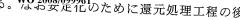Carbolic Acid Interaction with Common Industrial Catalysts
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Catalysis Background and Objectives
Carbolic acid, also known as phenol, has been a subject of significant interest in the field of industrial catalysis for decades. The interaction between carbolic acid and common industrial catalysts has played a crucial role in various chemical processes, particularly in the production of plastics, pharmaceuticals, and other high-value chemicals. This technological domain has witnessed substantial evolution since the early 20th century, driven by the increasing demand for more efficient and environmentally friendly production methods.
The development of catalytic processes involving carbolic acid has been closely tied to the growth of the petrochemical industry. As the demand for phenol-derived products surged, researchers and industry professionals sought to optimize catalytic reactions to improve yield, selectivity, and energy efficiency. The journey began with simple metal oxide catalysts and progressed towards more complex, multi-component catalytic systems.
One of the primary objectives in this field has been to enhance the conversion efficiency of carbolic acid in various reactions, such as alkylation, oxidation, and hydrogenation. Researchers have focused on developing catalysts that can operate under milder conditions, reduce byproduct formation, and exhibit longer lifespans. Additionally, there has been a growing emphasis on creating catalysts that are more resistant to deactivation caused by the corrosive nature of carbolic acid.
The environmental impact of carbolic acid catalysis has also become a significant concern in recent years. As a result, there is an increasing trend towards developing greener catalytic processes that minimize waste generation and reduce the use of hazardous substances. This has led to the exploration of bio-based catalysts and the integration of carbolic acid catalysis with other sustainable technologies.
Another key objective in this field is to improve the understanding of the fundamental mechanisms governing the interaction between carbolic acid and various catalysts. Advanced characterization techniques, such as in-situ spectroscopy and high-resolution microscopy, have enabled researchers to gain deeper insights into the surface chemistry and reaction kinetics involved in these catalytic processes.
Looking ahead, the field of carbolic acid catalysis aims to address several challenges, including the development of more selective catalysts for complex transformations, the design of catalytic systems capable of handling impure feedstocks, and the scaling up of novel catalytic processes for industrial applications. The integration of artificial intelligence and machine learning in catalyst design and process optimization is expected to accelerate progress in this domain, potentially leading to breakthrough innovations in the coming years.
The development of catalytic processes involving carbolic acid has been closely tied to the growth of the petrochemical industry. As the demand for phenol-derived products surged, researchers and industry professionals sought to optimize catalytic reactions to improve yield, selectivity, and energy efficiency. The journey began with simple metal oxide catalysts and progressed towards more complex, multi-component catalytic systems.
One of the primary objectives in this field has been to enhance the conversion efficiency of carbolic acid in various reactions, such as alkylation, oxidation, and hydrogenation. Researchers have focused on developing catalysts that can operate under milder conditions, reduce byproduct formation, and exhibit longer lifespans. Additionally, there has been a growing emphasis on creating catalysts that are more resistant to deactivation caused by the corrosive nature of carbolic acid.
The environmental impact of carbolic acid catalysis has also become a significant concern in recent years. As a result, there is an increasing trend towards developing greener catalytic processes that minimize waste generation and reduce the use of hazardous substances. This has led to the exploration of bio-based catalysts and the integration of carbolic acid catalysis with other sustainable technologies.
Another key objective in this field is to improve the understanding of the fundamental mechanisms governing the interaction between carbolic acid and various catalysts. Advanced characterization techniques, such as in-situ spectroscopy and high-resolution microscopy, have enabled researchers to gain deeper insights into the surface chemistry and reaction kinetics involved in these catalytic processes.
Looking ahead, the field of carbolic acid catalysis aims to address several challenges, including the development of more selective catalysts for complex transformations, the design of catalytic systems capable of handling impure feedstocks, and the scaling up of novel catalytic processes for industrial applications. The integration of artificial intelligence and machine learning in catalyst design and process optimization is expected to accelerate progress in this domain, potentially leading to breakthrough innovations in the coming years.
Industrial Demand for Carbolic Acid Catalytic Processes
The demand for carbolic acid catalytic processes in industrial applications has been steadily growing due to the versatile nature of carbolic acid, also known as phenol, in various manufacturing sectors. This compound serves as a crucial intermediate in the production of numerous products, including plastics, pharmaceuticals, and agrochemicals. The global phenol market size was valued at over $20 billion in 2020, with projections indicating continued growth in the coming years.
The primary driver for this demand is the expanding plastics industry, particularly in the production of bisphenol A (BPA), which is a key component in polycarbonate plastics and epoxy resins. These materials find extensive use in automotive, construction, and electronics industries. The increasing consumption of polycarbonate in lightweight automotive components and electronic devices has significantly boosted the demand for carbolic acid catalytic processes.
In the pharmaceutical sector, carbolic acid serves as a precursor for various drugs and antiseptics. The growing healthcare industry, coupled with the rising demand for over-the-counter medications, has further intensified the need for efficient carbolic acid production methods. Additionally, the agrochemical industry utilizes carbolic acid in the synthesis of herbicides and pesticides, contributing to the overall market demand.
The demand for carbolic acid catalytic processes is also influenced by environmental regulations and sustainability concerns. Industries are increasingly seeking more efficient and environmentally friendly production methods to reduce waste and energy consumption. This has led to a focus on developing improved catalysts and process technologies that can enhance yield, selectivity, and overall efficiency in carbolic acid production.
Geographically, Asia-Pacific region dominates the carbolic acid market, with China being the largest producer and consumer. The rapid industrialization and urbanization in emerging economies have significantly contributed to the growing demand in this region. North America and Europe also maintain substantial market shares, driven by their well-established chemical and pharmaceutical industries.
The industrial demand for carbolic acid catalytic processes is closely tied to economic factors and global industrial output. Economic downturns or disruptions in supply chains can impact the demand, as seen during the recent global pandemic. However, the long-term outlook remains positive, supported by the diverse applications of carbolic acid and ongoing technological advancements in catalytic processes.
The primary driver for this demand is the expanding plastics industry, particularly in the production of bisphenol A (BPA), which is a key component in polycarbonate plastics and epoxy resins. These materials find extensive use in automotive, construction, and electronics industries. The increasing consumption of polycarbonate in lightweight automotive components and electronic devices has significantly boosted the demand for carbolic acid catalytic processes.
In the pharmaceutical sector, carbolic acid serves as a precursor for various drugs and antiseptics. The growing healthcare industry, coupled with the rising demand for over-the-counter medications, has further intensified the need for efficient carbolic acid production methods. Additionally, the agrochemical industry utilizes carbolic acid in the synthesis of herbicides and pesticides, contributing to the overall market demand.
The demand for carbolic acid catalytic processes is also influenced by environmental regulations and sustainability concerns. Industries are increasingly seeking more efficient and environmentally friendly production methods to reduce waste and energy consumption. This has led to a focus on developing improved catalysts and process technologies that can enhance yield, selectivity, and overall efficiency in carbolic acid production.
Geographically, Asia-Pacific region dominates the carbolic acid market, with China being the largest producer and consumer. The rapid industrialization and urbanization in emerging economies have significantly contributed to the growing demand in this region. North America and Europe also maintain substantial market shares, driven by their well-established chemical and pharmaceutical industries.
The industrial demand for carbolic acid catalytic processes is closely tied to economic factors and global industrial output. Economic downturns or disruptions in supply chains can impact the demand, as seen during the recent global pandemic. However, the long-term outlook remains positive, supported by the diverse applications of carbolic acid and ongoing technological advancements in catalytic processes.
Current Challenges in Carbolic Acid-Catalyst Interactions
The interaction between carbolic acid and industrial catalysts presents several significant challenges that hinder optimal performance and efficiency in various chemical processes. One of the primary issues is catalyst deactivation due to the corrosive nature of carbolic acid. This phenomenon leads to reduced catalytic activity over time, necessitating frequent catalyst replacement and increasing operational costs.
Another major challenge is the formation of undesired by-products during reactions involving carbolic acid and catalysts. These side reactions not only decrease the yield of target compounds but also complicate downstream separation processes. The presence of impurities in the carbolic acid feedstock further exacerbates this problem, as they can interact with catalysts in unpredictable ways, leading to inconsistent product quality and reduced process reliability.
The high reactivity of carbolic acid also poses safety concerns in industrial settings. Its ability to rapidly react with certain catalysts can result in exothermic reactions, potentially leading to thermal runaway scenarios if not properly controlled. This necessitates stringent safety measures and sophisticated process control systems, adding complexity and cost to industrial operations.
Catalyst selectivity remains a significant challenge in carbolic acid-related processes. Achieving high selectivity towards desired products while minimizing unwanted side reactions is crucial for process efficiency. However, the strong interaction between carbolic acid and many catalysts often leads to poor selectivity, requiring extensive research and development efforts to design more specific and robust catalytic systems.
Mass transfer limitations present another hurdle in carbolic acid-catalyst interactions. The viscous nature of carbolic acid can impede efficient contact between reactants and catalyst surfaces, particularly in heterogeneous catalysis. This limitation can result in reduced reaction rates and lower overall process efficiency, necessitating innovative reactor designs and catalyst formulations to overcome these constraints.
The environmental impact of carbolic acid-catalyst interactions is also a growing concern. The potential for catalyst leaching and the generation of hazardous waste streams pose significant environmental risks. Developing green catalytic processes that minimize waste production and maximize atom economy remains a key challenge in this field.
Lastly, the scalability of laboratory-proven catalytic systems to industrial-scale operations presents numerous engineering challenges. Maintaining catalyst performance and stability under the harsh conditions of large-scale production, while ensuring economic viability, requires extensive process optimization and often involves trade-offs between efficiency, cost, and environmental considerations.
Another major challenge is the formation of undesired by-products during reactions involving carbolic acid and catalysts. These side reactions not only decrease the yield of target compounds but also complicate downstream separation processes. The presence of impurities in the carbolic acid feedstock further exacerbates this problem, as they can interact with catalysts in unpredictable ways, leading to inconsistent product quality and reduced process reliability.
The high reactivity of carbolic acid also poses safety concerns in industrial settings. Its ability to rapidly react with certain catalysts can result in exothermic reactions, potentially leading to thermal runaway scenarios if not properly controlled. This necessitates stringent safety measures and sophisticated process control systems, adding complexity and cost to industrial operations.
Catalyst selectivity remains a significant challenge in carbolic acid-related processes. Achieving high selectivity towards desired products while minimizing unwanted side reactions is crucial for process efficiency. However, the strong interaction between carbolic acid and many catalysts often leads to poor selectivity, requiring extensive research and development efforts to design more specific and robust catalytic systems.
Mass transfer limitations present another hurdle in carbolic acid-catalyst interactions. The viscous nature of carbolic acid can impede efficient contact between reactants and catalyst surfaces, particularly in heterogeneous catalysis. This limitation can result in reduced reaction rates and lower overall process efficiency, necessitating innovative reactor designs and catalyst formulations to overcome these constraints.
The environmental impact of carbolic acid-catalyst interactions is also a growing concern. The potential for catalyst leaching and the generation of hazardous waste streams pose significant environmental risks. Developing green catalytic processes that minimize waste production and maximize atom economy remains a key challenge in this field.
Lastly, the scalability of laboratory-proven catalytic systems to industrial-scale operations presents numerous engineering challenges. Maintaining catalyst performance and stability under the harsh conditions of large-scale production, while ensuring economic viability, requires extensive process optimization and often involves trade-offs between efficiency, cost, and environmental considerations.
Existing Catalytic Solutions for Carbolic Acid Processing
01 Historical use of carbolic acid in medical applications
Carbolic acid, also known as phenol, has a long history of use in medical applications. It was widely used as an antiseptic and disinfectant in the late 19th and early 20th centuries. Its properties made it effective for sterilizing surgical instruments and treating wounds, although its use has since been largely replaced by safer alternatives.- Carbolic acid in medical applications: Carbolic acid, also known as phenol, has been used in various medical applications due to its antiseptic properties. It has been utilized in disinfectants, wound treatments, and surgical procedures to prevent infections. The use of carbolic acid in medical settings has evolved over time, with improvements in formulations and application methods to enhance its effectiveness and safety.
- Carbolic acid in industrial processes: Carbolic acid finds applications in various industrial processes, including the production of plastics, resins, and other chemical compounds. It serves as a precursor or intermediate in the synthesis of numerous products. Industrial uses of carbolic acid often involve its incorporation into manufacturing processes or as a component in specialized formulations for specific industrial applications.
- Carbolic acid in water treatment: Carbolic acid has been employed in water treatment processes due to its disinfectant properties. It can be used to purify water and eliminate harmful microorganisms. The application of carbolic acid in water treatment systems often involves careful dosing and monitoring to ensure effective disinfection while maintaining water safety for consumption or other uses.
- Carbolic acid in personal care products: Carbolic acid has been used in various personal care products, including soaps, shampoos, and other hygiene items. Its antiseptic properties make it effective in cleansing and disinfecting the skin and hair. However, due to potential skin irritation, its use in personal care products has become limited, with alternative ingredients often preferred in modern formulations.
- Safety measures and handling of carbolic acid: Given the corrosive and toxic nature of carbolic acid, proper safety measures and handling procedures are crucial. This includes the use of protective equipment, specialized storage containers, and proper disposal methods. Safety protocols for working with carbolic acid often involve ventilation systems, emergency response plans, and employee training to minimize risks associated with its use in various applications.
02 Carbolic acid in industrial processes
Carbolic acid finds applications in various industrial processes. It is used in the production of plastics, resins, and other synthetic materials. Its chemical properties make it valuable in manufacturing processes, particularly in the creation of phenolic resins and as a precursor for other chemical compounds.Expand Specific Solutions03 Environmental and waste treatment applications
Carbolic acid and its derivatives are utilized in environmental and waste treatment applications. They can be employed in water purification processes, sewage treatment, and as components in certain types of air purification systems. These applications leverage the compound's disinfectant properties.Expand Specific Solutions04 Safety considerations and handling of carbolic acid
Due to its corrosive and toxic nature, special safety measures are required when handling carbolic acid. This includes the use of protective equipment, proper storage facilities, and specific handling procedures. Safety considerations are crucial in industrial settings where carbolic acid is used or produced.Expand Specific Solutions05 Modern alternatives and substitutes for carbolic acid
In many applications, safer alternatives have been developed to replace carbolic acid. These substitutes aim to provide similar functionality while reducing health and environmental risks. Research continues to find more environmentally friendly and less hazardous compounds that can perform the same roles as carbolic acid in various industries.Expand Specific Solutions
Major Players in Industrial Catalysis for Carbolic Acid
The competitive landscape for "Carbolic Acid Interaction with Common Industrial Catalysts" is characterized by a mature industry with established players and ongoing research. The market size is significant, given the widespread use of carbolic acid (phenol) in various industrial applications. Major petrochemical companies like China Petroleum & Chemical Corp., PetroChina, and Air Liquide are key players, leveraging their extensive resources and expertise. The technology's maturity is evident, with research institutions like Nanjing Tech University and East China Normal University contributing to advancements. Specialty chemical manufacturers such as Eastman Chemical and Sumitomo Chemical are also active in this field, focusing on developing innovative catalysts and processes for carbolic acid interactions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic systems for carbolic acid interactions in industrial processes. Their approach involves using modified zeolite catalysts with enhanced acidity and shape selectivity[1]. These catalysts demonstrate improved conversion rates of carbolic acid to valuable petrochemical products, such as phenol and cyclohexanone. Sinopec's research has also focused on optimizing reaction conditions, including temperature and pressure, to maximize yield and minimize byproduct formation[3]. Additionally, they have explored the use of noble metal-doped catalysts to enhance selectivity in carbolic acid hydrogenation reactions[5].
Strengths: Large-scale production capabilities, extensive R&D resources, and integrated supply chain. Weaknesses: Potential environmental concerns and dependence on fossil fuel feedstocks.
PetroChina Co., Ltd.
Technical Solution: PetroChina has invested in developing novel catalytic systems for carbolic acid conversion in petrochemical processes. Their approach focuses on heterogeneous catalysts with high surface area and tailored pore structures to enhance mass transfer and reaction kinetics[2]. PetroChina's researchers have made significant progress in designing bimetallic catalysts that exhibit synergistic effects, improving both activity and selectivity in carbolic acid transformations[4]. They have also explored the use of supported ionic liquid catalysts (SILCs) to facilitate carbolic acid esterification reactions, achieving higher yields and easier product separation[6].
Strengths: Strong government support, vast domestic market, and extensive oil and gas reserves. Weaknesses: Relatively newer to advanced catalyst research compared to some international competitors.
Key Innovations in Carbolic Acid-Catalyst Interactions
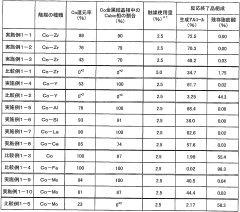
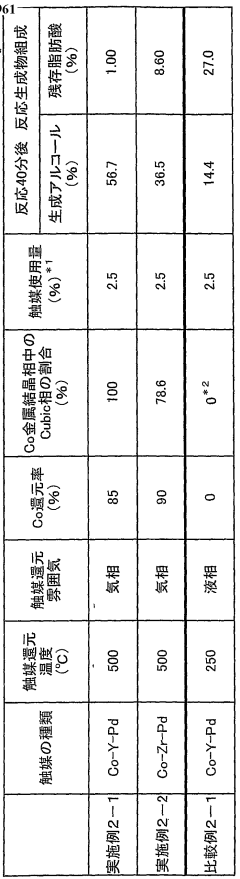
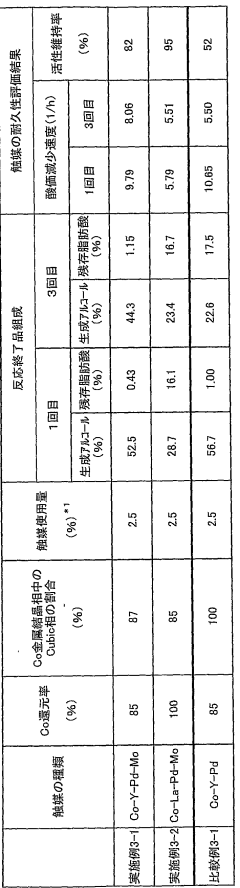
Catalyst for alcohol production
PatentWO2008099961A1
Innovation
- A catalyst comprising Co metal as an essential component, with Zr, Y, La, Ce, Si, Al, Sc, and V as cocatalyst components, and a Cubic phase content of 20% or more, produced under specific reduction conditions, enhancing catalytic activity and durability.
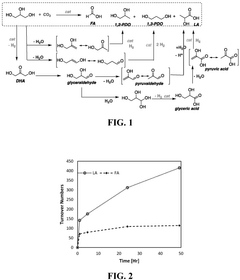
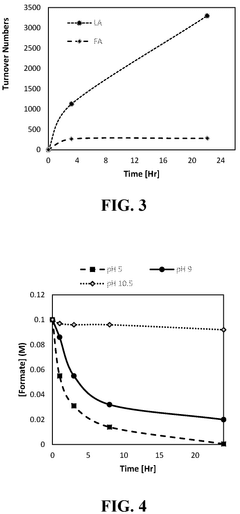
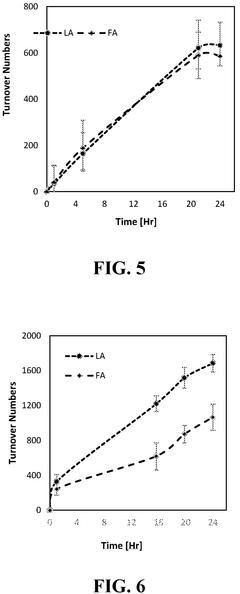
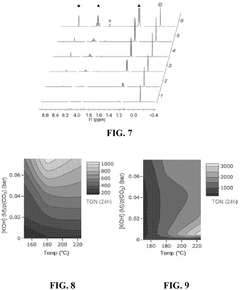
Catalysts for the transformation of carbon dioxide and glycerol to formic acid and lactic acid and methods of making the same
PatentActiveUS12194448B2
Innovation
- Development of water-soluble iridium (Ir) and ruthenium (Ru) N-heterocyclic carbene complexes as homogeneous catalysts or immobilized on solid substrates as heterogeneous catalysts for transforming CO2 or carbonate salts and glycerol into formic acid and lactic acid, optimizing reaction conditions like temperature, pressure, and base concentration to enhance efficiency.
Environmental Impact of Carbolic Acid Catalytic Processes
The environmental impact of carbolic acid catalytic processes is a critical consideration in industrial applications. These processes, while essential for various chemical productions, can have significant effects on ecosystems and human health if not properly managed.
Carbolic acid, also known as phenol, is a toxic compound that can cause severe environmental damage if released into water bodies or soil. When used in catalytic processes, there is a risk of emissions and waste generation that must be carefully controlled. The catalysts used in these processes, often metal-based, can also contribute to environmental contamination if not properly handled or disposed of.
One of the primary environmental concerns is the potential for air pollution. Volatile organic compounds (VOCs) may be released during carbolic acid catalytic reactions, contributing to smog formation and air quality degradation. Additionally, the production of byproducts and waste streams from these processes can lead to water pollution if not adequately treated before discharge.
Soil contamination is another significant risk associated with carbolic acid catalytic processes. Spills or improper disposal of waste materials can result in the accumulation of phenolic compounds in soil, affecting plant growth and soil microorganisms. This contamination can persist for extended periods and potentially enter the food chain through agricultural products.
The energy-intensive nature of many catalytic processes involving carbolic acid also contributes to their environmental footprint. The high temperatures and pressures often required in these reactions result in substantial energy consumption, typically derived from fossil fuel sources, leading to increased greenhouse gas emissions and climate change impacts.
However, advancements in green chemistry and sustainable catalysis are providing opportunities to mitigate these environmental concerns. The development of more efficient catalysts that operate under milder conditions can reduce energy requirements and minimize waste production. Additionally, the implementation of closed-loop systems and improved recycling techniques for both catalysts and reactants can significantly reduce the environmental burden of these processes.
Regulatory frameworks play a crucial role in managing the environmental impact of carbolic acid catalytic processes. Stringent emissions standards, waste management protocols, and monitoring requirements are essential for ensuring that industries employing these processes operate in an environmentally responsible manner. Furthermore, life cycle assessments of carbolic acid-based products and their production methods are increasingly being used to identify areas for environmental improvement throughout the entire value chain.
Carbolic acid, also known as phenol, is a toxic compound that can cause severe environmental damage if released into water bodies or soil. When used in catalytic processes, there is a risk of emissions and waste generation that must be carefully controlled. The catalysts used in these processes, often metal-based, can also contribute to environmental contamination if not properly handled or disposed of.
One of the primary environmental concerns is the potential for air pollution. Volatile organic compounds (VOCs) may be released during carbolic acid catalytic reactions, contributing to smog formation and air quality degradation. Additionally, the production of byproducts and waste streams from these processes can lead to water pollution if not adequately treated before discharge.
Soil contamination is another significant risk associated with carbolic acid catalytic processes. Spills or improper disposal of waste materials can result in the accumulation of phenolic compounds in soil, affecting plant growth and soil microorganisms. This contamination can persist for extended periods and potentially enter the food chain through agricultural products.
The energy-intensive nature of many catalytic processes involving carbolic acid also contributes to their environmental footprint. The high temperatures and pressures often required in these reactions result in substantial energy consumption, typically derived from fossil fuel sources, leading to increased greenhouse gas emissions and climate change impacts.
However, advancements in green chemistry and sustainable catalysis are providing opportunities to mitigate these environmental concerns. The development of more efficient catalysts that operate under milder conditions can reduce energy requirements and minimize waste production. Additionally, the implementation of closed-loop systems and improved recycling techniques for both catalysts and reactants can significantly reduce the environmental burden of these processes.
Regulatory frameworks play a crucial role in managing the environmental impact of carbolic acid catalytic processes. Stringent emissions standards, waste management protocols, and monitoring requirements are essential for ensuring that industries employing these processes operate in an environmentally responsible manner. Furthermore, life cycle assessments of carbolic acid-based products and their production methods are increasingly being used to identify areas for environmental improvement throughout the entire value chain.
Safety Protocols in Carbolic Acid Catalysis
Safety protocols in carbolic acid catalysis are of paramount importance due to the hazardous nature of the compound and its potential interactions with industrial catalysts. Carbolic acid, also known as phenol, is a highly corrosive and toxic substance that requires careful handling and stringent safety measures throughout the catalytic process.
Proper personal protective equipment (PPE) is essential for all personnel involved in carbolic acid catalysis. This includes chemical-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may also be necessary, depending on the specific process and potential for vapor exposure. Regular training and refresher courses on the proper use of PPE and emergency procedures should be mandatory for all workers.
Ventilation systems play a crucial role in maintaining a safe working environment. Adequate local exhaust ventilation should be installed to remove any vapors or fumes generated during the catalytic process. Regular maintenance and testing of these systems are necessary to ensure their effectiveness in preventing the accumulation of harmful concentrations of carbolic acid in the air.
Containment measures are essential to prevent spills and leaks. All equipment, including reactors, storage tanks, and transfer lines, should be designed with appropriate materials that are resistant to carbolic acid corrosion. Secondary containment systems, such as dikes or berms, should be in place to contain potential spills and prevent environmental contamination.
Emergency response protocols must be well-established and regularly practiced. This includes procedures for spill containment, decontamination, and first aid. Eyewash stations and safety showers should be readily accessible in all areas where carbolic acid is handled or stored. A comprehensive emergency response plan should be developed, detailing evacuation procedures and communication protocols in case of a major incident.
Proper storage and handling procedures are critical to prevent accidents. Carbolic acid should be stored in a cool, dry, well-ventilated area, away from incompatible materials and sources of ignition. Dedicated storage areas with restricted access should be used, and inventory management systems should be implemented to track the quantity and location of carbolic acid on-site.
Regular monitoring and testing of the catalytic process are necessary to detect any potential issues early. This includes monitoring reaction conditions, catalyst performance, and the integrity of equipment. Implementing a robust maintenance schedule can help prevent equipment failures that could lead to safety incidents.
Waste management is another crucial aspect of safety protocols in carbolic acid catalysis. Proper disposal methods for spent catalysts, contaminated materials, and waste products must be established and followed. This may involve neutralization, incineration, or other specialized treatment processes, depending on local regulations and the specific nature of the waste.
Proper personal protective equipment (PPE) is essential for all personnel involved in carbolic acid catalysis. This includes chemical-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection may also be necessary, depending on the specific process and potential for vapor exposure. Regular training and refresher courses on the proper use of PPE and emergency procedures should be mandatory for all workers.
Ventilation systems play a crucial role in maintaining a safe working environment. Adequate local exhaust ventilation should be installed to remove any vapors or fumes generated during the catalytic process. Regular maintenance and testing of these systems are necessary to ensure their effectiveness in preventing the accumulation of harmful concentrations of carbolic acid in the air.
Containment measures are essential to prevent spills and leaks. All equipment, including reactors, storage tanks, and transfer lines, should be designed with appropriate materials that are resistant to carbolic acid corrosion. Secondary containment systems, such as dikes or berms, should be in place to contain potential spills and prevent environmental contamination.
Emergency response protocols must be well-established and regularly practiced. This includes procedures for spill containment, decontamination, and first aid. Eyewash stations and safety showers should be readily accessible in all areas where carbolic acid is handled or stored. A comprehensive emergency response plan should be developed, detailing evacuation procedures and communication protocols in case of a major incident.
Proper storage and handling procedures are critical to prevent accidents. Carbolic acid should be stored in a cool, dry, well-ventilated area, away from incompatible materials and sources of ignition. Dedicated storage areas with restricted access should be used, and inventory management systems should be implemented to track the quantity and location of carbolic acid on-site.
Regular monitoring and testing of the catalytic process are necessary to detect any potential issues early. This includes monitoring reaction conditions, catalyst performance, and the integrity of equipment. Implementing a robust maintenance schedule can help prevent equipment failures that could lead to safety incidents.
Waste management is another crucial aspect of safety protocols in carbolic acid catalysis. Proper disposal methods for spent catalysts, contaminated materials, and waste products must be established and followed. This may involve neutralization, incineration, or other specialized treatment processes, depending on local regulations and the specific nature of the waste.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!