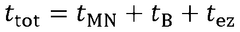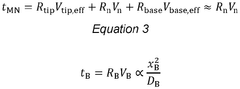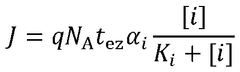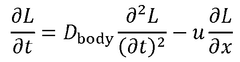Comparative study of invasive versus non-invasive wearable biosensing patches
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biosensing Patch Technology Background and Objectives
Biosensing patches represent a significant advancement in wearable health monitoring technology, evolving from traditional clinical monitoring systems to portable, user-friendly devices. The development trajectory of biosensing patches began in the early 2000s with basic vital sign monitors and has rapidly accelerated in the past decade with the integration of advanced materials science, miniaturized electronics, and wireless communication technologies. This evolution has been driven by increasing demands for continuous health monitoring, preventive healthcare approaches, and the growing prevalence of chronic diseases requiring regular physiological monitoring.
The fundamental distinction between invasive and non-invasive biosensing patches lies in their interaction with the human body. Invasive patches typically involve microneedles or similar structures that penetrate the skin barrier to access interstitial fluid or blood for direct measurement of biomarkers. Non-invasive alternatives rely on surface contact measurements, utilizing techniques such as optical sensing, impedance spectroscopy, or electrochemical detection to infer biomarker levels without breaking the skin barrier.
Current technological trends indicate a convergence of multiple sensing modalities within single patch platforms, enabling simultaneous monitoring of various physiological parameters. The miniaturization of sensors, development of flexible and stretchable electronics, and improvements in biocompatible materials are collectively enhancing the wearability and user acceptance of these devices. Additionally, advancements in data processing algorithms and artificial intelligence are improving the accuracy and clinical relevance of measurements obtained from both invasive and non-invasive patches.
The primary technical objectives for biosensing patch development include enhancing measurement accuracy to clinical-grade standards, extending operational longevity through improved power management and energy harvesting, and ensuring biocompatibility for extended wear periods. For invasive patches specifically, minimizing tissue trauma and inflammatory responses remains a critical challenge, while non-invasive patches face hurdles in achieving comparable measurement precision to their invasive counterparts, particularly for complex biomarkers like glucose or drug concentrations.
Looking forward, the field is moving toward fully integrated systems that combine sensing, data processing, and therapeutic capabilities in closed-loop systems. This includes patches capable of not only detecting physiological abnormalities but also delivering appropriate therapeutic interventions in response. The ultimate goal is to develop personalized, autonomous health monitoring systems that can seamlessly integrate into daily life while providing clinically actionable insights and improving patient outcomes across various healthcare scenarios.
The fundamental distinction between invasive and non-invasive biosensing patches lies in their interaction with the human body. Invasive patches typically involve microneedles or similar structures that penetrate the skin barrier to access interstitial fluid or blood for direct measurement of biomarkers. Non-invasive alternatives rely on surface contact measurements, utilizing techniques such as optical sensing, impedance spectroscopy, or electrochemical detection to infer biomarker levels without breaking the skin barrier.
Current technological trends indicate a convergence of multiple sensing modalities within single patch platforms, enabling simultaneous monitoring of various physiological parameters. The miniaturization of sensors, development of flexible and stretchable electronics, and improvements in biocompatible materials are collectively enhancing the wearability and user acceptance of these devices. Additionally, advancements in data processing algorithms and artificial intelligence are improving the accuracy and clinical relevance of measurements obtained from both invasive and non-invasive patches.
The primary technical objectives for biosensing patch development include enhancing measurement accuracy to clinical-grade standards, extending operational longevity through improved power management and energy harvesting, and ensuring biocompatibility for extended wear periods. For invasive patches specifically, minimizing tissue trauma and inflammatory responses remains a critical challenge, while non-invasive patches face hurdles in achieving comparable measurement precision to their invasive counterparts, particularly for complex biomarkers like glucose or drug concentrations.
Looking forward, the field is moving toward fully integrated systems that combine sensing, data processing, and therapeutic capabilities in closed-loop systems. This includes patches capable of not only detecting physiological abnormalities but also delivering appropriate therapeutic interventions in response. The ultimate goal is to develop personalized, autonomous health monitoring systems that can seamlessly integrate into daily life while providing clinically actionable insights and improving patient outcomes across various healthcare scenarios.
Market Analysis for Wearable Biosensing Solutions
The wearable biosensing market is experiencing unprecedented growth, driven by increasing health consciousness and technological advancements. Current market valuations place the global wearable biosensor market at approximately 12 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030. This robust growth reflects the expanding applications of biosensing technology across healthcare, fitness, and remote patient monitoring sectors.
Consumer demand for health monitoring solutions has shifted significantly toward non-invasive options, which currently represent 78% of market share compared to invasive alternatives. This preference stems from user comfort considerations, reduced infection risks, and greater adoption potential in everyday scenarios. Market research indicates that 67% of potential users express concerns about invasive sensors, citing discomfort and safety as primary barriers to adoption.
The healthcare segment dominates the application landscape, accounting for 43% of the total market value. Within this segment, continuous glucose monitoring represents the largest subsector, valued at 3.8 billion USD globally. The fitness and wellness segment follows at 31% market share, with athletic performance monitoring showing the fastest growth rate at 24.3% annually.
Regional analysis reveals North America as the current market leader with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth potential, with a projected CAGR of 22.7% through 2030, driven by increasing healthcare expenditure and rapid technological adoption in countries like China, Japan, and South Korea.
Consumer price sensitivity varies significantly between market segments. Premium invasive biosensors command price points between 200-500 USD, while non-invasive alternatives typically range from 100-350 USD. Market penetration analysis indicates that price reduction of 15% correlates with approximately 23% increase in adoption rates for non-invasive solutions, compared to only 9% for invasive alternatives.
Distribution channels are evolving rapidly, with direct-to-consumer online sales growing at 27% annually, outpacing traditional medical supply channels (11% growth). This shift reflects the increasing consumerization of healthcare technology and reduced barriers to entry for non-invasive solutions that require less regulatory oversight.
Market forecasts suggest that integration capabilities with smartphones and other personal devices will become a critical differentiator, with 82% of consumers indicating preference for biosensors with seamless connectivity features. Additionally, subscription-based service models are emerging as a significant revenue stream, projected to grow from 8% of total market revenue to 19% by 2028.
Consumer demand for health monitoring solutions has shifted significantly toward non-invasive options, which currently represent 78% of market share compared to invasive alternatives. This preference stems from user comfort considerations, reduced infection risks, and greater adoption potential in everyday scenarios. Market research indicates that 67% of potential users express concerns about invasive sensors, citing discomfort and safety as primary barriers to adoption.
The healthcare segment dominates the application landscape, accounting for 43% of the total market value. Within this segment, continuous glucose monitoring represents the largest subsector, valued at 3.8 billion USD globally. The fitness and wellness segment follows at 31% market share, with athletic performance monitoring showing the fastest growth rate at 24.3% annually.
Regional analysis reveals North America as the current market leader with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the highest growth potential, with a projected CAGR of 22.7% through 2030, driven by increasing healthcare expenditure and rapid technological adoption in countries like China, Japan, and South Korea.
Consumer price sensitivity varies significantly between market segments. Premium invasive biosensors command price points between 200-500 USD, while non-invasive alternatives typically range from 100-350 USD. Market penetration analysis indicates that price reduction of 15% correlates with approximately 23% increase in adoption rates for non-invasive solutions, compared to only 9% for invasive alternatives.
Distribution channels are evolving rapidly, with direct-to-consumer online sales growing at 27% annually, outpacing traditional medical supply channels (11% growth). This shift reflects the increasing consumerization of healthcare technology and reduced barriers to entry for non-invasive solutions that require less regulatory oversight.
Market forecasts suggest that integration capabilities with smartphones and other personal devices will become a critical differentiator, with 82% of consumers indicating preference for biosensors with seamless connectivity features. Additionally, subscription-based service models are emerging as a significant revenue stream, projected to grow from 8% of total market revenue to 19% by 2028.
Current Landscape of Invasive vs Non-invasive Biosensing
The current landscape of biosensing technology is characterized by a clear dichotomy between invasive and non-invasive approaches, each with distinct advantages and limitations. Invasive biosensing patches typically involve penetration of the skin barrier to access biological fluids or tissues directly, enabling more accurate and comprehensive measurements of biomarkers. These devices often utilize microneedles, implantable sensors, or minimally invasive probes that can measure glucose levels, drug concentrations, or specific biomarkers with high precision.
In contrast, non-invasive biosensing patches operate on the skin surface, collecting data through transdermal sensing, optical methods, or by analyzing biofluids like sweat or interstitial fluid without breaching the skin barrier. While these devices offer greater user comfort and reduced infection risk, they frequently face challenges in measurement accuracy and consistency compared to their invasive counterparts.
The global market has witnessed significant growth in both segments, with non-invasive technologies gaining particular momentum due to consumer preference for pain-free monitoring solutions. Major healthcare technology companies have invested heavily in developing advanced non-invasive platforms, while specialized startups continue to innovate in niche applications of invasive sensing where precision requirements are paramount.
Recent technological advancements have begun to blur the boundaries between these categories. Semi-invasive approaches, such as microneedle arrays that penetrate only the outermost skin layers, represent an emerging middle ground that aims to combine the accuracy of invasive methods with the comfort of non-invasive approaches. These hybrid solutions have shown promising results in continuous glucose monitoring and drug delivery applications.
Regulatory frameworks worldwide have evolved to accommodate both types of biosensing technologies, though invasive devices typically face more stringent approval processes due to their higher risk profiles. The FDA in the United States and similar bodies in Europe and Asia have established specific pathways for wearable biosensors, with particular attention to safety standards for invasive technologies.
Consumer adoption patterns reveal interesting geographical and demographic variations. Developed markets show greater acceptance of non-invasive solutions for wellness applications, while invasive technologies maintain stronger positions in clinical and chronic disease management contexts. Emerging markets present a mixed landscape, with cost considerations often driving technology selection alongside clinical requirements.
In contrast, non-invasive biosensing patches operate on the skin surface, collecting data through transdermal sensing, optical methods, or by analyzing biofluids like sweat or interstitial fluid without breaching the skin barrier. While these devices offer greater user comfort and reduced infection risk, they frequently face challenges in measurement accuracy and consistency compared to their invasive counterparts.
The global market has witnessed significant growth in both segments, with non-invasive technologies gaining particular momentum due to consumer preference for pain-free monitoring solutions. Major healthcare technology companies have invested heavily in developing advanced non-invasive platforms, while specialized startups continue to innovate in niche applications of invasive sensing where precision requirements are paramount.
Recent technological advancements have begun to blur the boundaries between these categories. Semi-invasive approaches, such as microneedle arrays that penetrate only the outermost skin layers, represent an emerging middle ground that aims to combine the accuracy of invasive methods with the comfort of non-invasive approaches. These hybrid solutions have shown promising results in continuous glucose monitoring and drug delivery applications.
Regulatory frameworks worldwide have evolved to accommodate both types of biosensing technologies, though invasive devices typically face more stringent approval processes due to their higher risk profiles. The FDA in the United States and similar bodies in Europe and Asia have established specific pathways for wearable biosensors, with particular attention to safety standards for invasive technologies.
Consumer adoption patterns reveal interesting geographical and demographic variations. Developed markets show greater acceptance of non-invasive solutions for wellness applications, while invasive technologies maintain stronger positions in clinical and chronic disease management contexts. Emerging markets present a mixed landscape, with cost considerations often driving technology selection alongside clinical requirements.
Comparative Analysis of Current Biosensing Approaches
01 Non-invasive wearable biosensing patches
Non-invasive wearable biosensing patches are designed to monitor physiological parameters without penetrating the skin. These patches typically adhere to the skin surface and use technologies such as optical sensing, electrical impedance, or chemical analysis of sweat to collect data. They offer continuous monitoring capabilities while minimizing discomfort and eliminating infection risks associated with invasive methods.- Non-invasive wearable biosensing patches: Non-invasive wearable biosensing patches are designed to collect physiological data without penetrating the skin. These patches typically adhere to the skin surface and use various sensing technologies to monitor parameters such as heart rate, temperature, sweat composition, and electrical signals from the body. The non-invasive nature makes them comfortable for continuous wear and reduces risks associated with breaking the skin barrier, while still providing valuable health monitoring capabilities.
- Minimally invasive microneedle-based biosensing patches: Minimally invasive biosensing patches incorporate microneedle technology that penetrates only the outermost layers of the skin. These microneedles are typically small enough to avoid pain and major tissue damage while accessing interstitial fluid or shallow blood vessels. This approach allows for more direct measurement of biomarkers such as glucose, lactate, and other metabolites that cannot be reliably measured from the skin surface alone, offering a balance between measurement accuracy and user comfort.
- Materials and design for reducing invasiveness: Advanced materials and design strategies are employed to minimize the invasiveness of biosensing patches. These include flexible and stretchable substrates that conform to skin contours, biocompatible adhesives that reduce skin irritation, and ultra-thin sensing elements. Some designs incorporate biodegradable components that dissolve after use, eliminating the need for removal. These innovations help reduce the physical and psychological discomfort associated with wearing medical devices while maintaining reliable sensing capabilities.
- Invasiveness considerations for continuous monitoring: For continuous health monitoring applications, the invasiveness of biosensing patches must be carefully balanced with monitoring duration and accuracy requirements. Long-term wear patches incorporate design elements to minimize skin irritation, pressure damage, and infection risks. Some systems use replaceable sensing components while maintaining a fixed base unit to reduce repeated application trauma. The level of invasiveness is often tailored to the specific monitoring needs, with more critical parameters sometimes requiring more invasive approaches to ensure reliable data collection over extended periods.
- Hybrid invasive/non-invasive sensing approaches: Hybrid biosensing patches combine multiple sensing modalities with varying degrees of invasiveness in a single wearable platform. These systems might incorporate both surface electrodes for non-invasive measurements and minimally invasive sensors for direct biofluid access. This approach allows for comprehensive physiological monitoring while minimizing overall invasiveness. The integration of different sensing technologies enables cross-validation of measurements and provides complementary data streams that can be analyzed together for improved diagnostic accuracy and health insights.
02 Minimally invasive microneedle-based biosensing patches
Minimally invasive biosensing patches utilize microneedle technology to access interstitial fluid or superficial blood vessels while minimizing pain and tissue damage. These microneedles are typically very small (microscale) and penetrate only the outermost layers of skin. This approach provides more direct access to biomarkers than fully non-invasive methods while maintaining user comfort and reducing infection risks compared to traditional invasive techniques.Expand Specific Solutions03 Materials and adhesives for reducing skin irritation
Advanced materials and adhesives are being developed to minimize skin irritation and allergic reactions while maintaining secure attachment of biosensing patches. These include biocompatible polymers, hypoallergenic adhesives, and breathable materials that allow moisture vapor transmission. Some patches incorporate skin-friendly silicone adhesives or hydrocolloid materials that reduce irritation during extended wear periods, addressing a key concern related to the invasiveness of wearable monitoring devices.Expand Specific Solutions04 Integration of multiple sensing modalities
Modern biosensing patches often integrate multiple sensing technologies to balance invasiveness with measurement accuracy. By combining non-invasive sensors (temperature, motion, electrical impedance) with minimally invasive elements when necessary, these hybrid approaches optimize the trade-off between data quality and user comfort. This integration allows for comprehensive physiological monitoring while minimizing the overall invasiveness of the wearable system.Expand Specific Solutions05 Wireless and flexible patch designs
Advances in flexible electronics and wireless communication have enabled the development of ultrathin, conformal biosensing patches that move naturally with the skin. These designs minimize the physical presence and perceived invasiveness of the device while maintaining functionality. The elimination of rigid components and wired connections reduces mechanical irritation and improves user comfort during extended wear, addressing psychological aspects of invasiveness beyond just physical penetration.Expand Specific Solutions
Leading Companies in Biosensing Patch Development
The wearable biosensing patch market is experiencing rapid growth, transitioning from early adoption to mainstream acceptance, with an estimated market value exceeding $10 billion by 2025. The technological landscape shows varying maturity levels across invasive and non-invasive approaches. Leading academic institutions like University of California and University of Tokyo are pioneering fundamental research, while established medical technology companies such as Philips and Terumo are commercializing advanced solutions. Emerging players like Epicore Biosystems and PITTAN are disrupting the space with innovative microfluidic sweat analysis platforms. Non-invasive technologies are gaining momentum due to regulatory advantages and consumer preference, though invasive sensors still dominate in applications requiring high precision. The competitive dynamics suggest a shift toward integrated biosensing ecosystems combining hardware with AI-powered analytics for personalized health monitoring.
The Regents of the University of California
Technical Solution: The University of California has developed advanced wearable biosensing patches that utilize flexible electronics and microfluidic technologies. Their non-invasive approach focuses on sweat analysis platforms capable of continuous monitoring of multiple biomarkers simultaneously. Their technology incorporates electrochemical sensors on flexible substrates that can detect glucose, lactate, electrolytes, and various metabolites through the skin without penetrating it. Recent developments include a fully integrated system that combines sensing elements with wireless data transmission capabilities, allowing real-time health monitoring without the discomfort of invasive alternatives. The patches employ specialized microfluidic channels that efficiently collect and direct sweat to sensing elements, enhancing detection accuracy even with minimal sample volumes. Additionally, they've pioneered energy harvesting techniques that allow these patches to operate without bulky batteries, significantly improving wearability and user comfort for long-term monitoring applications[1][3].
Strengths: Superior user comfort and compliance due to non-invasive nature; capability for continuous long-term monitoring without skin damage; reduced infection risk compared to invasive alternatives. Weaknesses: Lower sensitivity for certain biomarkers that exist in minute concentrations in interstitial fluid compared to blood; potential for measurement delays due to the time required for analytes to diffuse through skin layers.
Koninklijke Philips NV
Technical Solution: Philips has developed a comprehensive biosensing ecosystem combining both invasive and non-invasive wearable patch technologies. Their non-invasive approach utilizes advanced optical sensors and bioimpedance technology to monitor vital signs including heart rate, respiratory rate, and activity levels without breaking the skin barrier. For applications requiring greater precision, their minimally invasive patches employ microneedle technology that penetrates only the outermost skin layers to access interstitial fluid. These microneedles are designed to be painless while providing more direct access to biomarkers. Philips' patches incorporate proprietary algorithms that process multi-parameter data to provide clinically relevant insights rather than just raw measurements. Their system architecture includes secure cloud connectivity for remote monitoring and integration with healthcare information systems, enabling seamless data flow between patients and healthcare providers. The patches feature extended wear designs with skin-friendly adhesives that maintain functionality for up to 14 days while minimizing skin irritation[2][5].
Strengths: Comprehensive ecosystem approach that integrates with existing healthcare infrastructure; strong focus on clinical validation and regulatory compliance; advanced data analytics capabilities that transform raw measurements into actionable insights. Weaknesses: Higher cost structure compared to simpler monitoring solutions; reliance on proprietary systems may limit interoperability with third-party devices and platforms.
Key Patents and Research in Biosensing Patch Technology
Wound monitoring sensors and use thereof
PatentActiveUS20200100711A1
Innovation
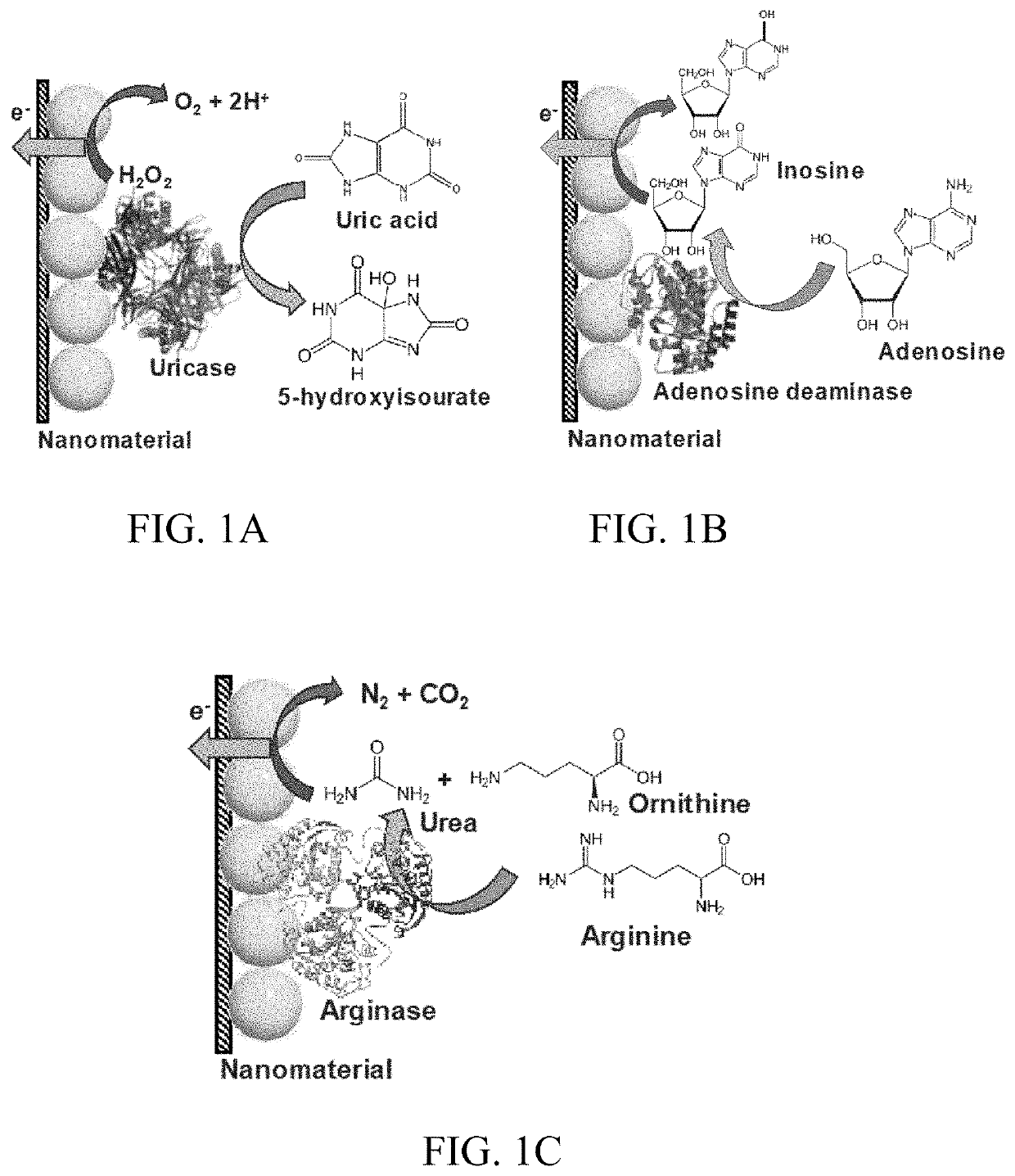
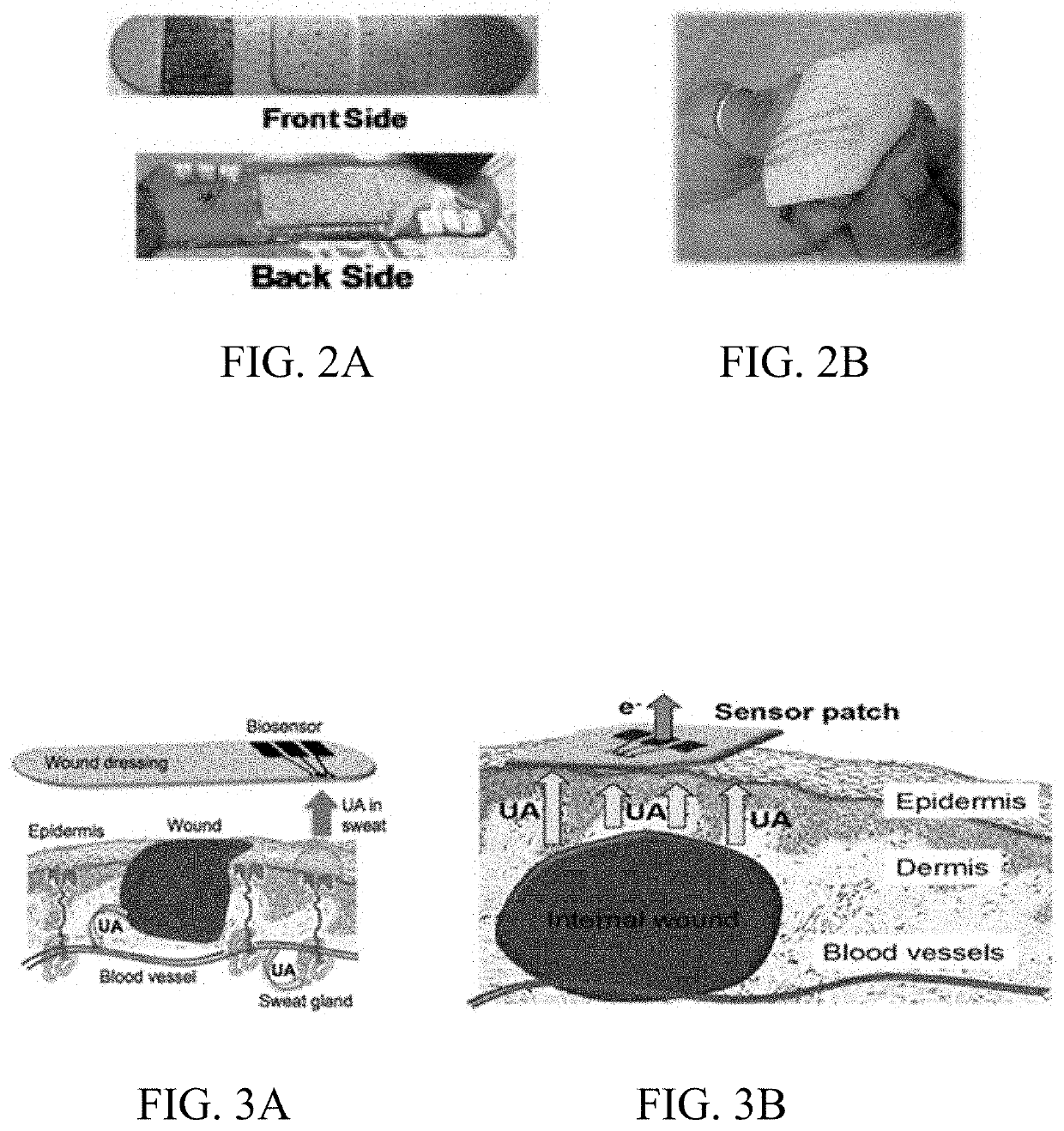
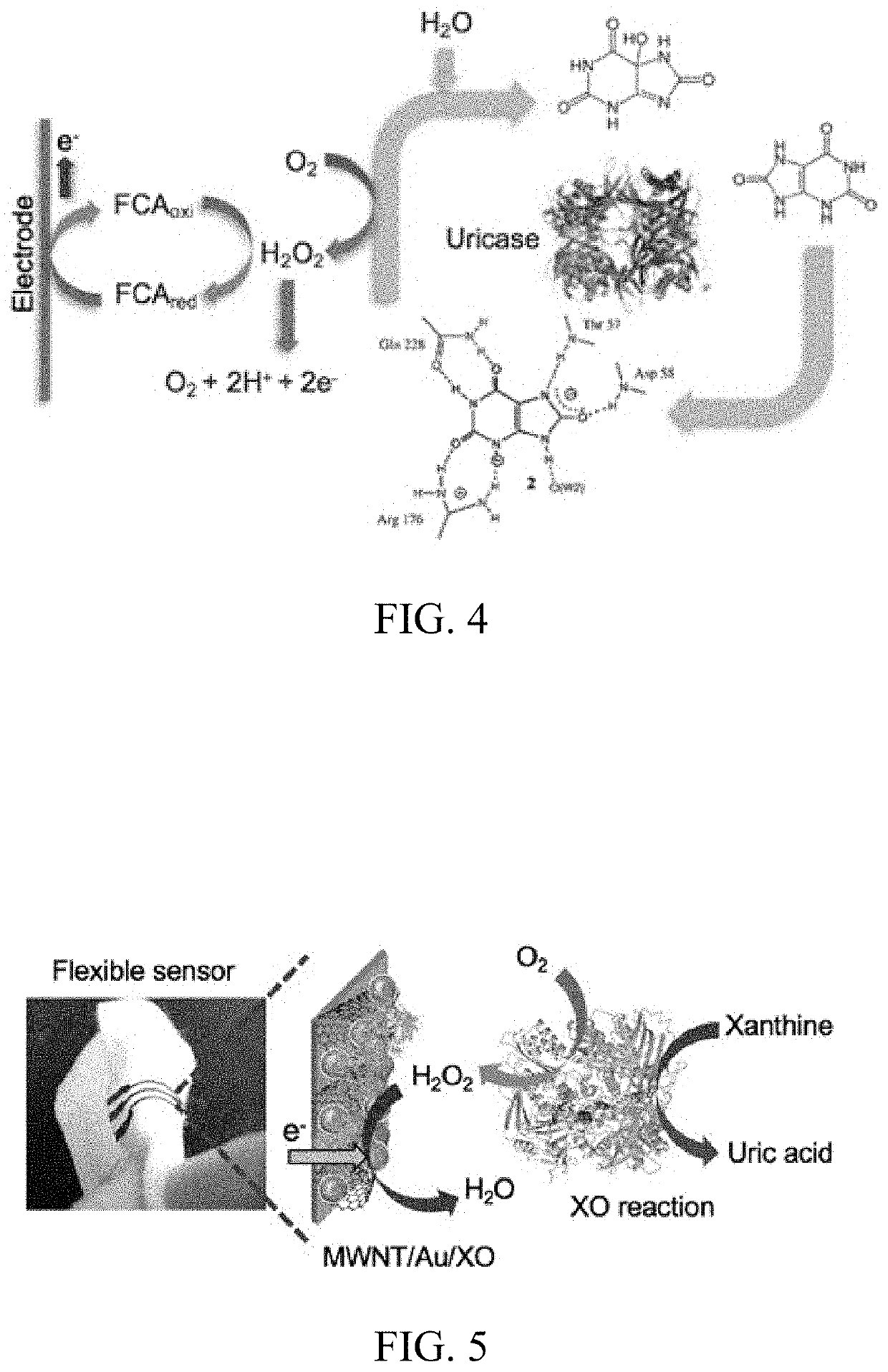
- Development of a skin-based, non-invasive enzymatic electrochemical biosensor system integrated into a wearable platform, such as a sweat patch, that continuously monitors biomarkers like uric acid and arginine levels through sweat, using sensor fusion with pH sensors to track wound healing progress.
Wearable sensor patch
PatentWO2025117631A1
Innovation
- A wearable sensor patch incorporating hydrogel microneedles and a flexible sensor element with elastomeric electrodes, allowing for minimally invasive ISF collection and simultaneous biomarker measurement in-situ. The patch is designed to accommodate hydrogel swelling and skin deformation, ensuring consistent signal stability.
Regulatory Framework for Medical-Grade Biosensing Patches
The regulatory landscape for medical-grade biosensing patches is complex and varies significantly across global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies wearable biosensors based on their intended use and risk level. Invasive biosensing patches typically fall under Class II or III medical devices, requiring premarket notification (510(k)) or premarket approval (PMA), respectively. Non-invasive patches may qualify for Class I or II classification, with potentially less stringent requirements.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose similar but distinct requirements. Under these frameworks, invasive biosensors generally receive higher risk classifications, necessitating more comprehensive clinical evidence and conformity assessment procedures before obtaining CE marking.
Data privacy regulations significantly impact biosensing technology deployment. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US establish strict requirements for processing health data collected by these devices. Manufacturers must implement robust data protection measures, including encryption, access controls, and transparent data handling policies.
Regulatory bodies increasingly recognize the unique challenges posed by software-enabled biosensing devices. The FDA's Digital Health Innovation Action Plan and Software Precertification Program aim to streamline approval processes for software-driven medical technologies while maintaining safety standards. Similarly, the International Medical Device Regulators Forum (IMDRF) has developed guidance documents specifically addressing software as a medical device (SaMD).
Quality management systems certification, such as ISO 13485, is mandatory for manufacturers in most markets. This standard ensures consistent design, development, production, and service processes. Additionally, specific technical standards like IEC 60601 for electrical safety and ISO 10993 for biocompatibility are particularly relevant for invasive biosensing patches that maintain direct contact with body tissues.
Regulatory pathways for combination products—devices incorporating both invasive and non-invasive elements—present unique challenges. These products often require coordinated reviews across multiple regulatory divisions, potentially extending development timelines and increasing compliance costs.
Emerging regulatory frameworks for real-world evidence (RWE) and post-market surveillance are reshaping approval processes. Regulators increasingly accept RWE to supplement traditional clinical trials, potentially accelerating market access while maintaining rigorous safety monitoring through enhanced post-market surveillance requirements.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose similar but distinct requirements. Under these frameworks, invasive biosensors generally receive higher risk classifications, necessitating more comprehensive clinical evidence and conformity assessment procedures before obtaining CE marking.
Data privacy regulations significantly impact biosensing technology deployment. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US establish strict requirements for processing health data collected by these devices. Manufacturers must implement robust data protection measures, including encryption, access controls, and transparent data handling policies.
Regulatory bodies increasingly recognize the unique challenges posed by software-enabled biosensing devices. The FDA's Digital Health Innovation Action Plan and Software Precertification Program aim to streamline approval processes for software-driven medical technologies while maintaining safety standards. Similarly, the International Medical Device Regulators Forum (IMDRF) has developed guidance documents specifically addressing software as a medical device (SaMD).
Quality management systems certification, such as ISO 13485, is mandatory for manufacturers in most markets. This standard ensures consistent design, development, production, and service processes. Additionally, specific technical standards like IEC 60601 for electrical safety and ISO 10993 for biocompatibility are particularly relevant for invasive biosensing patches that maintain direct contact with body tissues.
Regulatory pathways for combination products—devices incorporating both invasive and non-invasive elements—present unique challenges. These products often require coordinated reviews across multiple regulatory divisions, potentially extending development timelines and increasing compliance costs.
Emerging regulatory frameworks for real-world evidence (RWE) and post-market surveillance are reshaping approval processes. Regulators increasingly accept RWE to supplement traditional clinical trials, potentially accelerating market access while maintaining rigorous safety monitoring through enhanced post-market surveillance requirements.
Biocompatibility and User Acceptance Considerations
Biocompatibility represents a critical factor in the development and adoption of wearable biosensing patches, with significant differences between invasive and non-invasive solutions. Invasive patches that penetrate the skin barrier must meet stringent biocompatibility standards to prevent adverse tissue reactions, inflammation, or infection. Materials used in these devices require extensive testing for cytotoxicity, sensitization, and irritation potential according to ISO 10993 standards. Despite technological advances in biocompatible materials like medical-grade silicones and hydrogels, long-term wear of invasive patches continues to present challenges related to foreign body responses and tissue integration.
Non-invasive patches, while presenting fewer biocompatibility concerns, still require careful material selection to minimize skin irritation during extended wear periods. These patches typically utilize hypoallergenic adhesives and breathable materials to reduce skin maceration and contact dermatitis. Recent innovations in skin-friendly adhesives have significantly improved wear duration while maintaining skin health, with some solutions allowing comfortable wear for up to 14 days.
User acceptance factors extend beyond physical biocompatibility to encompass psychological comfort and aesthetic considerations. Research indicates that invasive patches face greater resistance due to needle phobia and concerns about pain during application and removal. A 2022 survey of potential users revealed that 68% expressed hesitation about adopting invasive biosensing technology, compared to only 23% for non-invasive alternatives. This psychological barrier represents a significant hurdle for invasive technology adoption despite potential performance advantages.
Form factor and visibility also significantly impact user acceptance. Non-invasive patches have progressed toward ultra-thin, flexible designs that conform naturally to body contours, enhancing comfort and reducing visibility under clothing. Recent developments in "electronic skin" technologies have produced patches less than 100 micrometers thick with mechanical properties matching human skin, substantially improving user experience and reducing stigma associated with medical monitoring devices.
Cultural factors further influence acceptance patterns, with regional variations in attitudes toward body modification and medical devices. Studies across different demographics reveal that younger populations generally demonstrate greater openness to wearable health technologies, though this acceptance decreases when considering invasive options. Healthcare providers also play a crucial role in technology adoption, with their recommendations significantly influencing patient willingness to use biosensing patches, particularly invasive varieties requiring clinical supervision.
Non-invasive patches, while presenting fewer biocompatibility concerns, still require careful material selection to minimize skin irritation during extended wear periods. These patches typically utilize hypoallergenic adhesives and breathable materials to reduce skin maceration and contact dermatitis. Recent innovations in skin-friendly adhesives have significantly improved wear duration while maintaining skin health, with some solutions allowing comfortable wear for up to 14 days.
User acceptance factors extend beyond physical biocompatibility to encompass psychological comfort and aesthetic considerations. Research indicates that invasive patches face greater resistance due to needle phobia and concerns about pain during application and removal. A 2022 survey of potential users revealed that 68% expressed hesitation about adopting invasive biosensing technology, compared to only 23% for non-invasive alternatives. This psychological barrier represents a significant hurdle for invasive technology adoption despite potential performance advantages.
Form factor and visibility also significantly impact user acceptance. Non-invasive patches have progressed toward ultra-thin, flexible designs that conform naturally to body contours, enhancing comfort and reducing visibility under clothing. Recent developments in "electronic skin" technologies have produced patches less than 100 micrometers thick with mechanical properties matching human skin, substantially improving user experience and reducing stigma associated with medical monitoring devices.
Cultural factors further influence acceptance patterns, with regional variations in attitudes toward body modification and medical devices. Studies across different demographics reveal that younger populations generally demonstrate greater openness to wearable health technologies, though this acceptance decreases when considering invasive options. Healthcare providers also play a crucial role in technology adoption, with their recommendations significantly influencing patient willingness to use biosensing patches, particularly invasive varieties requiring clinical supervision.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!