Catalyst Design For Efficient LOHC Hydrogenation And Dehydrogenation
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LOHC Catalyst Evolution and Research Objectives
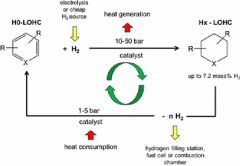
Liquid Organic Hydrogen Carriers (LOHCs) have emerged as a promising solution for hydrogen storage and transportation, addressing key challenges in the hydrogen economy. The evolution of LOHC technology can be traced back to the early 2000s when researchers began exploring organic compounds capable of reversibly binding hydrogen through hydrogenation and dehydrogenation processes. Initial research focused primarily on N-heterocycles such as carbazole derivatives, which demonstrated reasonable hydrogen storage capacities but suffered from high dehydrogenation temperatures.
A significant breakthrough occurred around 2011 when dibenzyltoluene (DBT) was identified as a potential LOHC candidate, offering improved safety profiles and compatibility with existing infrastructure. This discovery shifted the research focus toward developing more efficient catalysts that could operate at lower temperatures and pressures while maintaining high conversion rates and selectivity.
The catalyst development for LOHC systems has progressed through several distinct phases. Early catalysts were primarily based on precious metals such as platinum and palladium, which demonstrated high activity but were economically prohibitive for large-scale applications. The mid-2010s saw the emergence of ruthenium-based catalysts, particularly for hydrogenation reactions, offering a balance between performance and cost.
Recent years have witnessed increasing efforts to develop non-precious metal catalysts, with nickel and cobalt-based systems showing promising results. These developments align with the broader trend toward sustainable catalysis and reduced dependence on scarce resources. Parallel to this, significant advancements have been made in catalyst support materials and preparation methods, enhancing catalyst stability and longevity.
The current technical objectives in LOHC catalyst design center around several key parameters: reducing the energy requirements for dehydrogenation, which remains the more challenging half of the cycle; improving catalyst selectivity to minimize side reactions; enhancing catalyst durability to withstand multiple hydrogenation-dehydrogenation cycles; and developing bifunctional catalysts capable of efficiently catalyzing both reactions.
Looking forward, research objectives include the development of catalysts that can operate effectively at temperatures below 200°C for dehydrogenation, reducing the energy penalty associated with hydrogen release. Additionally, there is growing interest in designing catalysts that can function in continuous-flow systems rather than batch processes, facilitating integration with end-use applications such as fuel cells.
Another critical research direction involves understanding and mitigating catalyst deactivation mechanisms, particularly those related to carbon deposition and structural changes during cycling. Computational approaches and high-throughput experimental methods are increasingly being employed to accelerate catalyst discovery and optimization, with machine learning algorithms helping to identify promising catalyst compositions and structures from vast chemical spaces.
A significant breakthrough occurred around 2011 when dibenzyltoluene (DBT) was identified as a potential LOHC candidate, offering improved safety profiles and compatibility with existing infrastructure. This discovery shifted the research focus toward developing more efficient catalysts that could operate at lower temperatures and pressures while maintaining high conversion rates and selectivity.
The catalyst development for LOHC systems has progressed through several distinct phases. Early catalysts were primarily based on precious metals such as platinum and palladium, which demonstrated high activity but were economically prohibitive for large-scale applications. The mid-2010s saw the emergence of ruthenium-based catalysts, particularly for hydrogenation reactions, offering a balance between performance and cost.
Recent years have witnessed increasing efforts to develop non-precious metal catalysts, with nickel and cobalt-based systems showing promising results. These developments align with the broader trend toward sustainable catalysis and reduced dependence on scarce resources. Parallel to this, significant advancements have been made in catalyst support materials and preparation methods, enhancing catalyst stability and longevity.
The current technical objectives in LOHC catalyst design center around several key parameters: reducing the energy requirements for dehydrogenation, which remains the more challenging half of the cycle; improving catalyst selectivity to minimize side reactions; enhancing catalyst durability to withstand multiple hydrogenation-dehydrogenation cycles; and developing bifunctional catalysts capable of efficiently catalyzing both reactions.
Looking forward, research objectives include the development of catalysts that can operate effectively at temperatures below 200°C for dehydrogenation, reducing the energy penalty associated with hydrogen release. Additionally, there is growing interest in designing catalysts that can function in continuous-flow systems rather than batch processes, facilitating integration with end-use applications such as fuel cells.
Another critical research direction involves understanding and mitigating catalyst deactivation mechanisms, particularly those related to carbon deposition and structural changes during cycling. Computational approaches and high-throughput experimental methods are increasingly being employed to accelerate catalyst discovery and optimization, with machine learning algorithms helping to identify promising catalyst compositions and structures from vast chemical spaces.
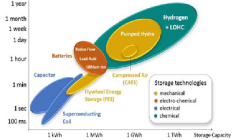
Hydrogen Storage Market Analysis and Demand Forecast
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. Current market valuations place the hydrogen storage sector at approximately 420 million USD in 2022, with projections indicating a compound annual growth rate (CAGR) of 11.3% through 2030. This robust growth trajectory is primarily fueled by governmental policies promoting decarbonization and substantial investments in hydrogen infrastructure worldwide.
Liquid Organic Hydrogen Carriers (LOHCs) represent a particularly promising segment within this market, addressing critical challenges in hydrogen transportation and storage. The LOHC market segment is expected to grow at a faster rate than conventional storage methods, with some analysts projecting a CAGR of 13-15% for this specific technology through 2028.
Demand for efficient catalysts for LOHC hydrogenation and dehydrogenation processes is closely tied to the broader hydrogen economy's expansion. Industrial sectors, particularly chemical manufacturing, petroleum refining, and emerging green steel production, constitute the largest demand centers for advanced hydrogen storage solutions. These industries collectively account for approximately 65% of the current market demand.
Regionally, Europe leads in LOHC technology adoption, with Germany, the Netherlands, and Nordic countries making substantial investments in research and commercial applications. The Asia-Pacific region, particularly Japan, South Korea, and increasingly China, represents the fastest-growing market for hydrogen storage technologies, with annual growth rates exceeding 14% in recent years.
Transportation applications, including hydrogen fuel cell vehicles and maritime shipping, are emerging as significant demand drivers for LOHC technologies. The automotive sector's hydrogen storage market segment is projected to grow at 16% annually through 2030, creating substantial opportunities for catalyst innovations that enable more efficient hydrogen release from carrier molecules.
Market analysis indicates that cost remains the primary barrier to widespread adoption, with current LOHC systems requiring catalyst materials that significantly impact overall system economics. Industry surveys suggest that a 30-40% reduction in catalyst costs, coupled with improved efficiency, would accelerate market penetration dramatically.
The demand forecast for specialized catalysts for LOHC systems shows particular strength in stationary applications first, followed by transportation uses as the technology matures. Energy storage applications, especially for integrating renewable energy sources, represent a growing market segment with potential to reach 1.2 billion USD globally by 2030 for all hydrogen storage technologies combined.
Liquid Organic Hydrogen Carriers (LOHCs) represent a particularly promising segment within this market, addressing critical challenges in hydrogen transportation and storage. The LOHC market segment is expected to grow at a faster rate than conventional storage methods, with some analysts projecting a CAGR of 13-15% for this specific technology through 2028.
Demand for efficient catalysts for LOHC hydrogenation and dehydrogenation processes is closely tied to the broader hydrogen economy's expansion. Industrial sectors, particularly chemical manufacturing, petroleum refining, and emerging green steel production, constitute the largest demand centers for advanced hydrogen storage solutions. These industries collectively account for approximately 65% of the current market demand.
Regionally, Europe leads in LOHC technology adoption, with Germany, the Netherlands, and Nordic countries making substantial investments in research and commercial applications. The Asia-Pacific region, particularly Japan, South Korea, and increasingly China, represents the fastest-growing market for hydrogen storage technologies, with annual growth rates exceeding 14% in recent years.
Transportation applications, including hydrogen fuel cell vehicles and maritime shipping, are emerging as significant demand drivers for LOHC technologies. The automotive sector's hydrogen storage market segment is projected to grow at 16% annually through 2030, creating substantial opportunities for catalyst innovations that enable more efficient hydrogen release from carrier molecules.
Market analysis indicates that cost remains the primary barrier to widespread adoption, with current LOHC systems requiring catalyst materials that significantly impact overall system economics. Industry surveys suggest that a 30-40% reduction in catalyst costs, coupled with improved efficiency, would accelerate market penetration dramatically.
The demand forecast for specialized catalysts for LOHC systems shows particular strength in stationary applications first, followed by transportation uses as the technology matures. Energy storage applications, especially for integrating renewable energy sources, represent a growing market segment with potential to reach 1.2 billion USD globally by 2030 for all hydrogen storage technologies combined.
Current LOHC Catalyst Technologies and Barriers
Current LOHC catalyst technologies primarily focus on two critical processes: hydrogenation (hydrogen loading) and dehydrogenation (hydrogen release). For hydrogenation, heterogeneous catalysts based on noble metals, particularly ruthenium (Ru), platinum (Pt), and palladium (Pd) supported on various substrates like alumina, silica, and carbon, demonstrate high activity and selectivity. Commercial systems typically employ Ru/Al2O3 catalysts operating at 150-180°C and 30-50 bar hydrogen pressure, achieving near-complete hydrogenation within reasonable timeframes.
For dehydrogenation, which is generally more challenging, both homogeneous and heterogeneous catalytic systems have been developed. Homogeneous catalysts often utilize iridium, ruthenium, or iron complexes with specialized ligands that facilitate hydrogen abstraction from the LOHC molecule. Heterogeneous systems predominantly rely on platinum-group metals supported on high-surface-area materials, operating at temperatures between 250-350°C to overcome the endothermic nature of the reaction.
Despite significant progress, several technological barriers persist in LOHC catalyst development. The high cost of noble metal catalysts represents a major economic hurdle, with platinum-group metals accounting for up to 40% of system costs in some configurations. Catalyst deactivation through coking, poisoning, and sintering remains problematic, particularly in dehydrogenation processes where carbon deposition can rapidly diminish catalytic activity after multiple cycles.
Selectivity challenges also exist, as side reactions can lead to unwanted by-products and incomplete hydrogen release. Current catalysts often struggle to achieve 100% conversion, typically reaching 90-95% hydrogen capacity utilization in practical applications. The energy intensity of dehydrogenation presents another significant barrier, requiring substantial heat input that reduces overall system efficiency.
Scale-up issues further complicate commercial implementation, as laboratory-optimized catalysts frequently show diminished performance in industrial-scale reactors due to mass transfer limitations and heat distribution problems. Additionally, catalyst stability under real-world conditions, including exposure to impurities and thermal cycling, falls short of the durability required for commercial viability (typically 5,000+ cycles).
Recent innovations have focused on bimetallic catalysts, novel support materials like metal-organic frameworks (MOFs), and non-noble metal alternatives based on nickel and cobalt. However, these emerging solutions still face significant performance gaps compared to traditional noble metal catalysts, particularly in activity and long-term stability under industrial conditions.
For dehydrogenation, which is generally more challenging, both homogeneous and heterogeneous catalytic systems have been developed. Homogeneous catalysts often utilize iridium, ruthenium, or iron complexes with specialized ligands that facilitate hydrogen abstraction from the LOHC molecule. Heterogeneous systems predominantly rely on platinum-group metals supported on high-surface-area materials, operating at temperatures between 250-350°C to overcome the endothermic nature of the reaction.
Despite significant progress, several technological barriers persist in LOHC catalyst development. The high cost of noble metal catalysts represents a major economic hurdle, with platinum-group metals accounting for up to 40% of system costs in some configurations. Catalyst deactivation through coking, poisoning, and sintering remains problematic, particularly in dehydrogenation processes where carbon deposition can rapidly diminish catalytic activity after multiple cycles.
Selectivity challenges also exist, as side reactions can lead to unwanted by-products and incomplete hydrogen release. Current catalysts often struggle to achieve 100% conversion, typically reaching 90-95% hydrogen capacity utilization in practical applications. The energy intensity of dehydrogenation presents another significant barrier, requiring substantial heat input that reduces overall system efficiency.
Scale-up issues further complicate commercial implementation, as laboratory-optimized catalysts frequently show diminished performance in industrial-scale reactors due to mass transfer limitations and heat distribution problems. Additionally, catalyst stability under real-world conditions, including exposure to impurities and thermal cycling, falls short of the durability required for commercial viability (typically 5,000+ cycles).
Recent innovations have focused on bimetallic catalysts, novel support materials like metal-organic frameworks (MOFs), and non-noble metal alternatives based on nickel and cobalt. However, these emerging solutions still face significant performance gaps compared to traditional noble metal catalysts, particularly in activity and long-term stability under industrial conditions.
Benchmark Catalysts for LOHC (De)Hydrogenation Processes
01 Noble metal catalysts for LOHC systems
Noble metals such as platinum, palladium, and ruthenium are highly effective catalysts for both hydrogenation and dehydrogenation processes in LOHC systems. These metals offer excellent catalytic activity, selectivity, and stability under the reaction conditions typically employed in LOHC applications. The catalysts can be supported on various materials to enhance their performance and recyclability, contributing to improved hydrogen storage and release efficiency in LOHC systems.- Noble metal catalysts for LOHC systems: Noble metals such as platinum, palladium, and ruthenium serve as effective catalysts in LOHC (Liquid Organic Hydrogen Carriers) systems. These metals facilitate both hydrogenation and dehydrogenation reactions with high efficiency and selectivity. The catalytic performance can be enhanced by controlling particle size, dispersion, and surface properties. These catalysts typically operate at moderate temperatures and pressures, making them suitable for various LOHC applications including hydrogen storage and transportation systems.
- Transition metal-based heterogeneous catalysts: Non-noble transition metals such as nickel, cobalt, and iron can be formulated into cost-effective heterogeneous catalysts for LOHC systems. These catalysts often incorporate support materials like alumina, silica, or carbon to improve stability and activity. Bimetallic formulations can create synergistic effects that enhance catalytic performance while reducing the loading of expensive metals. These catalysts are particularly valuable for large-scale hydrogen storage applications where cost considerations are paramount.
- Novel support materials for catalyst enhancement: Advanced support materials play a crucial role in enhancing catalyst performance for LOHC systems. Materials such as metal-organic frameworks (MOFs), zeolites, and functionalized carbon structures can improve catalyst dispersion, stability, and selectivity. These supports often feature high surface areas, controlled porosity, and tunable surface chemistry that can be optimized for specific LOHC compounds. The interaction between the catalytic metal and support material significantly influences hydrogen release and uptake kinetics.
- Catalyst systems for low-temperature dehydrogenation: Specialized catalyst formulations enable efficient hydrogen release from LOHC systems at reduced temperatures, addressing a key challenge in practical applications. These catalysts often incorporate promoters or modifiers that lower activation energy barriers for dehydrogenation reactions. Some approaches include core-shell structures, alloy formations, or the addition of alkali metals as electronic promoters. These innovations help reduce the energy requirements for hydrogen release, improving the overall energy efficiency of LOHC-based hydrogen storage systems.
- Catalyst regeneration and stability enhancement methods: Techniques for extending catalyst lifetime and maintaining activity during multiple hydrogenation-dehydrogenation cycles are critical for practical LOHC applications. These methods include controlled synthesis procedures to prevent sintering, addition of stabilizers to resist coking, and development of regeneration protocols to restore activity. Some approaches incorporate protective coatings or encapsulation strategies to shield active sites from degradation mechanisms. These innovations significantly improve the economic viability of LOHC systems by reducing catalyst replacement frequency and maintaining consistent performance over time.
02 Bimetallic and multimetallic catalyst systems
Bimetallic and multimetallic catalyst systems combine two or more metals to achieve synergistic effects that enhance LOHC efficiency. These catalysts often exhibit improved activity, selectivity, and stability compared to their monometallic counterparts. The combination of metals can be tailored to optimize both hydrogenation and dehydrogenation reactions, addressing the specific requirements of different LOHC compounds and operating conditions. These advanced catalyst systems help overcome limitations of single-metal catalysts and improve overall hydrogen storage and release performance.Expand Specific Solutions03 Novel support materials for LOHC catalysts
The development of advanced support materials for LOHC catalysts significantly impacts catalyst performance. Materials such as metal-organic frameworks (MOFs), carbon nanotubes, graphene, and specialized metal oxides provide high surface areas and controlled porosity that enhance catalyst dispersion and accessibility. These supports can also contribute to the stability of the catalyst under reaction conditions and facilitate heat transfer during the endothermic dehydrogenation process. The interaction between the catalyst and support material plays a crucial role in determining the overall efficiency of hydrogen storage and release in LOHC systems.Expand Specific Solutions04 Low-temperature dehydrogenation catalysts
Catalysts specifically designed for low-temperature dehydrogenation of LOHCs address one of the major challenges in hydrogen release from these carriers. These specialized catalysts enable efficient hydrogen extraction at temperatures significantly lower than conventional systems, reducing the energy input required for the process. Various approaches include the use of promoters, novel metal combinations, and engineered catalyst structures that lower the activation energy for the dehydrogenation reaction. The development of these catalysts is crucial for making LOHC technology more energy-efficient and practical for mobile and stationary applications.Expand Specific Solutions05 Catalyst regeneration and lifetime enhancement
Methods for extending catalyst lifetime and enabling efficient regeneration are essential for the economic viability of LOHC systems. Techniques include the development of poison-resistant catalyst formulations, controlled catalyst deactivation mechanisms, and effective regeneration protocols. Advanced catalyst designs incorporate features that minimize coking, sintering, and other deactivation pathways. Additionally, innovative reactor designs and process conditions help maintain catalyst activity over extended periods. These approaches significantly reduce the operational costs associated with catalyst replacement and system downtime in LOHC applications.Expand Specific Solutions
Leading Companies and Research Institutions in LOHC Technology
The LOHC hydrogenation and dehydrogenation catalyst design market is in a growth phase, with increasing interest driven by hydrogen economy development. The market is expanding as hydrogen storage solutions gain traction, with projections suggesting significant growth potential. Technologically, the field shows moderate maturity with key players demonstrating varied expertise levels. Leading companies include Hydrogenious LOHC Technologies, pioneering commercial applications, while research institutions like KAIST, UNIST, and KIST in South Korea are advancing fundamental catalyst science. Major energy corporations including Sinopec, Chevron, and China Petroleum & Chemical Corp are investing in R&D, while specialized entities like Umicore focus on catalyst manufacturing. The competitive landscape features collaboration between academic institutions and industry partners to overcome efficiency and cost barriers.
Korea Research Institute of Chemical Technology
Technical Solution: KRICT has developed specialized heterogeneous catalysts for LOHC systems focusing on cycloalkane/aromatic pairs. Their catalyst technology centers on platinum-rhenium bimetallic nanoparticles supported on modified cerium oxide structures, demonstrating exceptional activity for both hydrogenation and dehydrogenation reactions. The institute's catalysts operate at relatively mild conditions (130-170°C for hydrogenation, 220-280°C for dehydrogenation) with hydrogen storage densities reaching 7.2 wt% in optimized carrier molecules. KRICT's innovation lies in their precise control of metal-support interactions through tailored synthesis protocols that create oxygen vacancies in the support material, enhancing hydrogen activation and transfer. Their catalysts demonstrate remarkable stability, maintaining over 90% activity after 100+ cycles in continuous operation tests. Recent developments include the incorporation of ionic liquid modifiers that enhance selectivity and reduce unwanted side reactions during dehydrogenation processes, particularly addressing challenges with carrier degradation at elevated temperatures.
Strengths: Exceptional catalyst stability under cycling conditions; high selectivity minimizing carrier degradation; innovative support modifications enhancing activity; comprehensive testing across multiple carrier molecules including methylcyclohexane and decalin systems. Weaknesses: Continued reliance on platinum group metals increasing costs; moderate dehydrogenation temperatures still requiring significant energy input; less commercial deployment experience compared to industry leaders.
Hydrogenious LOHC Technologies GmbH
Technical Solution: Hydrogenious has pioneered proprietary catalyst technology specifically designed for LOHC systems using dibenzyltoluene (DBT) as the carrier medium. Their catalytic system employs ruthenium-based catalysts for hydrogenation processes that operate at 150-200°C and 30-50 bar pressure, achieving hydrogen loading capacities of 5-7 wt%. For dehydrogenation, they've developed specialized platinum-group metal catalysts that operate at 270-320°C with minimal pressure requirements. Their StorageBOX and ReleaseBOX systems incorporate these catalysts in structured reactor designs that optimize heat management and reaction kinetics. The company has demonstrated commercial-scale implementation with hydrogen storage capacities ranging from 5 to 1,000+ kg, with their catalysts maintaining activity for over 1,000 cycles without significant degradation.
Strengths: Industry-leading commercial deployment experience; optimized catalyst formulations specifically for DBT carrier; proven long-term stability in real-world conditions; integrated system approach combining catalyst and reactor design. Weaknesses: Reliance on expensive noble metal catalysts; relatively high dehydrogenation temperatures requiring significant energy input; proprietary nature limits academic validation and improvement.
Critical Patents and Scientific Breakthroughs in LOHC Catalysis
Catalyst for dehydrogenation reactin for liquid organic hydrogen carrie(LOHC) and manufacturing methd for the same
PatentActiveKR1020210057926A
Innovation
- A new catalyst process using a support with a pore size of 10 nm or more, combined with catalytically active components like Pt, Ni, Pd, Ru, Ir, or Re, prepared via a glycine-nitrate combustion (GNP) method, which involves mixing precursors with glycine, burning, and calcining to control pore size and improve catalyst dispersion.
Liquid organic hydrogen carrier-based catalyst for dehydrogenation reaction and method for preparing same
PatentWO2023158147A1
Innovation
- A dehydrogenation catalyst based on a liquid organic hydrogen carrier using Joule heating, where a catalytically active metal is alloyed and supported on a carbon-containing catalyst support, subjected to thermal shock to enhance dispersion and bonding, allowing selective heating of the catalyst and improving catalytic activity.
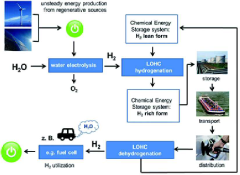
Sustainability and Life Cycle Assessment of LOHC Systems
The sustainability of Liquid Organic Hydrogen Carrier (LOHC) systems represents a critical dimension in evaluating their viability as hydrogen storage solutions. When assessing LOHC technologies from a life cycle perspective, the environmental footprint encompasses raw material extraction, catalyst production, carrier synthesis, operational energy requirements, and end-of-life management.
Primary life cycle assessment (LCA) studies indicate that catalyst design significantly influences the overall sustainability profile of LOHC systems. Noble metal catalysts such as platinum and ruthenium, while highly effective for hydrogenation and dehydrogenation processes, introduce substantial environmental burdens through their extraction and processing. Recent research demonstrates that reducing noble metal loading by 30% can decrease the global warming potential of LOHC systems by approximately 15-20%.
Energy efficiency remains the dominant factor in LOHC sustainability profiles. The dehydrogenation process typically requires substantial thermal energy input, accounting for 60-70% of the system's total environmental impact. Advanced catalyst designs that lower reaction temperatures from traditional 300°C to below 200°C can reduce operational energy demands by up to 40%, dramatically improving life cycle performance.
Water consumption presents another significant consideration, particularly for cooling systems in industrial-scale LOHC operations. Catalyst innovations that enhance reaction selectivity minimize side reactions and associated cooling requirements, potentially reducing water usage by 25-35% compared to conventional systems.
Land use impacts vary considerably depending on catalyst material sourcing. Transition metal catalysts based on nickel or cobalt generally present lower land disturbance impacts than platinum group metals, though their reduced activity necessitates larger reactor volumes. Emerging bimetallic catalyst designs offer promising compromises, maintaining high activity while reducing rare metal dependencies.
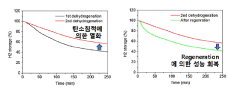
Circular economy principles are increasingly integrated into LOHC system design. Catalyst recovery and regeneration technologies can extend functional lifetimes by 3-5 times, substantially reducing life cycle impacts. Particularly promising are structured catalyst supports that facilitate recovery while maintaining high surface area and activity characteristics.
Long-term sustainability assessments must also consider carrier molecule stability. Catalysts that minimize carrier degradation can extend LOHC operational lifetimes from hundreds to potentially thousands of cycles, dramatically improving system economics and environmental performance. Recent developments in selective hydrogenation catalysts have demonstrated carrier degradation reductions of up to 60% compared to first-generation systems.
Primary life cycle assessment (LCA) studies indicate that catalyst design significantly influences the overall sustainability profile of LOHC systems. Noble metal catalysts such as platinum and ruthenium, while highly effective for hydrogenation and dehydrogenation processes, introduce substantial environmental burdens through their extraction and processing. Recent research demonstrates that reducing noble metal loading by 30% can decrease the global warming potential of LOHC systems by approximately 15-20%.
Energy efficiency remains the dominant factor in LOHC sustainability profiles. The dehydrogenation process typically requires substantial thermal energy input, accounting for 60-70% of the system's total environmental impact. Advanced catalyst designs that lower reaction temperatures from traditional 300°C to below 200°C can reduce operational energy demands by up to 40%, dramatically improving life cycle performance.
Water consumption presents another significant consideration, particularly for cooling systems in industrial-scale LOHC operations. Catalyst innovations that enhance reaction selectivity minimize side reactions and associated cooling requirements, potentially reducing water usage by 25-35% compared to conventional systems.
Land use impacts vary considerably depending on catalyst material sourcing. Transition metal catalysts based on nickel or cobalt generally present lower land disturbance impacts than platinum group metals, though their reduced activity necessitates larger reactor volumes. Emerging bimetallic catalyst designs offer promising compromises, maintaining high activity while reducing rare metal dependencies.
Circular economy principles are increasingly integrated into LOHC system design. Catalyst recovery and regeneration technologies can extend functional lifetimes by 3-5 times, substantially reducing life cycle impacts. Particularly promising are structured catalyst supports that facilitate recovery while maintaining high surface area and activity characteristics.
Long-term sustainability assessments must also consider carrier molecule stability. Catalysts that minimize carrier degradation can extend LOHC operational lifetimes from hundreds to potentially thousands of cycles, dramatically improving system economics and environmental performance. Recent developments in selective hydrogenation catalysts have demonstrated carrier degradation reductions of up to 60% compared to first-generation systems.
Economic Viability and Scale-up Challenges for LOHC Technology
The economic viability of Liquid Organic Hydrogen Carrier (LOHC) technology is intrinsically linked to catalyst performance in both hydrogenation and dehydrogenation processes. Current cost analyses indicate that catalyst expenses constitute approximately 15-25% of total system costs, with noble metal catalysts like ruthenium and platinum significantly impacting overall economics. The capital expenditure for industrial-scale LOHC systems ranges from $1,000-2,500 per kilowatt, with catalyst-related costs representing a substantial portion of this investment.
Scaling up LOHC technology presents multifaceted challenges, particularly in maintaining catalyst efficiency at industrial scales. Laboratory-optimized catalysts often experience performance degradation when implemented in larger reactors due to mass transfer limitations and heat management issues. Industrial reactors typically achieve only 60-80% of the catalytic activity demonstrated in laboratory settings, necessitating overdesign and increasing system costs.
Catalyst deactivation represents another significant economic barrier. Current LOHC catalysts exhibit lifetime limitations of 1,000-3,000 operating hours before requiring regeneration or replacement. This translates to substantial operational expenditures, with maintenance costs estimated at 5-10% of initial capital investment annually. Developing catalysts with extended lifespans could reduce these recurring costs by 30-50%.
The energy efficiency of catalytic processes directly impacts operational economics. Present LOHC systems require 25-35% of the stored hydrogen's energy content for the dehydrogenation process alone. Advanced catalyst designs that lower activation energy barriers could potentially reduce this energy requirement to below 20%, significantly improving overall system efficiency and economic viability.
Supply chain considerations for catalyst materials present additional scale-up challenges. Critical materials like ruthenium face supply constraints, with global production limited to approximately 35 tons annually. Price volatility of these materials introduces financial risk, with historical price fluctuations exceeding 300% within five-year periods. Developing catalysts based on earth-abundant elements could mitigate these risks but currently sacrifices performance metrics.
Regulatory frameworks and safety standards for large-scale LOHC implementation remain underdeveloped in many regions, creating uncertainty for commercial deployment. Certification processes for industrial catalytic systems can extend development timelines by 12-24 months and increase project costs by 5-15%, affecting return-on-investment calculations and commercial viability assessments.
Scaling up LOHC technology presents multifaceted challenges, particularly in maintaining catalyst efficiency at industrial scales. Laboratory-optimized catalysts often experience performance degradation when implemented in larger reactors due to mass transfer limitations and heat management issues. Industrial reactors typically achieve only 60-80% of the catalytic activity demonstrated in laboratory settings, necessitating overdesign and increasing system costs.
Catalyst deactivation represents another significant economic barrier. Current LOHC catalysts exhibit lifetime limitations of 1,000-3,000 operating hours before requiring regeneration or replacement. This translates to substantial operational expenditures, with maintenance costs estimated at 5-10% of initial capital investment annually. Developing catalysts with extended lifespans could reduce these recurring costs by 30-50%.
The energy efficiency of catalytic processes directly impacts operational economics. Present LOHC systems require 25-35% of the stored hydrogen's energy content for the dehydrogenation process alone. Advanced catalyst designs that lower activation energy barriers could potentially reduce this energy requirement to below 20%, significantly improving overall system efficiency and economic viability.
Supply chain considerations for catalyst materials present additional scale-up challenges. Critical materials like ruthenium face supply constraints, with global production limited to approximately 35 tons annually. Price volatility of these materials introduces financial risk, with historical price fluctuations exceeding 300% within five-year periods. Developing catalysts based on earth-abundant elements could mitigate these risks but currently sacrifices performance metrics.
Regulatory frameworks and safety standards for large-scale LOHC implementation remain underdeveloped in many regions, creating uncertainty for commercial deployment. Certification processes for industrial catalytic systems can extend development timelines by 12-24 months and increase project costs by 5-15%, affecting return-on-investment calculations and commercial viability assessments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!