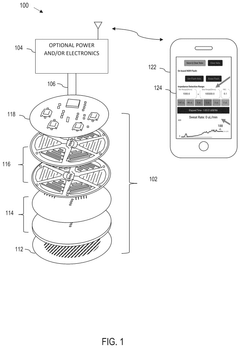
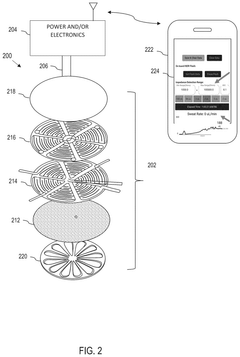
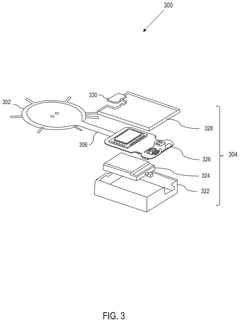
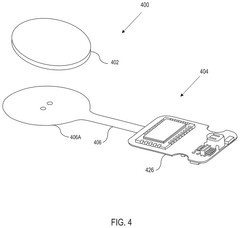
Wearable biosensing patches for drug monitoring applications
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Wearable Biosensing Patch Technology Background and Objectives
Wearable biosensing patches represent a revolutionary advancement in healthcare technology, emerging from the convergence of flexible electronics, biochemical sensing, and wireless communication technologies. These non-invasive, skin-adherent devices have evolved significantly over the past decade, transitioning from bulky, limited-function prototypes to sophisticated, miniaturized systems capable of continuous physiological monitoring. The trajectory of development has been characterized by progressive improvements in sensor sensitivity, biocompatibility, power efficiency, and data analytics capabilities.
The fundamental objective of wearable biosensing patches for drug monitoring applications is to provide real-time, continuous measurement of drug concentrations in bodily fluids, primarily interstitial fluid or sweat, without requiring blood draws or laboratory analysis. This technology aims to revolutionize therapeutic drug monitoring by enabling personalized dosing regimens, improving medication adherence, and reducing adverse drug reactions through immediate feedback on pharmacokinetics.
Historical development of these technologies began with simple electrochemical sensors for glucose monitoring, gradually expanding to encompass a wider range of analytes including various pharmaceutical compounds. Key technological milestones include the development of microneedle-based sensing platforms, implementation of enzymatic and aptamer-based recognition elements, and integration of microfluidic sampling systems that enhance reliability and extend operational lifetimes.
Current technological trends indicate movement toward multimodal sensing capabilities, where a single patch can simultaneously monitor multiple drug compounds and relevant physiological parameters. Additionally, there is growing emphasis on developing self-powered systems through energy harvesting techniques and improving biocompatibility through novel materials that minimize skin irritation during extended wear periods.
The ultimate technical goals for this technology include achieving clinically validated accuracy comparable to standard laboratory methods, extending continuous monitoring periods to weeks rather than days, developing universal sensing platforms adaptable to various drug classes, and creating fully integrated systems that combine sensing with automated drug delivery for closed-loop therapeutic systems.
Significant challenges remain in sensor selectivity, stability in complex biological matrices, and correlation of measurements from interstitial fluid or sweat with blood concentrations. Nevertheless, the field is progressing rapidly, driven by increasing demand for personalized medicine approaches and remote patient monitoring solutions, particularly for chronic conditions requiring complex medication regimens.
The fundamental objective of wearable biosensing patches for drug monitoring applications is to provide real-time, continuous measurement of drug concentrations in bodily fluids, primarily interstitial fluid or sweat, without requiring blood draws or laboratory analysis. This technology aims to revolutionize therapeutic drug monitoring by enabling personalized dosing regimens, improving medication adherence, and reducing adverse drug reactions through immediate feedback on pharmacokinetics.
Historical development of these technologies began with simple electrochemical sensors for glucose monitoring, gradually expanding to encompass a wider range of analytes including various pharmaceutical compounds. Key technological milestones include the development of microneedle-based sensing platforms, implementation of enzymatic and aptamer-based recognition elements, and integration of microfluidic sampling systems that enhance reliability and extend operational lifetimes.
Current technological trends indicate movement toward multimodal sensing capabilities, where a single patch can simultaneously monitor multiple drug compounds and relevant physiological parameters. Additionally, there is growing emphasis on developing self-powered systems through energy harvesting techniques and improving biocompatibility through novel materials that minimize skin irritation during extended wear periods.
The ultimate technical goals for this technology include achieving clinically validated accuracy comparable to standard laboratory methods, extending continuous monitoring periods to weeks rather than days, developing universal sensing platforms adaptable to various drug classes, and creating fully integrated systems that combine sensing with automated drug delivery for closed-loop therapeutic systems.
Significant challenges remain in sensor selectivity, stability in complex biological matrices, and correlation of measurements from interstitial fluid or sweat with blood concentrations. Nevertheless, the field is progressing rapidly, driven by increasing demand for personalized medicine approaches and remote patient monitoring solutions, particularly for chronic conditions requiring complex medication regimens.
Drug Monitoring Market Demand Analysis
The global drug monitoring market is experiencing significant growth driven by increasing prevalence of chronic diseases requiring medication management and rising concerns about medication adherence. Current projections indicate the drug therapeutic monitoring market will reach approximately $3.37 billion by 2025, growing at a CAGR of 7.1% from 2020. This growth trajectory reflects the expanding need for personalized medicine approaches and continuous patient monitoring solutions.
Healthcare providers are increasingly seeking real-time drug concentration data to optimize treatment protocols and minimize adverse effects. Traditional drug monitoring methods involving intermittent blood sampling fail to provide continuous insights into drug metabolism patterns, creating substantial demand for wearable biosensing technologies that can deliver ongoing monitoring capabilities without patient disruption.
Patient demographics are significantly influencing market dynamics, with aging populations in developed regions requiring more complex medication regimens. Statistics show that approximately 50% of patients with chronic conditions do not adhere to prescribed medication schedules, resulting in suboptimal treatment outcomes and increased healthcare costs estimated at $100-300 billion annually in the United States alone.
The home healthcare segment represents the fastest-growing application area for drug monitoring technologies, expanding at nearly 9% annually as healthcare systems worldwide shift toward outpatient and remote care models. This transition has been accelerated by the COVID-19 pandemic, which catalyzed adoption of telehealth solutions and remote patient monitoring technologies.
Geographically, North America currently dominates the drug monitoring market with approximately 40% market share, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the highest growth rates over the next five years due to improving healthcare infrastructure and increasing awareness about medication management.
Specific drug categories showing highest demand for continuous monitoring include anticoagulants, immunosuppressants, antiepileptics, and chemotherapeutic agents - medications with narrow therapeutic windows where maintaining optimal blood concentrations is critical for efficacy and safety. The oncology segment specifically represents about 25% of the current drug monitoring market demand.
Consumer preferences indicate strong interest in non-invasive monitoring solutions, with market surveys showing over 70% of patients would prefer wearable patches over traditional blood sampling methods if given the option. This consumer sentiment aligns with healthcare providers' interest in technologies that improve patient compliance and treatment outcomes while reducing overall healthcare utilization costs.
Healthcare providers are increasingly seeking real-time drug concentration data to optimize treatment protocols and minimize adverse effects. Traditional drug monitoring methods involving intermittent blood sampling fail to provide continuous insights into drug metabolism patterns, creating substantial demand for wearable biosensing technologies that can deliver ongoing monitoring capabilities without patient disruption.
Patient demographics are significantly influencing market dynamics, with aging populations in developed regions requiring more complex medication regimens. Statistics show that approximately 50% of patients with chronic conditions do not adhere to prescribed medication schedules, resulting in suboptimal treatment outcomes and increased healthcare costs estimated at $100-300 billion annually in the United States alone.
The home healthcare segment represents the fastest-growing application area for drug monitoring technologies, expanding at nearly 9% annually as healthcare systems worldwide shift toward outpatient and remote care models. This transition has been accelerated by the COVID-19 pandemic, which catalyzed adoption of telehealth solutions and remote patient monitoring technologies.
Geographically, North America currently dominates the drug monitoring market with approximately 40% market share, followed by Europe and Asia-Pacific. However, emerging economies in Asia and Latin America are expected to witness the highest growth rates over the next five years due to improving healthcare infrastructure and increasing awareness about medication management.
Specific drug categories showing highest demand for continuous monitoring include anticoagulants, immunosuppressants, antiepileptics, and chemotherapeutic agents - medications with narrow therapeutic windows where maintaining optimal blood concentrations is critical for efficacy and safety. The oncology segment specifically represents about 25% of the current drug monitoring market demand.
Consumer preferences indicate strong interest in non-invasive monitoring solutions, with market surveys showing over 70% of patients would prefer wearable patches over traditional blood sampling methods if given the option. This consumer sentiment aligns with healthcare providers' interest in technologies that improve patient compliance and treatment outcomes while reducing overall healthcare utilization costs.
Current Biosensing Patch Technologies and Challenges
Wearable biosensing patches represent a significant advancement in personalized healthcare monitoring, particularly for drug monitoring applications. Current technologies in this field can be broadly categorized into electrochemical, optical, and mechanical sensing modalities, each with distinct advantages and limitations for continuous drug monitoring.
Electrochemical biosensing patches dominate the current market, utilizing amperometric, potentiometric, or impedimetric detection methods. These patches typically incorporate enzyme-based sensors that generate electrical signals proportional to drug concentrations in interstitial fluid or sweat. Notable examples include microneedle array patches for minimally invasive sampling and solid-contact ion-selective electrodes for electrolyte monitoring. While offering high sensitivity and selectivity, these systems face challenges with enzyme stability in ambient conditions and signal drift during prolonged wear.
Optical biosensing technologies have emerged as promising alternatives, employing fluorescence, colorimetric, or surface plasmon resonance techniques. These patches often utilize chromophores or fluorophores that interact with target drug molecules to produce measurable optical signals. Recent innovations include smartphone-compatible colorimetric patches that enable visual drug concentration assessment without specialized equipment. However, optical approaches struggle with skin pigmentation interference and limited penetration depth.
Mechanical sensing modalities, including piezoelectric and acoustic wave sensors, detect mass changes or mechanical property alterations upon drug binding. These technologies offer label-free detection capabilities but typically demonstrate lower sensitivity compared to electrochemical alternatives.
A significant challenge across all biosensing patch technologies is biocompatibility and skin adhesion. Current adhesive technologies must balance secure attachment with minimal skin irritation during extended wear periods. Hydrogel-based interfaces show promise but often compromise sensor performance through analyte dilution effects.
Power management represents another critical limitation, with most advanced sensing systems requiring active electronics that necessitate batteries or energy harvesting components. Recent developments in low-power circuit design and flexible batteries have improved wearability but still restrict continuous monitoring duration.
Data processing and transmission capabilities vary widely among current technologies. Simple colorimetric patches offer binary or semi-quantitative results without digital connectivity, while advanced systems incorporate Bluetooth or NFC communication for real-time monitoring. The latter face challenges with secure data transmission and integration with electronic health record systems.
Manufacturing scalability remains problematic, particularly for complex multi-layer patches incorporating both sensing elements and electronics. Current fabrication approaches often involve manual assembly steps that limit mass production capabilities and increase unit costs, presenting barriers to widespread clinical adoption.
Electrochemical biosensing patches dominate the current market, utilizing amperometric, potentiometric, or impedimetric detection methods. These patches typically incorporate enzyme-based sensors that generate electrical signals proportional to drug concentrations in interstitial fluid or sweat. Notable examples include microneedle array patches for minimally invasive sampling and solid-contact ion-selective electrodes for electrolyte monitoring. While offering high sensitivity and selectivity, these systems face challenges with enzyme stability in ambient conditions and signal drift during prolonged wear.
Optical biosensing technologies have emerged as promising alternatives, employing fluorescence, colorimetric, or surface plasmon resonance techniques. These patches often utilize chromophores or fluorophores that interact with target drug molecules to produce measurable optical signals. Recent innovations include smartphone-compatible colorimetric patches that enable visual drug concentration assessment without specialized equipment. However, optical approaches struggle with skin pigmentation interference and limited penetration depth.
Mechanical sensing modalities, including piezoelectric and acoustic wave sensors, detect mass changes or mechanical property alterations upon drug binding. These technologies offer label-free detection capabilities but typically demonstrate lower sensitivity compared to electrochemical alternatives.
A significant challenge across all biosensing patch technologies is biocompatibility and skin adhesion. Current adhesive technologies must balance secure attachment with minimal skin irritation during extended wear periods. Hydrogel-based interfaces show promise but often compromise sensor performance through analyte dilution effects.
Power management represents another critical limitation, with most advanced sensing systems requiring active electronics that necessitate batteries or energy harvesting components. Recent developments in low-power circuit design and flexible batteries have improved wearability but still restrict continuous monitoring duration.
Data processing and transmission capabilities vary widely among current technologies. Simple colorimetric patches offer binary or semi-quantitative results without digital connectivity, while advanced systems incorporate Bluetooth or NFC communication for real-time monitoring. The latter face challenges with secure data transmission and integration with electronic health record systems.
Manufacturing scalability remains problematic, particularly for complex multi-layer patches incorporating both sensing elements and electronics. Current fabrication approaches often involve manual assembly steps that limit mass production capabilities and increase unit costs, presenting barriers to widespread clinical adoption.
Current Technical Solutions for Drug Monitoring Patches
01 Flexible and stretchable biosensing patches
Wearable biosensing patches designed with flexible and stretchable materials that conform to the body's contours for continuous monitoring. These patches incorporate elastic substrates and conductive materials that maintain functionality during movement and stretching, ensuring reliable data collection while providing comfort for long-term wear. The flexible design allows for application on various body locations while maintaining sensor accuracy and skin contact.- Flexible and adhesive biosensing patches: Wearable biosensing patches designed with flexible and adhesive materials that conform to the body's contours for continuous monitoring. These patches incorporate stretchable electronics and skin-friendly adhesives to ensure comfort during prolonged wear while maintaining reliable sensor contact with the skin. The flexible design allows for natural movement without compromising data collection accuracy or causing skin irritation.
- Sweat analysis and biomarker detection: Biosensing patches specifically designed to collect and analyze sweat for various biomarkers including electrolytes, metabolites, and hormones. These patches incorporate microfluidic channels to direct sweat to sensing elements that can detect multiple analytes simultaneously. The technology enables non-invasive monitoring of physiological parameters through perspiration, providing insights into hydration status, stress levels, and potential health conditions without the need for blood sampling.
- Continuous vital signs monitoring systems: Wearable patches that continuously monitor vital signs such as heart rate, respiratory rate, body temperature, and blood oxygen levels. These integrated systems use multiple sensing modalities including optical, electrical, and temperature sensors to provide comprehensive health data. The patches transmit information wirelessly to connected devices for real-time analysis and can alert users to abnormal readings that may require medical attention.
- Data processing and wireless communication capabilities: Advanced biosensing patches featuring integrated data processing units and wireless communication modules that enable real-time data transmission to smartphones, cloud platforms, or healthcare systems. These patches incorporate low-power microprocessors for on-device analytics and utilize protocols such as Bluetooth Low Energy, NFC, or cellular connectivity for efficient data transfer. The systems include encryption features to protect sensitive health information while allowing for remote monitoring and telemedicine applications.
- Energy harvesting and power management solutions: Innovative power solutions for wearable biosensing patches that extend operational lifetime through energy harvesting technologies and efficient power management. These patches utilize various energy sources including body heat, motion, or ambient light to supplement or replace traditional batteries. Advanced power management circuits optimize energy consumption by controlling sensor duty cycles and communication intervals, enabling extended continuous monitoring without frequent charging or replacement.
02 Sweat analysis biosensing patches
Biosensing patches specifically designed to collect and analyze sweat for health monitoring. These patches incorporate microfluidic channels and biochemical sensors that detect various biomarkers in sweat, including electrolytes, metabolites, and hormones. The technology enables non-invasive continuous monitoring of physiological parameters through sweat analysis, providing insights into hydration status, stress levels, and metabolic health without the need for blood sampling.Expand Specific Solutions03 Wireless data transmission in biosensing patches
Integration of wireless communication technologies in biosensing patches for real-time data transmission to smartphones or other devices. These patches incorporate low-power wireless protocols such as Bluetooth Low Energy, NFC, or custom RF solutions to transmit collected biometric data to monitoring systems. The wireless capability enables continuous remote monitoring while maintaining user mobility and comfort, with optimized power management systems to extend battery life for long-term use.Expand Specific Solutions04 Multi-parameter biosensing patches
Advanced biosensing patches capable of simultaneously monitoring multiple physiological parameters. These integrated systems combine various sensor types (electrochemical, optical, temperature, etc.) in a single patch platform to provide comprehensive health monitoring. The multi-parameter approach enables correlation between different biomarkers for more accurate health assessment, with sophisticated algorithms that process multiple data streams to provide holistic health insights.Expand Specific Solutions05 Energy harvesting for self-powered biosensing patches
Self-powered biosensing patch technologies that harvest energy from the body or environment. These patches incorporate energy harvesting mechanisms such as piezoelectric elements that convert body movement to electricity, thermoelectric generators utilizing body-ambient temperature differences, or photovoltaic cells for light energy conversion. The self-powering capability eliminates or reduces the need for batteries, enabling longer operational lifetimes and addressing sustainability concerns for continuous health monitoring applications.Expand Specific Solutions
Key Industry Players in Biosensing Patch Development
The wearable biosensing patch market for drug monitoring is in its growth phase, characterized by increasing adoption and technological advancements. The market is expanding rapidly, driven by rising chronic disease prevalence and demand for personalized healthcare solutions. Key players represent diverse sectors: academic institutions (University of California, North Carolina State University, USC), healthcare giants (Philips, Abbott Diabetes Care, Novo Nordisk, Eli Lilly), and specialized biosensor companies (LifeSignals, VivaLNK, One Health Biosensing). Technology maturity varies across applications, with glucose monitoring being most advanced while therapeutic drug monitoring remains emerging. Samsung, Intel, and ITRI contribute significant R&D capabilities, accelerating innovation in miniaturization, power efficiency, and wireless connectivity, positioning this technology for mainstream adoption in personalized medicine and remote patient monitoring.
The Regents of the University of California
Technical Solution: The University of California has developed advanced wearable biosensing patches that utilize microneedle technology for minimally invasive drug monitoring. Their platform incorporates electrochemical sensors with microneedle arrays that can penetrate the stratum corneum to access interstitial fluid without causing pain or bleeding. The system employs specialized biorecognition elements (enzymes, antibodies) immobilized on electrode surfaces to detect specific drug compounds and their metabolites in real-time[1]. Their patches feature wireless data transmission capabilities and flexible substrate materials that conform to skin contours, enhancing comfort during extended wear. UC researchers have demonstrated successful monitoring of various therapeutic drugs including antibiotics, chemotherapy agents, and anticonvulsants with clinically relevant sensitivity and specificity[2]. The patches incorporate on-board signal processing to filter noise and enhance detection limits, allowing for continuous monitoring over several days with minimal drift in sensor performance.
Strengths: Superior microneedle technology that achieves minimal invasiveness while maintaining high sampling efficiency; excellent biocompatibility with extended wear capability; demonstrated clinical validation across multiple drug classes. Weaknesses: Higher manufacturing complexity due to integration of multiple sensing modalities; potential challenges with scalability for mass production; requires periodic calibration for long-term monitoring applications.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed an innovative platform for wearable drug monitoring through their BioProcessor technology. Their biosensing patches integrate multiple sensor types (electrochemical, optical, and impedance-based) on a single flexible substrate with advanced system-on-chip architecture. The patches utilize a combination of microneedle arrays and iontophoresis to enhance drug extraction from interstitial fluid without causing discomfort[5]. Samsung's technology incorporates proprietary low-power electronics that enable continuous monitoring for up to 7 days on a single charge. Their patches feature Bluetooth Low Energy connectivity that seamlessly integrates with Samsung's health ecosystem, allowing for real-time data visualization and alert systems when drug levels fall outside therapeutic ranges. The company has implemented machine learning algorithms that improve measurement accuracy by accounting for individual physiological variables and environmental factors[6]. Samsung has demonstrated successful monitoring of cardiovascular medications, antipsychotics, and pain management drugs with their platform, achieving detection limits comparable to laboratory-based methods.
Strengths: Exceptional power management and miniaturization capabilities; seamless integration with existing mobile health platforms; advanced data analytics for personalized monitoring; robust manufacturing capabilities for mass production. Weaknesses: Higher cost compared to simpler monitoring systems; relatively new entrant to pharmaceutical monitoring space with less clinical validation history; potential regulatory challenges in different markets.
Core Biosensing Technologies and Patents Analysis
Wearable sensor patch
PatentWO2025117631A1
Innovation
- A wearable sensor patch incorporating hydrogel microneedles and a flexible sensor element with elastomeric electrodes, allowing for minimally invasive ISF collection and simultaneous biomarker measurement in-situ. The patch is designed to accommodate hydrogel swelling and skin deformation, ensuring consistent signal stability.
Wearable biosensor
PatentPendingUS20250228473A1
Innovation
- A modular, wearable biosensor patch with a multilayer structure comprising an adhesive layer, laser-induced graphene electrodes, and a PDMS microfluidic layer, featuring capillary burst valves and hydrogel valves to ensure sequential sweat collection and storage in discrete reservoirs, along with real-time monitoring capabilities.
Regulatory Framework for Medical Wearable Devices
The regulatory landscape for wearable biosensing patches in drug monitoring applications is complex and multifaceted, requiring careful navigation by manufacturers and healthcare providers. In the United States, the Food and Drug Administration (FDA) classifies these devices primarily under Class II medical devices, necessitating 510(k) clearance before market entry. However, devices with novel drug delivery mechanisms or those monitoring critical medications may face more stringent Class III requirements, including premarket approval (PMA) processes.
The European Union's regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR) in 2021, replacing the previous Medical Device Directive. Under MDR, wearable biosensing patches typically fall into Class IIa or IIb, depending on their specific functionality and risk profile. The regulation emphasizes post-market surveillance and requires manufacturers to implement robust quality management systems.
In Asia, regulatory approaches vary considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains stringent requirements similar to FDA standards, while China's National Medical Products Administration (NMPA) has recently updated its regulatory framework to accelerate approval for innovative medical technologies, including wearable biosensors.
Data privacy and security regulations represent another critical regulatory dimension. In Europe, the General Data Protection Regulation (GDPR) imposes strict requirements on the collection, processing, and storage of personal health data generated by wearable devices. Similarly, the Health Insurance Portability and Accountability Act (HIPAA) in the US establishes standards for protecting sensitive patient data, particularly relevant for biosensing patches that transmit medication monitoring data to healthcare providers.
Interoperability standards are increasingly important in the regulatory landscape. The International Medical Device Regulators Forum (IMDRF) has developed guidance documents addressing software as a medical device (SaMD), which often applies to the software components of wearable biosensing systems. Additionally, standards organizations like IEEE and ISO have established technical specifications for wearable medical devices, including requirements for biocompatibility, electrical safety, and wireless communication protocols.
Reimbursement pathways represent a significant regulatory challenge for wearable biosensing patches. In many jurisdictions, obtaining regulatory approval does not guarantee coverage by healthcare payers. Manufacturers must navigate complex health technology assessment processes to demonstrate not only safety and efficacy but also cost-effectiveness and clinical utility in drug monitoring applications.
Looking forward, regulatory frameworks are evolving to address the unique challenges posed by AI-enabled wearable biosensors. Both the FDA and European regulatory bodies are developing new guidelines for AI/ML-based medical devices, particularly those that employ continuous learning algorithms to improve drug monitoring accuracy over time.
The European Union's regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR) in 2021, replacing the previous Medical Device Directive. Under MDR, wearable biosensing patches typically fall into Class IIa or IIb, depending on their specific functionality and risk profile. The regulation emphasizes post-market surveillance and requires manufacturers to implement robust quality management systems.
In Asia, regulatory approaches vary considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains stringent requirements similar to FDA standards, while China's National Medical Products Administration (NMPA) has recently updated its regulatory framework to accelerate approval for innovative medical technologies, including wearable biosensors.
Data privacy and security regulations represent another critical regulatory dimension. In Europe, the General Data Protection Regulation (GDPR) imposes strict requirements on the collection, processing, and storage of personal health data generated by wearable devices. Similarly, the Health Insurance Portability and Accountability Act (HIPAA) in the US establishes standards for protecting sensitive patient data, particularly relevant for biosensing patches that transmit medication monitoring data to healthcare providers.
Interoperability standards are increasingly important in the regulatory landscape. The International Medical Device Regulators Forum (IMDRF) has developed guidance documents addressing software as a medical device (SaMD), which often applies to the software components of wearable biosensing systems. Additionally, standards organizations like IEEE and ISO have established technical specifications for wearable medical devices, including requirements for biocompatibility, electrical safety, and wireless communication protocols.
Reimbursement pathways represent a significant regulatory challenge for wearable biosensing patches. In many jurisdictions, obtaining regulatory approval does not guarantee coverage by healthcare payers. Manufacturers must navigate complex health technology assessment processes to demonstrate not only safety and efficacy but also cost-effectiveness and clinical utility in drug monitoring applications.
Looking forward, regulatory frameworks are evolving to address the unique challenges posed by AI-enabled wearable biosensors. Both the FDA and European regulatory bodies are developing new guidelines for AI/ML-based medical devices, particularly those that employ continuous learning algorithms to improve drug monitoring accuracy over time.
Biocompatibility and User Acceptance Factors
Biocompatibility represents a critical factor in the development and adoption of wearable biosensing patches for drug monitoring applications. These devices must maintain direct contact with the skin for extended periods without causing irritation, allergic reactions, or tissue damage. Current research indicates that approximately 15-20% of users experience some form of skin reaction when using adhesive-based wearable sensors, highlighting the importance of material selection in patch design.
Materials commonly employed in biocompatible patches include medical-grade silicones, polyurethanes, and hydrogels, each offering distinct advantages in terms of flexibility, breathability, and hypoallergenic properties. Recent advancements have focused on incorporating naturally derived materials such as cellulose derivatives and chitosan to further enhance biocompatibility while maintaining sensor functionality.
User acceptance factors extend beyond physical compatibility to encompass psychological and social dimensions of wearable technology adoption. Studies reveal that patch visibility, comfort during daily activities, and ease of application significantly influence user compliance. Notably, patches requiring replacement less frequently (every 7-14 days versus daily) demonstrate higher adherence rates, with approximately 78% of users preferring longer-wearing options despite potential trade-offs in accuracy.
Form factor considerations play a substantial role in user acceptance, with research indicating preferences for thin, flexible designs that conform naturally to body contours. The average thickness tolerance for continuous wear applications appears to be approximately 3mm, beyond which user discomfort increases exponentially. Additionally, patches with gradually tapering edges demonstrate superior comfort ratings compared to those with abrupt boundaries.
Adhesive performance presents a particular challenge in balancing secure attachment with comfortable removal. Novel adhesive systems incorporating moisture-responsive components have shown promise in maintaining attachment during exercise while reducing epidermal damage during removal. User studies indicate that perceived pain during removal represents a significant barrier to repeated use, with approximately 35% of first-time users citing removal discomfort as a reason for discontinuation.
Cultural and demographic factors further influence acceptance patterns, with variations observed across age groups, genders, and geographic regions. Younger demographics (18-35) generally demonstrate higher tolerance for visible patches, while older populations prioritize discretion. These insights underscore the importance of user-centered design approaches that consider both physiological compatibility and psychosocial acceptance factors in developing successful wearable drug monitoring technologies.
Materials commonly employed in biocompatible patches include medical-grade silicones, polyurethanes, and hydrogels, each offering distinct advantages in terms of flexibility, breathability, and hypoallergenic properties. Recent advancements have focused on incorporating naturally derived materials such as cellulose derivatives and chitosan to further enhance biocompatibility while maintaining sensor functionality.
User acceptance factors extend beyond physical compatibility to encompass psychological and social dimensions of wearable technology adoption. Studies reveal that patch visibility, comfort during daily activities, and ease of application significantly influence user compliance. Notably, patches requiring replacement less frequently (every 7-14 days versus daily) demonstrate higher adherence rates, with approximately 78% of users preferring longer-wearing options despite potential trade-offs in accuracy.
Form factor considerations play a substantial role in user acceptance, with research indicating preferences for thin, flexible designs that conform naturally to body contours. The average thickness tolerance for continuous wear applications appears to be approximately 3mm, beyond which user discomfort increases exponentially. Additionally, patches with gradually tapering edges demonstrate superior comfort ratings compared to those with abrupt boundaries.
Adhesive performance presents a particular challenge in balancing secure attachment with comfortable removal. Novel adhesive systems incorporating moisture-responsive components have shown promise in maintaining attachment during exercise while reducing epidermal damage during removal. User studies indicate that perceived pain during removal represents a significant barrier to repeated use, with approximately 35% of first-time users citing removal discomfort as a reason for discontinuation.
Cultural and demographic factors further influence acceptance patterns, with variations observed across age groups, genders, and geographic regions. Younger demographics (18-35) generally demonstrate higher tolerance for visible patches, while older populations prioritize discretion. These insights underscore the importance of user-centered design approaches that consider both physiological compatibility and psychosocial acceptance factors in developing successful wearable drug monitoring technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!