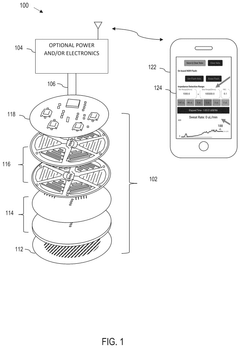
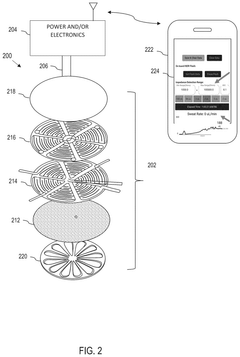
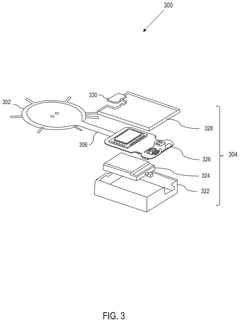
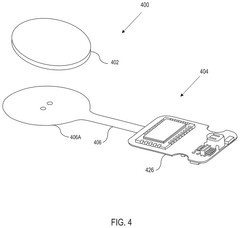
Wearable biosensing patches in cardiovascular health monitoring
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cardiovascular Monitoring Patches: Background and Objectives
Cardiovascular diseases remain the leading cause of mortality worldwide, accounting for approximately 17.9 million deaths annually according to the World Health Organization. Traditional monitoring methods such as Holter monitors and intermittent clinical assessments often fail to capture critical cardiac events that occur outside medical settings. This technological gap has driven the evolution of wearable biosensing patches specifically designed for continuous cardiovascular monitoring.
The development of cardiovascular monitoring patches traces back to the early 2000s, when advances in flexible electronics and miniaturized sensors first enabled the creation of skin-adherent monitoring devices. The field has since experienced accelerated growth, particularly in the last decade, with significant improvements in sensor accuracy, power efficiency, and data analytics capabilities. The convergence of nanotechnology, materials science, and artificial intelligence has further propelled innovation in this domain.
Current technological trends indicate a shift toward multimodal sensing platforms that can simultaneously monitor various cardiovascular parameters including heart rate, heart rate variability, blood pressure, electrocardiogram (ECG), and even biochemical markers such as troponin levels. Additionally, there is growing emphasis on developing patches with extended wear time, reduced skin irritation, and improved signal quality during physical activity.
The primary objective of cardiovascular monitoring patch technology is to enable continuous, non-invasive assessment of cardiac function in real-world settings, facilitating early detection of abnormalities and timely medical intervention. Specific technical goals include developing patches with enhanced sensitivity to detect subtle cardiac irregularities, improved specificity to reduce false alarms, and advanced algorithms for predictive analytics to identify deteriorating cardiac conditions before they become critical.
Another crucial objective is to overcome current limitations in power management, as extended monitoring periods demand efficient energy utilization. Research aims to develop energy harvesting technologies that can potentially enable self-powered patches, eliminating the need for frequent battery replacements or recharging.
From a clinical perspective, these technologies seek to bridge the gap between episodic clinical assessments and the continuous nature of cardiovascular health, providing healthcare providers with comprehensive longitudinal data for more informed decision-making. The ultimate goal is to shift cardiovascular care from reactive treatment to proactive management through personalized, data-driven interventions based on individual cardiac profiles and risk factors.
The development of cardiovascular monitoring patches traces back to the early 2000s, when advances in flexible electronics and miniaturized sensors first enabled the creation of skin-adherent monitoring devices. The field has since experienced accelerated growth, particularly in the last decade, with significant improvements in sensor accuracy, power efficiency, and data analytics capabilities. The convergence of nanotechnology, materials science, and artificial intelligence has further propelled innovation in this domain.
Current technological trends indicate a shift toward multimodal sensing platforms that can simultaneously monitor various cardiovascular parameters including heart rate, heart rate variability, blood pressure, electrocardiogram (ECG), and even biochemical markers such as troponin levels. Additionally, there is growing emphasis on developing patches with extended wear time, reduced skin irritation, and improved signal quality during physical activity.
The primary objective of cardiovascular monitoring patch technology is to enable continuous, non-invasive assessment of cardiac function in real-world settings, facilitating early detection of abnormalities and timely medical intervention. Specific technical goals include developing patches with enhanced sensitivity to detect subtle cardiac irregularities, improved specificity to reduce false alarms, and advanced algorithms for predictive analytics to identify deteriorating cardiac conditions before they become critical.
Another crucial objective is to overcome current limitations in power management, as extended monitoring periods demand efficient energy utilization. Research aims to develop energy harvesting technologies that can potentially enable self-powered patches, eliminating the need for frequent battery replacements or recharging.
From a clinical perspective, these technologies seek to bridge the gap between episodic clinical assessments and the continuous nature of cardiovascular health, providing healthcare providers with comprehensive longitudinal data for more informed decision-making. The ultimate goal is to shift cardiovascular care from reactive treatment to proactive management through personalized, data-driven interventions based on individual cardiac profiles and risk factors.
Market Analysis of Wearable Cardiovascular Monitoring Solutions
The global market for wearable cardiovascular monitoring solutions has experienced remarkable growth in recent years, driven by increasing prevalence of cardiovascular diseases, growing health consciousness among consumers, and technological advancements in biosensing capabilities. The market value reached approximately $13.7 billion in 2022 and is projected to grow at a CAGR of 23.4% through 2028, potentially reaching $47.5 billion by the end of the forecast period.
Consumer demand for continuous, non-invasive monitoring solutions has created significant market opportunities for wearable biosensing patches. These devices address the critical need for early detection and management of cardiovascular conditions outside traditional clinical settings. The aging global population, coupled with rising incidence of lifestyle-related heart conditions, has expanded the potential user base substantially.
Market segmentation reveals distinct categories within this space: consumer-grade wellness devices, clinical-grade remote monitoring solutions, and hybrid products that bridge both markets. Consumer-grade devices currently dominate market share at approximately 65%, though clinical applications are growing at a faster rate due to increasing adoption of remote patient monitoring programs by healthcare providers.
Regional analysis indicates North America holds the largest market share (42%), followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region demonstrates the highest growth potential, with China and India emerging as key markets due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about preventive healthcare.
Key demand drivers include the shift toward preventive healthcare models, insurance reimbursement policies supporting remote monitoring, and consumer preference for non-invasive, comfortable monitoring solutions. The COVID-19 pandemic accelerated market growth by highlighting the importance of remote health monitoring capabilities and reducing hospital visits for routine checkups.
Pricing analysis reveals significant variation across market segments, with consumer devices ranging from $50-300, while clinical-grade solutions command premium prices of $300-1,200 depending on functionality and certification level. Subscription-based revenue models are gaining traction, particularly for solutions offering advanced analytics and clinical interpretation services.
Distribution channels are evolving rapidly, with direct-to-consumer online sales growing fastest (37% annual growth), followed by healthcare provider procurement systems and retail pharmacy chains. Strategic partnerships between technology companies and healthcare providers have emerged as a critical success factor for market penetration.
Consumer demand for continuous, non-invasive monitoring solutions has created significant market opportunities for wearable biosensing patches. These devices address the critical need for early detection and management of cardiovascular conditions outside traditional clinical settings. The aging global population, coupled with rising incidence of lifestyle-related heart conditions, has expanded the potential user base substantially.
Market segmentation reveals distinct categories within this space: consumer-grade wellness devices, clinical-grade remote monitoring solutions, and hybrid products that bridge both markets. Consumer-grade devices currently dominate market share at approximately 65%, though clinical applications are growing at a faster rate due to increasing adoption of remote patient monitoring programs by healthcare providers.
Regional analysis indicates North America holds the largest market share (42%), followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region demonstrates the highest growth potential, with China and India emerging as key markets due to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about preventive healthcare.
Key demand drivers include the shift toward preventive healthcare models, insurance reimbursement policies supporting remote monitoring, and consumer preference for non-invasive, comfortable monitoring solutions. The COVID-19 pandemic accelerated market growth by highlighting the importance of remote health monitoring capabilities and reducing hospital visits for routine checkups.
Pricing analysis reveals significant variation across market segments, with consumer devices ranging from $50-300, while clinical-grade solutions command premium prices of $300-1,200 depending on functionality and certification level. Subscription-based revenue models are gaining traction, particularly for solutions offering advanced analytics and clinical interpretation services.
Distribution channels are evolving rapidly, with direct-to-consumer online sales growing fastest (37% annual growth), followed by healthcare provider procurement systems and retail pharmacy chains. Strategic partnerships between technology companies and healthcare providers have emerged as a critical success factor for market penetration.
Technical Challenges in Biosensing Patch Development
The development of wearable biosensing patches for cardiovascular monitoring faces numerous technical challenges that must be overcome to achieve reliable, continuous, and non-invasive health monitoring. Current biosensing patch technologies encounter significant limitations in sensor miniaturization while maintaining high sensitivity and specificity for cardiovascular biomarkers. The integration of multiple sensing modalities (ECG, PPG, temperature, motion) into a single compact patch presents complex design challenges related to component placement and signal interference.
Material biocompatibility remains a critical hurdle, as prolonged skin contact requires materials that minimize irritation while maintaining adhesion in various environmental conditions. Many existing patches cause skin reactions after extended wear periods, limiting their practical application for continuous monitoring. Additionally, achieving reliable adhesion that withstands movement, perspiration, and daily activities without compromising comfort presents significant engineering challenges.
Power management constitutes another major technical barrier. The limited surface area of patches restricts battery size, while continuous sensing and data transmission are power-intensive operations. Current energy harvesting technologies (kinetic, thermal, solar) have not yet reached sufficient efficiency for completely self-powered operation in most cardiovascular monitoring applications.
Signal processing and artifact rejection represent sophisticated technical challenges. Movement artifacts, electromagnetic interference, and biological signal variations significantly impact data quality. Real-time filtering algorithms must be computationally efficient while effectively distinguishing between physiological signals and noise—a particularly difficult task given the low-power constraints of wearable devices.
Data transmission protocols face the competing demands of energy efficiency, security, and reliability. Continuous wireless transmission quickly depletes batteries, while intermittent transmission may miss critical cardiovascular events. Furthermore, the secure transmission of sensitive health data requires robust encryption that must operate within the computational constraints of miniaturized hardware.
Sensor degradation over time affects measurement accuracy and reliability. Chemical sensors for biomarkers like lactate or glucose experience drift and reduced sensitivity with extended use. Physical sensors may become less effective due to biofouling or mechanical stress, necessitating either frequent replacement or advanced self-calibration mechanisms that are not yet fully realized in current technologies.
Manufacturing scalability presents additional challenges, as mass production of complex, multi-layer flexible electronic systems with integrated sensing components requires specialized processes that are currently costly and difficult to standardize. The integration of rigid electronic components with flexible substrates creates stress points that can lead to device failure during normal use conditions.
Material biocompatibility remains a critical hurdle, as prolonged skin contact requires materials that minimize irritation while maintaining adhesion in various environmental conditions. Many existing patches cause skin reactions after extended wear periods, limiting their practical application for continuous monitoring. Additionally, achieving reliable adhesion that withstands movement, perspiration, and daily activities without compromising comfort presents significant engineering challenges.
Power management constitutes another major technical barrier. The limited surface area of patches restricts battery size, while continuous sensing and data transmission are power-intensive operations. Current energy harvesting technologies (kinetic, thermal, solar) have not yet reached sufficient efficiency for completely self-powered operation in most cardiovascular monitoring applications.
Signal processing and artifact rejection represent sophisticated technical challenges. Movement artifacts, electromagnetic interference, and biological signal variations significantly impact data quality. Real-time filtering algorithms must be computationally efficient while effectively distinguishing between physiological signals and noise—a particularly difficult task given the low-power constraints of wearable devices.
Data transmission protocols face the competing demands of energy efficiency, security, and reliability. Continuous wireless transmission quickly depletes batteries, while intermittent transmission may miss critical cardiovascular events. Furthermore, the secure transmission of sensitive health data requires robust encryption that must operate within the computational constraints of miniaturized hardware.
Sensor degradation over time affects measurement accuracy and reliability. Chemical sensors for biomarkers like lactate or glucose experience drift and reduced sensitivity with extended use. Physical sensors may become less effective due to biofouling or mechanical stress, necessitating either frequent replacement or advanced self-calibration mechanisms that are not yet fully realized in current technologies.
Manufacturing scalability presents additional challenges, as mass production of complex, multi-layer flexible electronic systems with integrated sensing components requires specialized processes that are currently costly and difficult to standardize. The integration of rigid electronic components with flexible substrates creates stress points that can lead to device failure during normal use conditions.
Current Biosensing Patch Solutions for Cardiac Monitoring
01 Flexible and adhesive biosensing patches
Wearable biosensing patches designed with flexible and adhesive materials that conform to the body's contours for continuous monitoring. These patches incorporate stretchable electronics and skin-friendly adhesives to ensure comfort during extended wear while maintaining reliable sensor contact with the skin. The flexible design allows for natural movement without compromising data collection accuracy or causing discomfort to the wearer.- Flexible and stretchable biosensing patches: Wearable biosensing patches designed with flexible and stretchable materials that conform to the body's contours for continuous monitoring. These patches incorporate elastic substrates and conductive materials that maintain functionality during movement and physical activity. The flexibility allows for comfortable long-term wear while ensuring consistent sensor contact with the skin for accurate biosignal detection.
- Sweat analysis and biomarker detection: Biosensing patches specifically designed to collect and analyze sweat for various biomarkers including electrolytes, metabolites, and hormones. These patches incorporate microfluidic channels for sweat collection and specialized sensors that can detect multiple analytes simultaneously. The technology enables non-invasive monitoring of health conditions through continuous analysis of sweat composition and biomarker concentrations.
- Wireless data transmission and connectivity: Integration of wireless communication technologies in biosensing patches for real-time data transmission to smartphones, tablets, or cloud platforms. These patches incorporate low-power wireless protocols such as Bluetooth, NFC, or RFID to transmit collected biometric data. The wireless connectivity enables continuous remote monitoring, immediate alerts for abnormal readings, and integration with healthcare information systems.
- Energy harvesting and power management: Advanced power management systems and energy harvesting technologies for extended operation of wearable biosensing patches. These patches utilize various energy sources including body heat, motion, or ambient light to supplement or replace traditional batteries. The integration of ultra-low-power electronics and efficient power management circuits enables continuous monitoring for extended periods without frequent recharging or replacement.
- Multi-parameter physiological monitoring: Comprehensive biosensing patches capable of simultaneously monitoring multiple physiological parameters such as heart rate, temperature, blood oxygen levels, and respiratory rate. These patches incorporate various sensor types within a single wearable platform to provide holistic health monitoring. The integration of multiple sensing modalities enables correlation between different physiological parameters for more accurate health assessment and early detection of abnormalities.
02 Sweat analysis and biomarker detection
Biosensing patches capable of analyzing sweat composition to detect various biomarkers including electrolytes, metabolites, and hormones. These patches collect and analyze sweat in real-time, providing insights into hydration status, stress levels, and potential health concerns. The technology incorporates microfluidic channels and specialized sensors that can detect minute changes in biomarker concentrations, enabling early detection of physiological changes.Expand Specific Solutions03 Wireless data transmission and connectivity
Integration of wireless communication technologies in biosensing patches for seamless data transmission to smartphones, tablets, or cloud platforms. These patches utilize Bluetooth, NFC, or other wireless protocols to transmit collected biometric data in real-time or at scheduled intervals. The connectivity features enable remote monitoring by healthcare providers and integration with health management applications, allowing for timely interventions and personalized health insights.Expand Specific Solutions04 Energy harvesting and power management
Advanced power management systems and energy harvesting technologies for extended operation of wearable biosensing patches. These patches incorporate thin-film batteries, solar cells, or motion-based energy harvesting to reduce the need for frequent charging or battery replacement. Power optimization algorithms adjust sensor sampling rates and transmission frequencies based on activity levels and monitoring priorities, maximizing operational lifespan while maintaining essential monitoring functions.Expand Specific Solutions05 Multi-parameter physiological monitoring
Comprehensive biosensing patches capable of simultaneously monitoring multiple physiological parameters such as heart rate, body temperature, blood oxygen levels, and physical activity. These multi-functional patches integrate various sensor types within a single wearable device, providing a holistic view of the wearer's health status. Advanced algorithms process the multi-parameter data to identify correlations between different physiological indicators and detect potential health issues that might not be apparent from single-parameter monitoring.Expand Specific Solutions
Key Industry Players in Biosensing Patch Market
The wearable biosensing patches market for cardiovascular health monitoring is in a growth phase, with increasing adoption driven by rising cardiovascular disease prevalence globally. The market size is expanding rapidly, projected to reach several billion dollars by 2025, fueled by growing demand for remote patient monitoring solutions. Technologically, the field shows varying maturity levels across companies. Industry leaders like Philips, Baxter International, and Edwards Lifesciences have established robust platforms with FDA-cleared products, while innovative startups such as Bardy Diagnostics, LifeSignals, and Seers Technology are advancing specialized solutions with novel features. Academic institutions including Texas A&M University and Rowan University are contributing breakthrough research in materials and sensor technologies, accelerating the development of next-generation patches with enhanced accuracy and comfort.
Koninklijke Philips NV
Technical Solution: Philips has pioneered advanced wearable biosensing patch technology for cardiovascular monitoring through their Philips Wearable Biosensor platform. Their patches incorporate multiple sensing modalities including ECG, respiratory rate, skin temperature, and accelerometry in a lightweight (12g), water-resistant design that can be worn for up to 14 days[2]. The system utilizes proprietary algorithms to process physiological signals and detect early signs of patient deterioration, with particular focus on arrhythmia detection and heart failure management. Philips' patches employ a unique electrode design that maintains skin contact during movement while minimizing irritation through breathable materials and hypoallergenic adhesives[4]. Their technology includes automated data transmission to centralized monitoring systems via Bluetooth, with edge computing capabilities that pre-process data to conserve battery life and bandwidth. The patches integrate with Philips' broader healthcare ecosystem, enabling seamless data flow into electronic health records and clinical decision support systems[5].
Strengths: Comprehensive integration with existing hospital systems and EHRs enhances clinical workflow; Long wear time (up to 14 days) provides extended monitoring periods; Multi-parameter monitoring offers more complete physiological assessment beyond basic ECG. Weaknesses: Higher initial cost compared to traditional monitoring solutions; Reliance on Bluetooth connectivity may create limitations in certain healthcare environments with connectivity restrictions or interference.
LumiraDx UK Ltd.
Technical Solution: LumiraDx has developed an innovative platform for wearable cardiovascular monitoring through their multi-parameter biosensing patches. Their technology utilizes a proprietary electrochemical sensing array that can simultaneously measure multiple cardiac biomarkers in addition to traditional ECG monitoring[2]. The patches incorporate microfluidic channels that can collect and analyze small volumes of interstitial fluid to detect markers of cardiac stress and damage, such as troponin and BNP, providing early warning of cardiac events before symptoms appear. LumiraDx's system employs a unique low-power sensing architecture that enables continuous monitoring for up to 7 days on a single charge, with selective high-frequency sampling during detected events of interest[4]. Their patches utilize a proprietary adhesive technology that maintains skin contact while minimizing irritation, even in patients with sensitive skin or during physical activity. The system includes automated data analysis with machine learning algorithms that can identify subtle patterns indicating deterioration in cardiac function, with results transmitted securely to healthcare providers through cellular connectivity independent of patient smartphones[7].
Strengths: Unique combination of electrophysiological monitoring with biochemical marker detection provides more comprehensive cardiac assessment; Independent cellular connectivity eliminates reliance on patient smartphones or home WiFi; Advanced analytics can detect subtle deterioration patterns before clinical symptoms appear. Weaknesses: More complex technology may result in higher manufacturing costs and end-user pricing; Biochemical sensing components may have more stringent storage requirements than purely electronic monitoring systems.
Critical Patents and Innovations in Patch-Based Cardiac Sensing
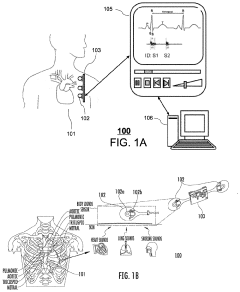
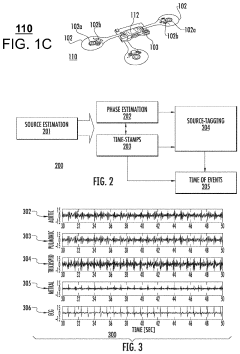
System and method of marking cardiac time intervals from the heart valve signals using a Near-Field Communication based patch biosensor
PatentPendingUS20230072872A1
Innovation
- A wearable, non-invasive biosensor system using near-field communication (NFC) technology that captures electrocardiogram (ECG) signals and composite vibration data to monitor cardiac time intervals, allowing for a battery-less, single-patch design that powers itself with radio-frequency signals, enabling hemodynamic assessments and heart-lung function analysis on a smartphone or remote monitoring station.
Wearable biosensor
PatentPendingUS20250228473A1
Innovation
- A modular, wearable biosensor patch with a multilayer structure comprising an adhesive layer, laser-induced graphene electrodes, and a PDMS microfluidic layer, featuring capillary burst valves and hydrogel valves to ensure sequential sweat collection and storage in discrete reservoirs, along with real-time monitoring capabilities.
Regulatory Framework for Medical-Grade Wearable Devices
The regulatory landscape for medical-grade wearable devices, particularly biosensing patches for cardiovascular monitoring, presents a complex framework that manufacturers must navigate to bring products to market. In the United States, the Food and Drug Administration (FDA) classifies these devices based on risk levels, with most cardiovascular monitoring patches falling under Class II, requiring 510(k) clearance. This process demands substantial clinical validation and demonstration of substantial equivalence to predicate devices.
The European Union's regulatory approach centers on the Medical Device Regulation (MDR), which replaced the Medical Device Directive in 2021, imposing more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Cardiovascular monitoring patches typically fall under Class IIa or IIb, depending on their specific functionality and invasiveness level.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains rigorous standards similar to FDA requirements, while China's National Medical Products Administration (NMPA) has recently strengthened its regulatory framework, particularly for innovative medical technologies. These regional variations create significant challenges for global market entry strategies.
Data privacy regulations intersect critically with medical device regulations. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on handling patient health data collected by wearable patches, necessitating robust data security measures and transparent privacy policies.
Reimbursement pathways represent another regulatory consideration, as coverage decisions by Medicare, Medicaid, and private insurers in the US, or national health services in other countries, significantly impact market adoption. Currently, only a limited number of wearable cardiovascular monitoring solutions have achieved reimbursement status.
The regulatory framework continues to evolve in response to rapid technological advancement. The FDA's Digital Health Innovation Action Plan and Software Precertification Program aim to streamline approval processes for software-driven medical devices, while international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) seek to establish consistent global standards for wearable medical technologies.
Manufacturers must implement comprehensive quality management systems compliant with ISO 13485 standards and maintain vigilance regarding post-market surveillance requirements, which have become increasingly stringent across all major markets, requiring systematic monitoring and reporting of adverse events.
The European Union's regulatory approach centers on the Medical Device Regulation (MDR), which replaced the Medical Device Directive in 2021, imposing more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. Cardiovascular monitoring patches typically fall under Class IIa or IIb, depending on their specific functionality and invasiveness level.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains rigorous standards similar to FDA requirements, while China's National Medical Products Administration (NMPA) has recently strengthened its regulatory framework, particularly for innovative medical technologies. These regional variations create significant challenges for global market entry strategies.
Data privacy regulations intersect critically with medical device regulations. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on handling patient health data collected by wearable patches, necessitating robust data security measures and transparent privacy policies.
Reimbursement pathways represent another regulatory consideration, as coverage decisions by Medicare, Medicaid, and private insurers in the US, or national health services in other countries, significantly impact market adoption. Currently, only a limited number of wearable cardiovascular monitoring solutions have achieved reimbursement status.
The regulatory framework continues to evolve in response to rapid technological advancement. The FDA's Digital Health Innovation Action Plan and Software Precertification Program aim to streamline approval processes for software-driven medical devices, while international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) seek to establish consistent global standards for wearable medical technologies.
Manufacturers must implement comprehensive quality management systems compliant with ISO 13485 standards and maintain vigilance regarding post-market surveillance requirements, which have become increasingly stringent across all major markets, requiring systematic monitoring and reporting of adverse events.
User Adoption Barriers and Clinical Integration Challenges
Despite the promising potential of wearable biosensing patches for cardiovascular health monitoring, several significant barriers impede widespread user adoption. Patient comfort represents a primary concern, as many current devices cause skin irritation during prolonged wear periods. The adhesive technologies used in these patches often struggle to maintain consistent skin contact while balancing comfort, particularly for users with sensitive skin or those requiring extended monitoring sessions spanning multiple days or weeks.
Technical literacy presents another substantial adoption barrier, especially among elderly populations who might benefit most from cardiovascular monitoring. Many users report difficulties in properly applying patches, interpreting data, or troubleshooting basic technical issues. This challenge is compounded by concerns regarding data privacy and security, with patients expressing hesitation about continuous health data collection and transmission to cloud-based platforms.
From a clinical integration perspective, healthcare systems face considerable challenges incorporating wearable patch data into established workflows. Electronic Health Record (EHR) compatibility remains problematic, with limited standardization for data formats from various wearable devices. This fragmentation creates significant obstacles for clinicians attempting to incorporate patch-generated data into patient records or clinical decision-making processes.
Reimbursement structures present another substantial hurdle, as many healthcare systems lack clear billing codes or payment pathways for remote monitoring using wearable patches. Without established reimbursement mechanisms, healthcare providers have limited financial incentives to adopt these technologies despite their potential clinical benefits.
Clinical validation represents perhaps the most critical challenge, with many physicians expressing skepticism about data accuracy and reliability from consumer-grade wearable patches. The variable quality of cardiovascular measurements across different devices and conditions (such as during physical activity or sleep) raises concerns about making clinical decisions based on potentially inconsistent data. This skepticism is reinforced by the limited number of large-scale clinical trials validating these technologies against gold-standard measurement methods.
Regulatory frameworks further complicate integration, as many wearable patches exist in a gray area between consumer wellness products and medical devices. This regulatory uncertainty creates hesitation among healthcare providers regarding liability and compliance issues when incorporating patch data into formal care protocols.
Technical literacy presents another substantial adoption barrier, especially among elderly populations who might benefit most from cardiovascular monitoring. Many users report difficulties in properly applying patches, interpreting data, or troubleshooting basic technical issues. This challenge is compounded by concerns regarding data privacy and security, with patients expressing hesitation about continuous health data collection and transmission to cloud-based platforms.
From a clinical integration perspective, healthcare systems face considerable challenges incorporating wearable patch data into established workflows. Electronic Health Record (EHR) compatibility remains problematic, with limited standardization for data formats from various wearable devices. This fragmentation creates significant obstacles for clinicians attempting to incorporate patch-generated data into patient records or clinical decision-making processes.
Reimbursement structures present another substantial hurdle, as many healthcare systems lack clear billing codes or payment pathways for remote monitoring using wearable patches. Without established reimbursement mechanisms, healthcare providers have limited financial incentives to adopt these technologies despite their potential clinical benefits.
Clinical validation represents perhaps the most critical challenge, with many physicians expressing skepticism about data accuracy and reliability from consumer-grade wearable patches. The variable quality of cardiovascular measurements across different devices and conditions (such as during physical activity or sleep) raises concerns about making clinical decisions based on potentially inconsistent data. This skepticism is reinforced by the limited number of large-scale clinical trials validating these technologies against gold-standard measurement methods.
Regulatory frameworks further complicate integration, as many wearable patches exist in a gray area between consumer wellness products and medical devices. This regulatory uncertainty creates hesitation among healthcare providers regarding liability and compliance issues when incorporating patch data into formal care protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!