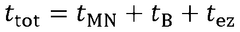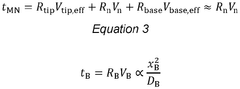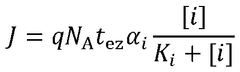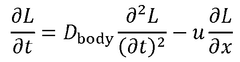Standards and regulations for wearable biosensing patches in healthcare
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Biosensing Patch Regulatory Landscape
The regulatory landscape for wearable biosensing patches in healthcare is characterized by a complex framework of standards and regulations that vary significantly across different regions. In the United States, the Food and Drug Administration (FDA) classifies these devices based on their intended use and risk level, with most wearable biosensors falling under Class II medical devices requiring 510(k) clearance. The FDA's Digital Health Innovation Action Plan specifically addresses software as a medical device (SaMD), which is particularly relevant for patches with data processing capabilities.
The European Union implements the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These regulations introduced more stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Wearable biosensing patches typically fall under Class IIa or IIb, depending on their functionality and invasiveness level.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks. Japan follows a classification system similar to the FDA, while China has implemented a more centralized approval process with specific requirements for foreign manufacturers.
International standards play a crucial role in harmonizing these diverse regulatory approaches. ISO 13485 for quality management systems in medical devices serves as a foundational standard globally. For electrical safety and electromagnetic compatibility, IEC 60601 series standards are widely adopted. The ISO 14971 standard for risk management is particularly important for wearable patches due to their direct contact with patients.
Data privacy and security regulations add another layer of complexity. In the EU, the General Data Protection Regulation (GDPR) imposes strict requirements on health data processing. The Health Insurance Portability and Accountability Act (HIPAA) serves a similar function in the US, while other regions have developed their own frameworks, such as Japan's Act on the Protection of Personal Information.
Emerging standards specifically addressing wearable technology include IEEE P1752 for mobile health data and ASTM F3461 for skin-contacting wearables. These standards focus on interoperability, biocompatibility, and performance metrics tailored to the unique characteristics of wearable biosensing patches.
Regulatory bodies are increasingly adopting a risk-based approach to accommodate rapid technological innovation while maintaining patient safety. This includes expedited review pathways for breakthrough technologies and pre-certification programs that evaluate a company's quality processes rather than individual products, allowing for more agile iterations of software-driven wearable devices.
The European Union implements the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which came into full effect in 2021 and 2022 respectively. These regulations introduced more stringent requirements for clinical evidence, post-market surveillance, and unique device identification. Wearable biosensing patches typically fall under Class IIa or IIb, depending on their functionality and invasiveness level.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks. Japan follows a classification system similar to the FDA, while China has implemented a more centralized approval process with specific requirements for foreign manufacturers.
International standards play a crucial role in harmonizing these diverse regulatory approaches. ISO 13485 for quality management systems in medical devices serves as a foundational standard globally. For electrical safety and electromagnetic compatibility, IEC 60601 series standards are widely adopted. The ISO 14971 standard for risk management is particularly important for wearable patches due to their direct contact with patients.
Data privacy and security regulations add another layer of complexity. In the EU, the General Data Protection Regulation (GDPR) imposes strict requirements on health data processing. The Health Insurance Portability and Accountability Act (HIPAA) serves a similar function in the US, while other regions have developed their own frameworks, such as Japan's Act on the Protection of Personal Information.
Emerging standards specifically addressing wearable technology include IEEE P1752 for mobile health data and ASTM F3461 for skin-contacting wearables. These standards focus on interoperability, biocompatibility, and performance metrics tailored to the unique characteristics of wearable biosensing patches.
Regulatory bodies are increasingly adopting a risk-based approach to accommodate rapid technological innovation while maintaining patient safety. This includes expedited review pathways for breakthrough technologies and pre-certification programs that evaluate a company's quality processes rather than individual products, allowing for more agile iterations of software-driven wearable devices.
Healthcare Market Demand for Biosensing Patches
The global market for wearable biosensing patches in healthcare is experiencing unprecedented growth, driven by increasing prevalence of chronic diseases and the shift toward remote patient monitoring. Current market analyses indicate that the wearable medical device market, which includes biosensing patches, is projected to reach $85.6 billion by 2027, with a compound annual growth rate of 18.3% from 2020. Biosensing patches specifically represent one of the fastest-growing segments within this broader market.
Healthcare providers are increasingly seeking solutions that enable continuous monitoring of patients' vital signs and physiological parameters outside traditional clinical settings. This demand stems from the need to reduce hospital readmissions, decrease healthcare costs, and improve patient outcomes through early intervention. The COVID-19 pandemic has significantly accelerated this trend, creating a surge in demand for remote monitoring technologies that minimize unnecessary physical contact while maintaining high standards of care.
Insurance companies and healthcare systems have begun recognizing the cost-effectiveness of preventive monitoring through biosensing patches, leading to improved reimbursement policies that further drive market adoption. Studies indicate that implementation of remote monitoring solutions can reduce hospital readmissions by up to 38% for certain chronic conditions, representing substantial cost savings for healthcare systems.
The aging global population presents another significant market driver, with individuals over 65 years expected to number 1.5 billion by 2050. This demographic typically requires more intensive healthcare monitoring and management of multiple chronic conditions, creating sustained demand for non-invasive monitoring solutions like biosensing patches.
Consumer demand is equally important, with patient surveys indicating that 64% of chronic disease patients express interest in using wearable technology to manage their conditions. The preference for unobtrusive, comfortable monitoring solutions that integrate seamlessly into daily life has positioned biosensing patches as an attractive alternative to traditional wearable devices.
Emerging markets in Asia-Pacific and Latin America represent significant growth opportunities, with healthcare modernization initiatives and increasing middle-class populations driving adoption. China's healthcare wearables market alone is growing at 28.6% annually, outpacing global averages.
Industry forecasts suggest specialized biosensing patches for specific conditions will see particularly strong growth, with patches designed for diabetes management, cardiovascular monitoring, and neurological assessment leading market development. The convergence of artificial intelligence with biosensing technology is expected to further expand market applications by enabling more sophisticated data analysis and personalized healthcare interventions.
Healthcare providers are increasingly seeking solutions that enable continuous monitoring of patients' vital signs and physiological parameters outside traditional clinical settings. This demand stems from the need to reduce hospital readmissions, decrease healthcare costs, and improve patient outcomes through early intervention. The COVID-19 pandemic has significantly accelerated this trend, creating a surge in demand for remote monitoring technologies that minimize unnecessary physical contact while maintaining high standards of care.
Insurance companies and healthcare systems have begun recognizing the cost-effectiveness of preventive monitoring through biosensing patches, leading to improved reimbursement policies that further drive market adoption. Studies indicate that implementation of remote monitoring solutions can reduce hospital readmissions by up to 38% for certain chronic conditions, representing substantial cost savings for healthcare systems.
The aging global population presents another significant market driver, with individuals over 65 years expected to number 1.5 billion by 2050. This demographic typically requires more intensive healthcare monitoring and management of multiple chronic conditions, creating sustained demand for non-invasive monitoring solutions like biosensing patches.
Consumer demand is equally important, with patient surveys indicating that 64% of chronic disease patients express interest in using wearable technology to manage their conditions. The preference for unobtrusive, comfortable monitoring solutions that integrate seamlessly into daily life has positioned biosensing patches as an attractive alternative to traditional wearable devices.
Emerging markets in Asia-Pacific and Latin America represent significant growth opportunities, with healthcare modernization initiatives and increasing middle-class populations driving adoption. China's healthcare wearables market alone is growing at 28.6% annually, outpacing global averages.
Industry forecasts suggest specialized biosensing patches for specific conditions will see particularly strong growth, with patches designed for diabetes management, cardiovascular monitoring, and neurological assessment leading market development. The convergence of artificial intelligence with biosensing technology is expected to further expand market applications by enabling more sophisticated data analysis and personalized healthcare interventions.
Current Regulatory Challenges for Medical Wearables
The regulatory landscape for wearable biosensing patches in healthcare is currently characterized by significant fragmentation and inconsistency across different regions. In the United States, the FDA has established a risk-based classification system for medical devices, with most wearable biosensors falling under Class II (moderate risk) requiring 510(k) clearance. However, the rapid pace of technological innovation has created a gap between existing regulatory frameworks and emerging wearable technologies, particularly those with multiple functionalities or novel sensing capabilities.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. These regulations have created substantial challenges for manufacturers of wearable biosensing patches, especially smaller companies with limited regulatory resources. The transition period for compliance has proven particularly difficult, with many manufacturers struggling to meet the new requirements within the specified timeframes.
Data privacy and security regulations present another significant challenge. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on the collection, processing, and storage of health data. Wearable biosensing patches, which continuously collect sensitive physiological data, must implement robust data protection measures while maintaining functionality and user experience.
Interoperability standards represent a critical regulatory gap. Despite efforts from organizations like IEEE, HL7, and the Continua Health Alliance, there remains a lack of universally accepted standards for data exchange between wearable devices and healthcare systems. This fragmentation impedes the integration of wearable data into clinical workflows and electronic health records, limiting their utility in healthcare settings.
Regulatory bodies are also grappling with the validation of software algorithms embedded in wearable biosensing patches. The FDA's Digital Health Software Precertification Program represents an attempt to address this challenge, but questions remain about how to effectively validate AI and machine learning algorithms that may evolve over time through continuous learning.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to align regulatory approaches across different jurisdictions. However, significant differences persist, creating compliance burdens for manufacturers seeking global market access. These disparities are particularly pronounced in emerging markets, where regulatory frameworks for wearable medical technologies may be underdeveloped or entirely absent.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. These regulations have created substantial challenges for manufacturers of wearable biosensing patches, especially smaller companies with limited regulatory resources. The transition period for compliance has proven particularly difficult, with many manufacturers struggling to meet the new requirements within the specified timeframes.
Data privacy and security regulations present another significant challenge. The General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the US impose strict requirements on the collection, processing, and storage of health data. Wearable biosensing patches, which continuously collect sensitive physiological data, must implement robust data protection measures while maintaining functionality and user experience.
Interoperability standards represent a critical regulatory gap. Despite efforts from organizations like IEEE, HL7, and the Continua Health Alliance, there remains a lack of universally accepted standards for data exchange between wearable devices and healthcare systems. This fragmentation impedes the integration of wearable data into clinical workflows and electronic health records, limiting their utility in healthcare settings.
Regulatory bodies are also grappling with the validation of software algorithms embedded in wearable biosensing patches. The FDA's Digital Health Software Precertification Program represents an attempt to address this challenge, but questions remain about how to effectively validate AI and machine learning algorithms that may evolve over time through continuous learning.
International harmonization efforts, such as the International Medical Device Regulators Forum (IMDRF), are working to align regulatory approaches across different jurisdictions. However, significant differences persist, creating compliance burdens for manufacturers seeking global market access. These disparities are particularly pronounced in emerging markets, where regulatory frameworks for wearable medical technologies may be underdeveloped or entirely absent.
Compliance Frameworks for Wearable Medical Devices
01 Flexible and adhesive biosensing patches
Wearable biosensing patches designed with flexible and adhesive materials that conform to the body's contours for continuous monitoring. These patches incorporate stretchable electronics and skin-friendly adhesives to ensure comfort during prolonged wear while maintaining reliable sensor contact with the skin. The flexible design allows for natural movement without compromising data collection accuracy or causing skin irritation.- Wearable biosensing patches for health monitoring: Wearable biosensing patches designed for continuous health monitoring can track various physiological parameters such as heart rate, temperature, and blood glucose levels. These patches typically incorporate flexible electronics and sensors that adhere to the skin, allowing for non-invasive monitoring. The data collected can be transmitted wirelessly to smartphones or healthcare systems for real-time analysis, enabling early detection of health issues and personalized healthcare management.
- Materials and adhesives for skin-friendly biosensing patches: Advanced materials and adhesives are crucial for developing skin-friendly biosensing patches that can be worn comfortably for extended periods. These materials include biocompatible polymers, stretchable substrates, and hypoallergenic adhesives that minimize skin irritation while maintaining secure attachment. The patches are designed to be breathable, waterproof, and capable of conforming to body contours, ensuring reliable sensor contact with the skin while allowing for normal daily activities including showering and exercise.
- Sweat analysis and biomarker detection technologies: Wearable biosensing patches can analyze sweat composition to detect various biomarkers indicative of health conditions. These patches incorporate electrochemical sensors, colorimetric assays, or optical detection methods to measure electrolytes, metabolites, hormones, and other biomolecules present in sweat. The technology enables non-invasive monitoring of conditions such as dehydration, stress levels, and metabolic disorders, providing valuable insights for personalized health management and athletic performance optimization.
- Energy harvesting and power management for long-term use: Energy harvesting technologies are integrated into wearable biosensing patches to extend their operational lifespan without frequent battery replacements. These patches can generate power from body heat, movement, or ambient light using thermoelectric generators, piezoelectric materials, or photovoltaic cells. Advanced power management systems optimize energy consumption by implementing sleep modes, efficient data processing algorithms, and selective sensing schedules, enabling continuous monitoring for days or weeks without external charging.
- Data processing and wireless communication systems: Sophisticated data processing and wireless communication systems are essential components of wearable biosensing patches. These systems include miniaturized microcontrollers that perform on-device signal processing and analytics to extract meaningful health insights from raw sensor data. Low-power wireless technologies such as Bluetooth Low Energy, NFC, or custom RF protocols enable secure transmission of data to smartphones or cloud platforms. Advanced encryption and authentication mechanisms protect sensitive health information while ensuring reliable connectivity for continuous monitoring applications.
02 Multimodal physiological monitoring systems
Advanced biosensing patches capable of simultaneously monitoring multiple physiological parameters such as heart rate, temperature, sweat composition, and blood glucose levels. These integrated systems combine various sensor types within a single patch platform to provide comprehensive health insights. The multimodal approach enables correlation between different biomarkers for more accurate health assessment and early detection of medical conditions.Expand Specific Solutions03 Data processing and wireless communication capabilities
Biosensing patches equipped with integrated microprocessors for on-device data processing and wireless communication modules for real-time data transmission to smartphones or cloud platforms. These patches incorporate low-power electronics and efficient algorithms to analyze biosignals locally before transmission, reducing power consumption and extending battery life. The wireless connectivity enables remote monitoring by healthcare providers and integration with telehealth systems.Expand Specific Solutions04 Specialized biomarker detection technologies
Innovative sensing technologies designed for specific biomarker detection, including electrochemical sensors, optical sensors, and enzymatic biosensors. These specialized detection methods enable monitoring of targeted health indicators such as stress hormones, inflammatory markers, or specific disease biomarkers. The technologies incorporate novel materials and detection principles to achieve high sensitivity and selectivity for accurate biomarker quantification.Expand Specific Solutions05 Energy harvesting and power management solutions
Self-powered biosensing patches that utilize energy harvesting technologies such as piezoelectric generators, thermoelectric converters, or photovoltaic cells to extend operational lifetime. These patches incorporate advanced power management circuits to optimize energy usage and storage. The energy harvesting capabilities reduce or eliminate the need for battery replacement, enabling truly long-term wearable health monitoring solutions.Expand Specific Solutions
Key Regulatory Bodies and Industry Players
The wearable biosensing patch market in healthcare is currently in a growth phase, characterized by increasing adoption and technological advancements. The global market size is expanding rapidly, projected to reach significant valuation as healthcare systems embrace remote monitoring solutions. Technologically, the field shows varying maturity levels across players. Companies like Philips, Samsung, and Intel bring substantial resources and established healthcare credentials, while specialized firms such as VitalConnect, LifeSignals, and VivaLNK demonstrate advanced innovation in patch-specific technologies. Academic institutions (USC, Texas A&M) and research organizations (Industrial Technology Research Institute) contribute foundational research. The regulatory landscape remains complex, with major players like Philips and Samsung better positioned to navigate compliance requirements, while newer entrants focus on developing compliant technologies that can scale within evolving standards frameworks.
Koninklijke Philips NV
Technical Solution: Philips has developed an extensive portfolio of wearable biosensing patches that adhere to rigorous medical device regulations globally. Their BioSensor platform incorporates wireless vital signs monitoring technology that complies with IEC 60601-1-11 standards for home healthcare environments. Philips has been instrumental in establishing industry standards through their participation in the Continua Health Alliance and Personal Connected Health Alliance, helping develop IEEE 11073 standards for personal health device communication. Their wearable patches implement advanced algorithms validated through clinical trials to ensure measurement accuracy according to AAMI standards. Philips' regulatory strategy encompasses both traditional medical device pathways and newer regulatory frameworks specifically designed for Software as a Medical Device (SaMD), allowing their connected biosensing solutions to meet FDA, MDR (EU), and MDSAP requirements simultaneously. The company has also pioneered cybersecurity standards for connected medical devices, implementing NIST frameworks for threat modeling and vulnerability management.
Strengths: Extensive global regulatory expertise and established quality management systems; strong integration capabilities with hospital information systems; comprehensive clinical validation protocols. Weaknesses: Higher price points compared to specialized startups; complex product portfolio may result in longer regulatory approval timelines for new features.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed an integrated approach to wearable biosensing that bridges consumer electronics and medical device regulatory frameworks. Their Samsung Health platform incorporates both consumer wearables and medical-grade patches that comply with different regulatory tiers based on intended use. For their medical-grade biosensors, Samsung implements IEC 62304 software lifecycle processes and has established quality management systems that meet both ISO 13485 and FDA requirements. The company has been actively involved in standards development through participation in the Bluetooth Special Interest Group's Medical Devices Working Group, contributing to the development of Bluetooth Low Energy profiles optimized for medical sensor data transmission. Samsung's regulatory strategy leverages their consumer electronics expertise to address usability requirements (IEC 62366) while implementing medical device data systems that comply with healthcare interoperability standards like HL7 FHIR. Their biosensing patches incorporate Samsung Knox security architecture adapted for healthcare applications, meeting both FDA cybersecurity guidance and HIPAA requirements for protected health information.
Strengths: Extensive manufacturing capabilities with established supply chain management; strong consumer electronics integration creating comprehensive health ecosystems; advanced security implementations. Weaknesses: Primary focus on consumer market may limit depth of specialized clinical applications; complex organizational structure can slow regulatory approval processes for purely medical applications.
Critical Technical Standards for Biosensing Accuracy
Wearable sensor patch
PatentWO2025117631A1
Innovation
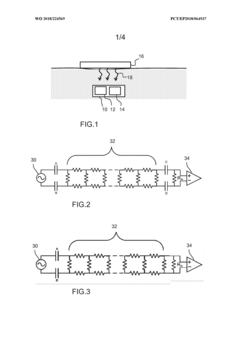
- A wearable sensor patch incorporating hydrogel microneedles and a flexible sensor element with elastomeric electrodes, allowing for minimally invasive ISF collection and simultaneous biomarker measurement in-situ. The patch is designed to accommodate hydrogel swelling and skin deformation, ensuring consistent signal stability.
Wearable or implantable sensor or actuator device
PatentWO2018224569A1
Innovation
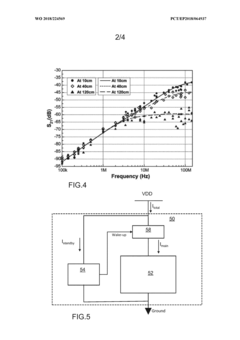
- A sensor or actuator device with a dormant mode and a self-powered wake up circuit that uses externally received wireless signals to switch from dormant to active mode, eliminating standby power consumption by disconnecting the battery and utilizing RF or optical energy harvesting for wake up signals.
Patient Data Privacy and Security Requirements
Patient data privacy and security represent critical concerns in the implementation of wearable biosensing patches in healthcare. These devices continuously collect sensitive physiological data, necessitating robust protection mechanisms that comply with international regulations. The Health Insurance Portability and Accountability Act (HIPAA) in the United States establishes stringent requirements for safeguarding protected health information (PHI), mandating encryption, access controls, and audit trails for all patient data collected through wearable patches.
Similarly, the European Union's General Data Protection Regulation (GDPR) imposes comprehensive data protection requirements applicable to wearable biosensing technologies. These regulations require explicit patient consent for data collection, processing transparency, and the right to data portability and deletion. Manufacturers must implement privacy by design principles, ensuring security measures are integrated into devices from the conceptual stage rather than added as afterthoughts.
The ISO/IEC 27001 and ISO/IEC 27799 standards provide frameworks specifically for information security management in healthcare contexts. These standards outline requirements for establishing, implementing, maintaining, and continually improving information security management systems. For wearable biosensing patches, compliance with these standards involves implementing secure data transmission protocols, robust authentication mechanisms, and comprehensive risk assessment procedures.
Technical security requirements for these devices include end-to-end encryption for all data transmission, secure storage mechanisms for on-device data, and strong authentication protocols to prevent unauthorized access. Many regulatory bodies now require multi-factor authentication for accessing sensitive health data and mandate regular security updates to address emerging vulnerabilities.
Data minimization principles must be incorporated into device design, ensuring only necessary health information is collected and retained. This approach reduces potential exposure in case of security breaches while maintaining clinical utility. Manufacturers must also implement breach detection systems and establish incident response protocols to address security incidents promptly.
Patient consent management represents another critical security requirement. Systems must provide clear mechanisms for patients to understand what data is being collected, how it will be used, and options to revoke consent. This includes granular permission settings allowing patients to control which specific data types are shared with different healthcare providers or third parties.
Regulatory frameworks increasingly emphasize accountability through comprehensive documentation of security measures, regular compliance audits, and vulnerability assessments. Manufacturers must maintain detailed records demonstrating ongoing compliance with applicable regulations and standards. This documentation becomes particularly important during regulatory reviews and in the aftermath of security incidents.
Similarly, the European Union's General Data Protection Regulation (GDPR) imposes comprehensive data protection requirements applicable to wearable biosensing technologies. These regulations require explicit patient consent for data collection, processing transparency, and the right to data portability and deletion. Manufacturers must implement privacy by design principles, ensuring security measures are integrated into devices from the conceptual stage rather than added as afterthoughts.
The ISO/IEC 27001 and ISO/IEC 27799 standards provide frameworks specifically for information security management in healthcare contexts. These standards outline requirements for establishing, implementing, maintaining, and continually improving information security management systems. For wearable biosensing patches, compliance with these standards involves implementing secure data transmission protocols, robust authentication mechanisms, and comprehensive risk assessment procedures.
Technical security requirements for these devices include end-to-end encryption for all data transmission, secure storage mechanisms for on-device data, and strong authentication protocols to prevent unauthorized access. Many regulatory bodies now require multi-factor authentication for accessing sensitive health data and mandate regular security updates to address emerging vulnerabilities.
Data minimization principles must be incorporated into device design, ensuring only necessary health information is collected and retained. This approach reduces potential exposure in case of security breaches while maintaining clinical utility. Manufacturers must also implement breach detection systems and establish incident response protocols to address security incidents promptly.
Patient consent management represents another critical security requirement. Systems must provide clear mechanisms for patients to understand what data is being collected, how it will be used, and options to revoke consent. This includes granular permission settings allowing patients to control which specific data types are shared with different healthcare providers or third parties.
Regulatory frameworks increasingly emphasize accountability through comprehensive documentation of security measures, regular compliance audits, and vulnerability assessments. Manufacturers must maintain detailed records demonstrating ongoing compliance with applicable regulations and standards. This documentation becomes particularly important during regulatory reviews and in the aftermath of security incidents.
Clinical Validation Protocols for Biosensing Patches
Clinical validation protocols for biosensing patches represent a critical framework for ensuring these wearable healthcare technologies deliver accurate, reliable, and clinically meaningful data. The validation process typically follows a multi-phase approach, beginning with laboratory testing under controlled conditions to establish baseline performance metrics, followed by small-scale human subject testing, and culminating in larger clinical trials that reflect real-world usage scenarios.
The primary validation parameters include accuracy (comparison against gold standard medical devices), precision (reproducibility of measurements), sensitivity (ability to detect subtle physiological changes), and specificity (ability to distinguish between similar but distinct biological signals). These parameters must be rigorously tested across diverse patient populations, considering variables such as skin types, age groups, activity levels, and environmental conditions.
Temporal validation is particularly important for continuous monitoring applications, requiring assessment of drift characteristics, calibration stability, and long-term performance degradation. This includes evaluating how factors such as perspiration, movement artifacts, and skin interface changes affect measurement reliability over extended wear periods ranging from 24 hours to several weeks.
User compliance validation constitutes another essential protocol component, examining how patch design, comfort, and usability influence patient adherence. This includes structured assessments of skin irritation, allergic reactions, and overall comfort during normal daily activities, sleep, and exercise.
Data processing algorithms require separate validation protocols to ensure that signal processing, artifact rejection, and analytical methods accurately transform raw sensor data into clinically actionable information. This often involves comparing algorithm outputs against manual analysis by clinical experts across diverse physiological states and patient conditions.
Validation protocols must also address specific clinical contexts, establishing performance requirements for different use cases such as chronic disease management, post-surgical monitoring, or preventive health screening. Each application demands tailored validation approaches that reflect the specific physiological parameters being monitored and the clinical decisions that will be informed by the data.
Regulatory bodies increasingly require validation studies to demonstrate not only technical performance but also clinical utility and patient outcomes. This necessitates the development of standardized protocols that can establish clear connections between biosensor data quality and meaningful health impacts, supporting both regulatory approval processes and clinical adoption pathways.
The primary validation parameters include accuracy (comparison against gold standard medical devices), precision (reproducibility of measurements), sensitivity (ability to detect subtle physiological changes), and specificity (ability to distinguish between similar but distinct biological signals). These parameters must be rigorously tested across diverse patient populations, considering variables such as skin types, age groups, activity levels, and environmental conditions.
Temporal validation is particularly important for continuous monitoring applications, requiring assessment of drift characteristics, calibration stability, and long-term performance degradation. This includes evaluating how factors such as perspiration, movement artifacts, and skin interface changes affect measurement reliability over extended wear periods ranging from 24 hours to several weeks.
User compliance validation constitutes another essential protocol component, examining how patch design, comfort, and usability influence patient adherence. This includes structured assessments of skin irritation, allergic reactions, and overall comfort during normal daily activities, sleep, and exercise.
Data processing algorithms require separate validation protocols to ensure that signal processing, artifact rejection, and analytical methods accurately transform raw sensor data into clinically actionable information. This often involves comparing algorithm outputs against manual analysis by clinical experts across diverse physiological states and patient conditions.
Validation protocols must also address specific clinical contexts, establishing performance requirements for different use cases such as chronic disease management, post-surgical monitoring, or preventive health screening. Each application demands tailored validation approaches that reflect the specific physiological parameters being monitored and the clinical decisions that will be informed by the data.
Regulatory bodies increasingly require validation studies to demonstrate not only technical performance but also clinical utility and patient outcomes. This necessitates the development of standardized protocols that can establish clear connections between biosensor data quality and meaningful health impacts, supporting both regulatory approval processes and clinical adoption pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!