMS design and implementation.
The regulatory framework also encompasses specific guidance documents issued by regulatory bodies. For instance, the FDA has published guidance on "Battery-Powered Medical Devices: Safety Risk Management for Design and Post-Market Surveillance," which outlines best practices for managing risks associated with batteries in medical devices throughout their lifecycle.
Manufacturers must navigate the premarket approval (PMA) or 510(k) clearance processes, depending on the device classification. For BMS in high-risk medical devices, a more rigorous PMA process is typically required, necessitating extensive clinical data and thorough safety evaluations.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to implement systems for monitoring the performance and safety of their devices in real-world settings, including the BMS components. This involves reporting adverse events, conducting recalls if necessary, and continuously improving the safety and effectiveness of their products.
The regulatory landscape is not static, and it continues to evolve in response to technological advancements and emerging safety concerns. For instance, the increasing integration of wireless charging and smart battery technologies in medical devices has prompted regulatory bodies to develop new guidelines and standards to address these innovations.
Compliance with cybersecurity regulations is becoming increasingly important for BMS in medical devices, especially as these systems become more connected and data-driven. The FDA and other regulatory bodies are actively developing and updating guidelines to address the unique cybersecurity challenges posed by smart battery systems in medical devices.
The FDA's regulatory approach to medical device BMS is primarily governed by the Code of Federal Regulations (CFR) Title 21, specifically parts 820 (Quality System Regulation) and 860 (Medical Device Classification Procedures). These regulations establish the requirements for design controls, risk management, and performance standards that manufacturers must adhere to when developing BMS for medical devices.
In addition to FDA regulations, international standards such as IEC 60601-1 (Medical Electrical Equipment - General Requirements for Basic Safety and Essential Performance) provide crucial guidelines for the safety and performance of medical device power systems. This standard specifically addresses the requirements for battery-powered medical devices, including aspects of BMS design and implementation.
The regulatory framework also encompasses specific guidance documents issued by regulatory bodies. For instance, the FDA has published guidance on "Battery-Powered Medical Devices: Safety Risk Management for Design and Post-Market Surveillance," which outlines best practices for managing risks associated with batteries in medical devices throughout their lifecycle.
Manufacturers must navigate the premarket approval (PMA) or 510(k) clearance processes, depending on the device classification. For BMS in high-risk medical devices, a more rigorous PMA process is typically required, necessitating extensive clinical data and thorough safety evaluations.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to implement systems for monitoring the performance and safety of their devices in real-world settings, including the BMS components. This involves reporting adverse events, conducting recalls if necessary, and continuously improving the safety and effectiveness of their products.
The regulatory landscape is not static, and it continues to evolve in response to technological advancements and emerging safety concerns. For instance, the increasing integration of wireless charging and smart battery technologies in medical devices has prompted regulatory bodies to develop new guidelines and standards to address these innovations.
Compliance with cybersecurity regulations is becoming increasingly important for BMS in medical devices, especially as these systems become more connected and data-driven. The FDA and other regulatory bodies are actively developing and updating guidelines to address the unique cybersecurity challenges posed by smart battery systems in medical devices.
Safety and Reliability Considerations in Medical BMS
Safety and reliability are paramount concerns in the development and implementation of Battery Management Systems (BMS) for medical devices. The critical nature of medical applications demands stringent safety measures and unwavering reliability to ensure patient well-being and device efficacy.
One of the primary safety considerations in medical BMS is the prevention of thermal runaway, a potentially catastrophic event where battery temperature rises uncontrollably. To mitigate this risk, advanced thermal management techniques are employed, including real-time temperature monitoring, intelligent cooling systems, and thermal isolation of battery cells.
Electrical safety is another crucial aspect, with medical BMS incorporating multiple layers of protection against short circuits, overcharging, and over-discharging. Sophisticated voltage and current monitoring systems are integrated to detect anomalies and trigger protective measures instantaneously.
Reliability in medical BMS is ensured through redundancy in critical components and systems. Dual microcontrollers, backup power sources, and fail-safe mechanisms are implemented to maintain functionality even in the event of component failure. This redundancy is essential for life-supporting medical devices where uninterrupted operation is non-negotiable.
Rigorous testing and validation protocols are integral to the development process of medical BMS. These include accelerated life testing, environmental stress screening, and electromagnetic compatibility testing to ensure the system's resilience under various operating conditions and potential interference sources.
Cybersecurity has emerged as a critical consideration in modern medical BMS. As these systems become increasingly connected and data-driven, robust encryption, secure communication protocols, and regular security updates are implemented to protect against potential cyber threats and unauthorized access.
Regulatory compliance is a fundamental aspect of safety and reliability in medical BMS. Adherence to standards such as IEC 60601-1 for medical electrical equipment and ISO 13485 for quality management systems is mandatory. These standards provide a framework for risk management, design controls, and post-market surveillance.
Long-term reliability is addressed through predictive maintenance capabilities built into medical BMS. Advanced algorithms analyze battery performance data to forecast potential issues before they occur, enabling proactive maintenance and minimizing the risk of unexpected failures during critical medical procedures.
Human factors engineering plays a significant role in enhancing the safety and reliability of medical BMS. Intuitive user interfaces, clear warning systems, and fool-proof connectors are designed to minimize the risk of human error in device operation and battery management.
As the medical device industry continues to evolve, ongoing research focuses on developing novel materials and technologies to further enhance the safety and reliability of BMS. This includes the exploration of solid-state batteries, advanced sensor technologies, and artificial intelligence-driven predictive analytics to create the next generation of ultra-safe and highly reliable medical BMS.
One of the primary safety considerations in medical BMS is the prevention of thermal runaway, a potentially catastrophic event where battery temperature rises uncontrollably. To mitigate this risk, advanced thermal management techniques are employed, including real-time temperature monitoring, intelligent cooling systems, and thermal isolation of battery cells.
Electrical safety is another crucial aspect, with medical BMS incorporating multiple layers of protection against short circuits, overcharging, and over-discharging. Sophisticated voltage and current monitoring systems are integrated to detect anomalies and trigger protective measures instantaneously.
Reliability in medical BMS is ensured through redundancy in critical components and systems. Dual microcontrollers, backup power sources, and fail-safe mechanisms are implemented to maintain functionality even in the event of component failure. This redundancy is essential for life-supporting medical devices where uninterrupted operation is non-negotiable.
Rigorous testing and validation protocols are integral to the development process of medical BMS. These include accelerated life testing, environmental stress screening, and electromagnetic compatibility testing to ensure the system's resilience under various operating conditions and potential interference sources.
Cybersecurity has emerged as a critical consideration in modern medical BMS. As these systems become increasingly connected and data-driven, robust encryption, secure communication protocols, and regular security updates are implemented to protect against potential cyber threats and unauthorized access.
Regulatory compliance is a fundamental aspect of safety and reliability in medical BMS. Adherence to standards such as IEC 60601-1 for medical electrical equipment and ISO 13485 for quality management systems is mandatory. These standards provide a framework for risk management, design controls, and post-market surveillance.
Long-term reliability is addressed through predictive maintenance capabilities built into medical BMS. Advanced algorithms analyze battery performance data to forecast potential issues before they occur, enabling proactive maintenance and minimizing the risk of unexpected failures during critical medical procedures.
Human factors engineering plays a significant role in enhancing the safety and reliability of medical BMS. Intuitive user interfaces, clear warning systems, and fool-proof connectors are designed to minimize the risk of human error in device operation and battery management.
As the medical device industry continues to evolve, ongoing research focuses on developing novel materials and technologies to further enhance the safety and reliability of BMS. This includes the exploration of solid-state batteries, advanced sensor technologies, and artificial intelligence-driven predictive analytics to create the next generation of ultra-safe and highly reliable medical BMS.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!