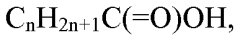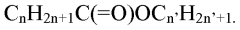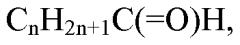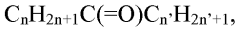Carbolic Acid’s Role in Higher Yield Chemical Processes
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Overview
Carbolic acid, also known as phenol, is a crucial organic compound with significant applications in various chemical processes. Its molecular formula, C6H5OH, consists of a hydroxyl group (-OH) attached to a benzene ring. This unique structure grants carbolic acid its distinctive properties, making it invaluable in industrial and scientific applications.
The compound was first isolated from coal tar in the 1830s by German chemist Friedlieb Ferdinand Runge. Its discovery marked a significant milestone in organic chemistry and paved the way for numerous advancements in chemical synthesis. Carbolic acid's importance in higher yield chemical processes stems from its versatile reactivity and its role as a precursor for many other valuable compounds.
In its pure form, carbolic acid appears as colorless needle-shaped crystals that melt at approximately 41°C (106°F). It has a characteristic sweet, tarry odor and is highly corrosive to human tissue. Despite its hazardous nature, when properly handled, carbolic acid serves as a vital reagent in various industrial processes.
One of the primary applications of carbolic acid is in the production of phenolic resins, which are widely used in the manufacture of plywood, construction materials, and automotive parts. These resins offer excellent heat resistance and electrical insulation properties, making them indispensable in many industries. Additionally, carbolic acid serves as a starting material for the synthesis of numerous pharmaceuticals, including aspirin and various antiseptics.
In the realm of higher yield chemical processes, carbolic acid plays a crucial role as both a reactant and a catalyst. Its ability to undergo electrophilic aromatic substitution reactions makes it an excellent building block for synthesizing more complex organic compounds. Furthermore, its acidic properties allow it to catalyze certain reactions, enhancing yields and reducing reaction times in various chemical processes.
The production of carbolic acid itself has evolved over time, with modern methods focusing on more efficient and environmentally friendly processes. Currently, the most common industrial method involves the partial oxidation of cumene, known as the cumene process. This method not only produces carbolic acid but also acetone as a valuable co-product, significantly improving the overall economic viability of the process.
As environmental concerns grow, researchers are exploring greener alternatives for carbolic acid production and utilization. These efforts include developing bio-based routes for phenol synthesis and investigating novel catalytic systems to enhance reaction efficiency while minimizing waste generation. Such advancements are crucial for ensuring the sustainable use of carbolic acid in future chemical processes.
The compound was first isolated from coal tar in the 1830s by German chemist Friedlieb Ferdinand Runge. Its discovery marked a significant milestone in organic chemistry and paved the way for numerous advancements in chemical synthesis. Carbolic acid's importance in higher yield chemical processes stems from its versatile reactivity and its role as a precursor for many other valuable compounds.
In its pure form, carbolic acid appears as colorless needle-shaped crystals that melt at approximately 41°C (106°F). It has a characteristic sweet, tarry odor and is highly corrosive to human tissue. Despite its hazardous nature, when properly handled, carbolic acid serves as a vital reagent in various industrial processes.
One of the primary applications of carbolic acid is in the production of phenolic resins, which are widely used in the manufacture of plywood, construction materials, and automotive parts. These resins offer excellent heat resistance and electrical insulation properties, making them indispensable in many industries. Additionally, carbolic acid serves as a starting material for the synthesis of numerous pharmaceuticals, including aspirin and various antiseptics.
In the realm of higher yield chemical processes, carbolic acid plays a crucial role as both a reactant and a catalyst. Its ability to undergo electrophilic aromatic substitution reactions makes it an excellent building block for synthesizing more complex organic compounds. Furthermore, its acidic properties allow it to catalyze certain reactions, enhancing yields and reducing reaction times in various chemical processes.
The production of carbolic acid itself has evolved over time, with modern methods focusing on more efficient and environmentally friendly processes. Currently, the most common industrial method involves the partial oxidation of cumene, known as the cumene process. This method not only produces carbolic acid but also acetone as a valuable co-product, significantly improving the overall economic viability of the process.
As environmental concerns grow, researchers are exploring greener alternatives for carbolic acid production and utilization. These efforts include developing bio-based routes for phenol synthesis and investigating novel catalytic systems to enhance reaction efficiency while minimizing waste generation. Such advancements are crucial for ensuring the sustainable use of carbolic acid in future chemical processes.
Market Demand Analysis
The market demand for carbolic acid, also known as phenol, in higher yield chemical processes has been steadily increasing due to its versatile applications across various industries. The global phenol market size was valued at USD 23.3 billion in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 3.5% from 2021 to 2028. This growth is primarily driven by the rising demand for phenol derivatives in end-use industries such as automotive, construction, and electronics.
In the chemical industry, carbolic acid plays a crucial role in the production of bisphenol A (BPA), which is a key raw material for polycarbonates and epoxy resins. The increasing demand for these materials in automotive and construction sectors is expected to fuel the market growth for carbolic acid. Additionally, the growing use of phenolic resins in the electronics industry for printed circuit boards and semiconductor encapsulation is further boosting the demand.
The pharmaceutical sector is another significant consumer of carbolic acid, utilizing it in the synthesis of various drugs and antiseptics. With the global pharmaceutical market expanding, particularly in emerging economies, the demand for carbolic acid in this sector is anticipated to rise. Furthermore, the personal care and cosmetics industry is increasingly incorporating phenol derivatives in products such as hair dyes, sunscreens, and skin lightening agents, contributing to the overall market growth.
Environmental concerns and stringent regulations regarding the use of phenol-based products have led to a shift towards bio-based alternatives. However, the superior properties and cost-effectiveness of carbolic acid continue to maintain its dominance in the market. Manufacturers are focusing on developing eco-friendly production processes and exploring new applications to sustain market growth.
Geographically, Asia Pacific is expected to witness the highest growth in carbolic acid demand, driven by rapid industrialization, urbanization, and increasing disposable incomes in countries like China and India. North America and Europe are mature markets but continue to show steady growth due to ongoing research and development activities in high-performance materials and specialty chemicals.
The COVID-19 pandemic initially disrupted the supply chain and manufacturing activities, leading to a temporary decline in demand. However, the market has shown resilience and is recovering, with increased demand for disinfectants and medical equipment contributing to the rebound. As economies recover and industrial activities resume, the market for carbolic acid in higher yield chemical processes is expected to regain its growth trajectory.
In the chemical industry, carbolic acid plays a crucial role in the production of bisphenol A (BPA), which is a key raw material for polycarbonates and epoxy resins. The increasing demand for these materials in automotive and construction sectors is expected to fuel the market growth for carbolic acid. Additionally, the growing use of phenolic resins in the electronics industry for printed circuit boards and semiconductor encapsulation is further boosting the demand.
The pharmaceutical sector is another significant consumer of carbolic acid, utilizing it in the synthesis of various drugs and antiseptics. With the global pharmaceutical market expanding, particularly in emerging economies, the demand for carbolic acid in this sector is anticipated to rise. Furthermore, the personal care and cosmetics industry is increasingly incorporating phenol derivatives in products such as hair dyes, sunscreens, and skin lightening agents, contributing to the overall market growth.
Environmental concerns and stringent regulations regarding the use of phenol-based products have led to a shift towards bio-based alternatives. However, the superior properties and cost-effectiveness of carbolic acid continue to maintain its dominance in the market. Manufacturers are focusing on developing eco-friendly production processes and exploring new applications to sustain market growth.
Geographically, Asia Pacific is expected to witness the highest growth in carbolic acid demand, driven by rapid industrialization, urbanization, and increasing disposable incomes in countries like China and India. North America and Europe are mature markets but continue to show steady growth due to ongoing research and development activities in high-performance materials and specialty chemicals.
The COVID-19 pandemic initially disrupted the supply chain and manufacturing activities, leading to a temporary decline in demand. However, the market has shown resilience and is recovering, with increased demand for disinfectants and medical equipment contributing to the rebound. As economies recover and industrial activities resume, the market for carbolic acid in higher yield chemical processes is expected to regain its growth trajectory.
Current Challenges
The use of carbolic acid in higher yield chemical processes faces several significant challenges that hinder its widespread adoption and optimal utilization. One of the primary obstacles is the corrosive nature of carbolic acid, which poses substantial risks to equipment and infrastructure. This corrosivity necessitates the use of specialized materials and protective coatings, significantly increasing production costs and maintenance requirements.
Another major challenge is the toxicity of carbolic acid, which presents serious health and safety concerns for workers in chemical plants. Stringent safety protocols and protective measures must be implemented, leading to increased operational complexity and potential productivity losses. Furthermore, the environmental impact of carbolic acid is a growing concern, as improper handling or accidental release can have severe consequences on ecosystems and water sources.
The volatility of carbolic acid at room temperature presents additional challenges in terms of storage, transportation, and process control. This volatility not only increases the risk of exposure but also complicates the maintenance of precise reaction conditions necessary for high-yield processes. Achieving consistent purity levels of carbolic acid is another significant hurdle, as impurities can dramatically affect reaction outcomes and product quality.
From a process engineering perspective, the integration of carbolic acid into existing chemical processes often requires substantial modifications to equipment and workflows. This retrofitting can be both costly and time-consuming, potentially disrupting production schedules and requiring extensive validation procedures. Additionally, the energy-intensive nature of many carbolic acid-based processes contributes to higher operational costs and conflicts with growing sustainability initiatives in the chemical industry.
The regulatory landscape surrounding the use of carbolic acid is becoming increasingly stringent, with new guidelines and restrictions being implemented globally. Compliance with these regulations often necessitates significant investments in monitoring systems, waste treatment facilities, and documentation processes. This regulatory burden can be particularly challenging for smaller chemical manufacturers, potentially limiting innovation and market competition.
Lastly, the development of alternative, less hazardous chemicals and processes poses a competitive challenge to the continued use of carbolic acid in high-yield applications. As research progresses in green chemistry and sustainable manufacturing, there is growing pressure to find safer and more environmentally friendly substitutes. This shift in focus could potentially reduce investment in carbolic acid-based technologies, slowing down advancements in its application for higher yield processes.
Another major challenge is the toxicity of carbolic acid, which presents serious health and safety concerns for workers in chemical plants. Stringent safety protocols and protective measures must be implemented, leading to increased operational complexity and potential productivity losses. Furthermore, the environmental impact of carbolic acid is a growing concern, as improper handling or accidental release can have severe consequences on ecosystems and water sources.
The volatility of carbolic acid at room temperature presents additional challenges in terms of storage, transportation, and process control. This volatility not only increases the risk of exposure but also complicates the maintenance of precise reaction conditions necessary for high-yield processes. Achieving consistent purity levels of carbolic acid is another significant hurdle, as impurities can dramatically affect reaction outcomes and product quality.
From a process engineering perspective, the integration of carbolic acid into existing chemical processes often requires substantial modifications to equipment and workflows. This retrofitting can be both costly and time-consuming, potentially disrupting production schedules and requiring extensive validation procedures. Additionally, the energy-intensive nature of many carbolic acid-based processes contributes to higher operational costs and conflicts with growing sustainability initiatives in the chemical industry.
The regulatory landscape surrounding the use of carbolic acid is becoming increasingly stringent, with new guidelines and restrictions being implemented globally. Compliance with these regulations often necessitates significant investments in monitoring systems, waste treatment facilities, and documentation processes. This regulatory burden can be particularly challenging for smaller chemical manufacturers, potentially limiting innovation and market competition.
Lastly, the development of alternative, less hazardous chemicals and processes poses a competitive challenge to the continued use of carbolic acid in high-yield applications. As research progresses in green chemistry and sustainable manufacturing, there is growing pressure to find safer and more environmentally friendly substitutes. This shift in focus could potentially reduce investment in carbolic acid-based technologies, slowing down advancements in its application for higher yield processes.
Existing Applications
01 Improved production methods for carbolic acid
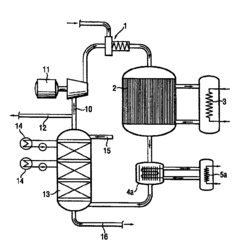
Various improved methods and apparatus for producing carbolic acid with higher yields have been developed. These include optimized reaction conditions, novel catalysts, and more efficient extraction techniques to increase the overall yield of carbolic acid from raw materials.- Improved production methods for carbolic acid: Various methods have been developed to enhance the yield of carbolic acid production. These include optimizing reaction conditions, using catalysts, and improving separation techniques. Such advancements aim to increase efficiency and reduce production costs in industrial settings.
- Utilization of novel catalysts: Research has focused on developing and implementing new catalysts to improve carbolic acid yield. These catalysts can enhance reaction rates, increase selectivity, and allow for milder reaction conditions, ultimately leading to higher product yields and purity.
- Equipment design for carbolic acid production: Specialized equipment has been designed to optimize carbolic acid production processes. This includes reactors, distillation columns, and separation units tailored to the specific requirements of carbolic acid synthesis and purification, resulting in improved yields and product quality.
- Process integration and optimization: Integrating various stages of carbolic acid production and optimizing the overall process flow can lead to significant improvements in yield. This approach involves careful analysis of each step, from raw material preparation to final product purification, to identify and eliminate bottlenecks and inefficiencies.
- Waste reduction and byproduct utilization: Strategies for reducing waste and utilizing byproducts in carbolic acid production have been developed to improve overall yield and sustainability. These methods focus on recycling unreacted materials, converting byproducts into valuable compounds, and minimizing environmental impact while maximizing resource efficiency.
02 Purification and separation techniques
Advanced purification and separation techniques have been implemented to enhance the yield and quality of carbolic acid. These may involve innovative distillation processes, crystallization methods, or membrane separation technologies to isolate and concentrate carbolic acid from reaction mixtures.Expand Specific Solutions03 Continuous flow reactors for carbolic acid production
Continuous flow reactor systems have been designed specifically for carbolic acid synthesis. These systems allow for better control of reaction parameters, improved heat transfer, and more efficient use of raw materials, leading to increased yields compared to batch processes.Expand Specific Solutions04 Waste reduction and byproduct utilization
Methods for reducing waste and utilizing byproducts in carbolic acid production have been developed. These approaches aim to increase overall yield by recovering valuable compounds from waste streams and optimizing resource utilization throughout the production process.Expand Specific Solutions05 Process monitoring and control systems
Advanced monitoring and control systems have been implemented to optimize carbolic acid production. These systems use real-time data analysis, predictive modeling, and automated adjustments to maintain optimal reaction conditions and maximize yield throughout the production process.Expand Specific Solutions
Key Industry Players
The carbolic acid market in higher yield chemical processes is in a mature stage, with established players and well-defined applications. The global market size for carbolic acid (phenol) is substantial, driven by its versatile use in various industries. Technologically, the production processes are well-developed, with major companies like BASF Corp., Sumitomo Chemical Co., Ltd., and Bayer Intellectual Property GmbH leading the field. These companies have invested heavily in R&D to optimize production methods and improve yields. Emerging players such as Resonac Corp. and NextChem SpA are also making strides in developing innovative approaches to carbolic acid production, potentially disrupting the market with more efficient and sustainable processes.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed an innovative approach to carbolic acid production that focuses on process intensification and yield improvement. Their technology combines the traditional cumene process with advanced membrane separation techniques[10]. By integrating pervaporation membranes into the reaction system, Sumitomo has achieved in-situ removal of water formed during the oxidation step, driving the equilibrium towards higher phenol yields[11]. Additionally, they have implemented a novel catalytic system that enhances the selectivity of the cumene hydroperoxide decomposition step, reducing the formation of unwanted byproducts. Sumitomo has also developed a proprietary heat integration system that optimizes energy utilization across the entire production process, significantly reducing overall energy consumption[12].
Strengths: Enhanced yield through equilibrium shift, improved energy efficiency, reduced byproduct formation. Weaknesses: Complexity of integrated membrane systems, potential membrane fouling issues.
BASF Corp.
Technical Solution: BASF has developed an innovative process for the production of carbolic acid (phenol) using cumene as a starting material. This process, known as the cumene process or Hock process, involves the oxidation of cumene to cumene hydroperoxide, followed by acid-catalyzed rearrangement to phenol and acetone[1]. BASF has optimized this process to achieve higher yields and improved energy efficiency. They have implemented advanced catalysts and reactor designs to enhance the selectivity of the oxidation step, reducing byproduct formation[2]. Additionally, BASF has integrated heat recovery systems to utilize the exothermic nature of the reactions, significantly reducing overall energy consumption[3].
Strengths: High yield, energy-efficient process, reduced byproduct formation. Weaknesses: Dependence on cumene availability, potential safety concerns due to handling of explosive intermediates.
Core Innovations
Process and apparatus for the production of carboylic acids with one to four carbon atoms
PatentInactiveEP1035101A3
Innovation
- A process involving partial recycling of the reaction starting gas mixture, which has been largely freed of acids through aqueous countercurrent scrubbing, reduces acid concentration before returning it to the reactor, allowing for high yields of saturated carboxylic acids by maintaining low partial pressure of organic acids and recycling unreacted hydrocarbons and intermediates.
Hydrogenation of carboxylic acids to increase yield of aromatics
PatentWO2014190124A1
Innovation
- A two-step process involving hydrogenation of carboxylic acids with a hydrogenation catalyst to produce an oxygenate mixture with a targeted hydrogen to carbon effective ratio, followed by exposure to a condensation catalyst to enhance the yield of aromatic hydrocarbons, utilizing catalysts such as zeolites and multi-functional catalysts like copper-loaded silica-bound ZSM-5.
Environmental Impact
The environmental impact of carbolic acid (phenol) in higher yield chemical processes is a critical consideration for sustainable industrial practices. Carbolic acid, while essential in various chemical syntheses, poses significant environmental risks if not properly managed.
In production processes, carbolic acid can contribute to air pollution through volatile organic compound (VOC) emissions. These emissions not only affect local air quality but also contribute to the formation of ground-level ozone, a key component of smog. Industrial facilities utilizing carbolic acid must implement robust air pollution control measures, such as thermal oxidizers or carbon adsorption systems, to mitigate these emissions effectively.
Water pollution is another major concern associated with carbolic acid use. Effluents containing phenol can severely impact aquatic ecosystems, as it is toxic to many organisms even at low concentrations. Advanced wastewater treatment technologies, including activated carbon filtration and advanced oxidation processes, are crucial for removing phenol from industrial wastewater before discharge.
Soil contamination can occur through accidental spills or improper disposal of carbolic acid-containing waste. This contamination can persist in the environment, affecting soil microorganisms and potentially entering the food chain. Proper handling, storage, and disposal protocols are essential to prevent such incidents and protect soil ecosystems.
The production of carbolic acid itself has environmental implications. Traditional methods often involve energy-intensive processes and the use of fossil fuel-derived feedstocks. However, recent advancements in green chemistry have led to more sustainable production routes, such as the use of biomass-derived feedstocks and catalytic processes that operate under milder conditions, reducing overall energy consumption and carbon footprint.
In terms of lifecycle assessment, the environmental impact of carbolic acid extends beyond its immediate use. The production of derivatives and end products must also be considered. For instance, the use of carbolic acid in the production of plastics and resins contributes to the broader issue of plastic pollution and microplastics in the environment.
Regulatory frameworks play a crucial role in mitigating the environmental impact of carbolic acid. Many countries have implemented strict regulations on its production, use, and disposal. These regulations often mandate the use of best available technologies for pollution control and set limits on emissions and effluent quality.
As industries strive for higher yield chemical processes involving carbolic acid, there is an increasing focus on developing cleaner technologies and circular economy approaches. This includes research into bio-based alternatives, improved recycling methods for phenol-containing waste streams, and the development of more efficient catalysts that can operate at lower temperatures and pressures, thereby reducing overall energy consumption and environmental footprint.
In production processes, carbolic acid can contribute to air pollution through volatile organic compound (VOC) emissions. These emissions not only affect local air quality but also contribute to the formation of ground-level ozone, a key component of smog. Industrial facilities utilizing carbolic acid must implement robust air pollution control measures, such as thermal oxidizers or carbon adsorption systems, to mitigate these emissions effectively.
Water pollution is another major concern associated with carbolic acid use. Effluents containing phenol can severely impact aquatic ecosystems, as it is toxic to many organisms even at low concentrations. Advanced wastewater treatment technologies, including activated carbon filtration and advanced oxidation processes, are crucial for removing phenol from industrial wastewater before discharge.
Soil contamination can occur through accidental spills or improper disposal of carbolic acid-containing waste. This contamination can persist in the environment, affecting soil microorganisms and potentially entering the food chain. Proper handling, storage, and disposal protocols are essential to prevent such incidents and protect soil ecosystems.
The production of carbolic acid itself has environmental implications. Traditional methods often involve energy-intensive processes and the use of fossil fuel-derived feedstocks. However, recent advancements in green chemistry have led to more sustainable production routes, such as the use of biomass-derived feedstocks and catalytic processes that operate under milder conditions, reducing overall energy consumption and carbon footprint.
In terms of lifecycle assessment, the environmental impact of carbolic acid extends beyond its immediate use. The production of derivatives and end products must also be considered. For instance, the use of carbolic acid in the production of plastics and resins contributes to the broader issue of plastic pollution and microplastics in the environment.
Regulatory frameworks play a crucial role in mitigating the environmental impact of carbolic acid. Many countries have implemented strict regulations on its production, use, and disposal. These regulations often mandate the use of best available technologies for pollution control and set limits on emissions and effluent quality.
As industries strive for higher yield chemical processes involving carbolic acid, there is an increasing focus on developing cleaner technologies and circular economy approaches. This includes research into bio-based alternatives, improved recycling methods for phenol-containing waste streams, and the development of more efficient catalysts that can operate at lower temperatures and pressures, thereby reducing overall energy consumption and environmental footprint.
Safety Regulations
The use of carbolic acid (phenol) in chemical processes necessitates strict adherence to safety regulations due to its corrosive and toxic nature. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established comprehensive guidelines for handling carbolic acid in industrial settings.
These regulations typically mandate the use of appropriate personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, and protective clothing. Proper ventilation systems are required to minimize exposure to phenol vapors, with specific air exchange rates and fume hood specifications outlined in safety protocols.
Storage and handling regulations for carbolic acid are particularly stringent. The chemical must be stored in tightly sealed containers in well-ventilated areas, away from sources of heat or ignition. Specific requirements for container materials, such as stainless steel or certain plastics, are often specified to prevent degradation and potential leaks.
Emergency response procedures are a critical component of safety regulations. Facilities using carbolic acid must have clearly defined spill response protocols, including the availability of neutralizing agents and absorbent materials. Eye wash stations and safety showers must be readily accessible in areas where phenol is handled or stored.
Waste disposal regulations for carbolic acid are equally important. The chemical is classified as hazardous waste in many jurisdictions, requiring specialized disposal methods. Incineration is often the preferred method for disposing of phenol-containing waste, with strict emission control requirements.
Worker training is a fundamental aspect of safety regulations. Employees handling carbolic acid must receive comprehensive training on its hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety audits are typically mandated to ensure ongoing compliance and safety awareness.
Environmental regulations also play a significant role in the use of carbolic acid. Effluent discharge limits are strictly controlled, with many jurisdictions requiring advanced treatment processes to remove phenol from wastewater before release. Air quality regulations may impose limits on phenol emissions, necessitating the use of scrubbers or other air pollution control devices.
As the chemical industry continues to evolve, safety regulations for carbolic acid use are subject to ongoing review and updates. Regulatory bodies increasingly emphasize risk assessment and management approaches, encouraging companies to implement comprehensive safety management systems that go beyond mere compliance to foster a culture of safety and continuous improvement.
These regulations typically mandate the use of appropriate personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, and protective clothing. Proper ventilation systems are required to minimize exposure to phenol vapors, with specific air exchange rates and fume hood specifications outlined in safety protocols.
Storage and handling regulations for carbolic acid are particularly stringent. The chemical must be stored in tightly sealed containers in well-ventilated areas, away from sources of heat or ignition. Specific requirements for container materials, such as stainless steel or certain plastics, are often specified to prevent degradation and potential leaks.
Emergency response procedures are a critical component of safety regulations. Facilities using carbolic acid must have clearly defined spill response protocols, including the availability of neutralizing agents and absorbent materials. Eye wash stations and safety showers must be readily accessible in areas where phenol is handled or stored.
Waste disposal regulations for carbolic acid are equally important. The chemical is classified as hazardous waste in many jurisdictions, requiring specialized disposal methods. Incineration is often the preferred method for disposing of phenol-containing waste, with strict emission control requirements.
Worker training is a fundamental aspect of safety regulations. Employees handling carbolic acid must receive comprehensive training on its hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety audits are typically mandated to ensure ongoing compliance and safety awareness.
Environmental regulations also play a significant role in the use of carbolic acid. Effluent discharge limits are strictly controlled, with many jurisdictions requiring advanced treatment processes to remove phenol from wastewater before release. Air quality regulations may impose limits on phenol emissions, necessitating the use of scrubbers or other air pollution control devices.
As the chemical industry continues to evolve, safety regulations for carbolic acid use are subject to ongoing review and updates. Regulatory bodies increasingly emphasize risk assessment and management approaches, encouraging companies to implement comprehensive safety management systems that go beyond mere compliance to foster a culture of safety and continuous improvement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!