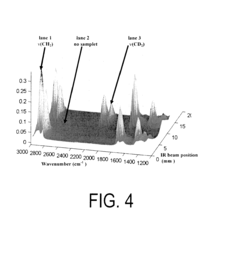
How ATR-FTIR Achieves Quantitative Results With ATR Correction And Proper Standards?
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ATR-FTIR Technology Background and Objectives
Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) has evolved significantly since its inception in the 1960s. This analytical technique combines the principles of internal reflection spectroscopy with the computational power of Fourier transform algorithms to provide rapid, non-destructive analysis of samples across various states of matter. The technology leverages the phenomenon of evanescent waves that penetrate beyond the reflecting surface when infrared radiation undergoes total internal reflection at the interface between the ATR crystal and the sample.
The historical development of ATR-FTIR has been marked by continuous improvements in hardware components, including more sensitive detectors, higher quality optical elements, and more powerful computational capabilities. These advancements have transformed ATR-FTIR from a qualitative analytical tool to one capable of delivering precise quantitative measurements, particularly when proper correction factors and calibration standards are applied.
Current technological trends in ATR-FTIR focus on enhancing quantitative accuracy through improved ATR correction algorithms that account for variations in penetration depth across different wavelengths. These corrections are essential because the evanescent wave penetrates the sample to different depths depending on the wavelength of light, the refractive indices of both the crystal and sample, and the angle of incidence.
The primary objective of modern ATR-FTIR technology is to achieve reliable quantitative results that are comparable to those obtained from transmission FTIR methods. This requires addressing several technical challenges, including the development of standardized ATR correction procedures that can be universally applied across different instrument configurations and sample types.
Another critical goal is the establishment of proper calibration standards that accurately represent the matrix effects and concentration ranges encountered in real-world samples. These standards must be stable, reproducible, and traceable to ensure the validity of quantitative measurements across different laboratories and over time.
The technology aims to expand its application scope beyond traditional laboratory settings into field-deployable systems for real-time monitoring and analysis. This expansion necessitates the development of more robust, miniaturized components and simplified user interfaces that maintain quantitative accuracy while being accessible to non-specialist users.
Furthermore, there is a growing emphasis on integrating ATR-FTIR with other analytical techniques and data processing methods, such as machine learning algorithms, to enhance the extraction of quantitative information from complex spectral data. This integration represents a significant frontier in the evolution of ATR-FTIR technology, potentially enabling more sophisticated analysis of multicomponent systems and trace-level quantification.
The historical development of ATR-FTIR has been marked by continuous improvements in hardware components, including more sensitive detectors, higher quality optical elements, and more powerful computational capabilities. These advancements have transformed ATR-FTIR from a qualitative analytical tool to one capable of delivering precise quantitative measurements, particularly when proper correction factors and calibration standards are applied.
Current technological trends in ATR-FTIR focus on enhancing quantitative accuracy through improved ATR correction algorithms that account for variations in penetration depth across different wavelengths. These corrections are essential because the evanescent wave penetrates the sample to different depths depending on the wavelength of light, the refractive indices of both the crystal and sample, and the angle of incidence.
The primary objective of modern ATR-FTIR technology is to achieve reliable quantitative results that are comparable to those obtained from transmission FTIR methods. This requires addressing several technical challenges, including the development of standardized ATR correction procedures that can be universally applied across different instrument configurations and sample types.
Another critical goal is the establishment of proper calibration standards that accurately represent the matrix effects and concentration ranges encountered in real-world samples. These standards must be stable, reproducible, and traceable to ensure the validity of quantitative measurements across different laboratories and over time.
The technology aims to expand its application scope beyond traditional laboratory settings into field-deployable systems for real-time monitoring and analysis. This expansion necessitates the development of more robust, miniaturized components and simplified user interfaces that maintain quantitative accuracy while being accessible to non-specialist users.
Furthermore, there is a growing emphasis on integrating ATR-FTIR with other analytical techniques and data processing methods, such as machine learning algorithms, to enhance the extraction of quantitative information from complex spectral data. This integration represents a significant frontier in the evolution of ATR-FTIR technology, potentially enabling more sophisticated analysis of multicomponent systems and trace-level quantification.
Market Applications and Demand Analysis for Quantitative ATR-FTIR
The global market for Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) spectroscopy continues to expand significantly, driven by increasing demand for accurate quantitative analysis across multiple industries. The pharmaceutical sector represents one of the largest market segments, with an estimated annual growth rate exceeding 7% as companies seek reliable methods for drug formulation analysis, raw material verification, and quality control processes that comply with stringent regulatory requirements.
In the chemical manufacturing industry, quantitative ATR-FTIR has become essential for process monitoring and quality assurance. Companies increasingly implement in-line and at-line ATR-FTIR systems to provide real-time quantitative feedback during production, reducing waste and optimizing manufacturing efficiency. This application segment has seen particularly strong adoption in polymer production, where precise quantitative measurements of chemical compositions directly impact product performance.
The food and beverage industry demonstrates growing reliance on quantitative ATR-FTIR for authentication testing and compositional analysis. With consumers demanding greater transparency regarding product ingredients and authenticity, manufacturers increasingly utilize ATR-FTIR's quantitative capabilities to verify product composition and detect potential adulterants or contaminants.
Environmental monitoring represents another rapidly expanding application area. Government agencies and environmental consultancies deploy portable ATR-FTIR systems with quantitative capabilities for field analysis of soil contaminants, water pollutants, and atmospheric particulates. The ability to obtain accurate concentration measurements in real-time provides significant advantages over traditional laboratory-based testing methods.
Academic and research institutions constitute a stable market segment, utilizing quantitative ATR-FTIR for diverse applications ranging from materials science to biological research. The demand for instruments capable of delivering reproducible quantitative results continues to grow as researchers seek to publish defensible data in competitive research environments.
Market analysis indicates a clear trend toward miniaturization and portability in quantitative ATR-FTIR instrumentation. Companies developing handheld devices with reliable quantitative capabilities are experiencing substantial market growth, particularly in applications requiring field deployment or point-of-use testing. This trend aligns with broader industry movements toward decentralized testing and real-time decision making.
The healthcare diagnostics sector represents an emerging application area with significant growth potential. Early research demonstrates promising results using quantitative ATR-FTIR for biological fluid analysis, potentially enabling rapid, non-invasive diagnostic procedures. While still developing, this application could substantially expand the market for quantitative ATR-FTIR technologies in coming years.
In the chemical manufacturing industry, quantitative ATR-FTIR has become essential for process monitoring and quality assurance. Companies increasingly implement in-line and at-line ATR-FTIR systems to provide real-time quantitative feedback during production, reducing waste and optimizing manufacturing efficiency. This application segment has seen particularly strong adoption in polymer production, where precise quantitative measurements of chemical compositions directly impact product performance.
The food and beverage industry demonstrates growing reliance on quantitative ATR-FTIR for authentication testing and compositional analysis. With consumers demanding greater transparency regarding product ingredients and authenticity, manufacturers increasingly utilize ATR-FTIR's quantitative capabilities to verify product composition and detect potential adulterants or contaminants.
Environmental monitoring represents another rapidly expanding application area. Government agencies and environmental consultancies deploy portable ATR-FTIR systems with quantitative capabilities for field analysis of soil contaminants, water pollutants, and atmospheric particulates. The ability to obtain accurate concentration measurements in real-time provides significant advantages over traditional laboratory-based testing methods.
Academic and research institutions constitute a stable market segment, utilizing quantitative ATR-FTIR for diverse applications ranging from materials science to biological research. The demand for instruments capable of delivering reproducible quantitative results continues to grow as researchers seek to publish defensible data in competitive research environments.
Market analysis indicates a clear trend toward miniaturization and portability in quantitative ATR-FTIR instrumentation. Companies developing handheld devices with reliable quantitative capabilities are experiencing substantial market growth, particularly in applications requiring field deployment or point-of-use testing. This trend aligns with broader industry movements toward decentralized testing and real-time decision making.
The healthcare diagnostics sector represents an emerging application area with significant growth potential. Early research demonstrates promising results using quantitative ATR-FTIR for biological fluid analysis, potentially enabling rapid, non-invasive diagnostic procedures. While still developing, this application could substantially expand the market for quantitative ATR-FTIR technologies in coming years.
Current Challenges in ATR-FTIR Quantitative Analysis
Despite the widespread adoption of ATR-FTIR spectroscopy for quantitative analysis, several significant challenges continue to impede its full potential. One of the primary obstacles is the depth of penetration variability, which depends on wavelength, refractive indices, and incident angle. This variability creates a non-linear relationship between concentration and absorbance, complicating the application of Beer-Lambert law without appropriate corrections.
Sample-to-crystal contact presents another formidable challenge. Inconsistent pressure application during measurements can lead to varying degrees of contact between the sample and ATR crystal, resulting in significant reproducibility issues. This is particularly problematic for solid samples where surface irregularities can create air gaps that distort spectral data.
The ATR correction algorithms themselves introduce complexities. Current correction methods often rely on simplified assumptions about sample properties, leading to systematic errors when analyzing complex matrices or heterogeneous samples. The correction factors may not adequately account for all physical interactions at the sample-crystal interface, especially for samples with varying densities or compositions.
Reference standards pose additional difficulties in quantitative ATR-FTIR analysis. Unlike transmission FTIR, where matrix-matched standards are well-established, ATR-FTIR requires standards that not only match the chemical composition but also the physical properties of the sample. The scarcity of certified reference materials specifically designed for ATR-FTIR quantification limits the accuracy and traceability of results.
Spectral preprocessing methods present their own challenges. Techniques such as baseline correction, normalization, and derivative transformations can significantly impact quantitative results. The lack of standardized preprocessing protocols leads to inconsistencies across laboratories and hinders method transferability.
Environmental factors further complicate ATR-FTIR quantitative analysis. Temperature fluctuations can affect both the sample properties and the performance of the ATR crystal, leading to spectral shifts and intensity variations. Humidity changes can alter sample hydration states, particularly for hygroscopic materials, affecting the reliability of quantitative measurements.
Instrument-to-instrument variability represents another significant challenge. Differences in optical configurations, detector sensitivities, and ATR crystal materials between instruments can lead to systematic differences in spectral data, complicating method transfer and standardization efforts across different laboratory settings.
Sample-to-crystal contact presents another formidable challenge. Inconsistent pressure application during measurements can lead to varying degrees of contact between the sample and ATR crystal, resulting in significant reproducibility issues. This is particularly problematic for solid samples where surface irregularities can create air gaps that distort spectral data.
The ATR correction algorithms themselves introduce complexities. Current correction methods often rely on simplified assumptions about sample properties, leading to systematic errors when analyzing complex matrices or heterogeneous samples. The correction factors may not adequately account for all physical interactions at the sample-crystal interface, especially for samples with varying densities or compositions.
Reference standards pose additional difficulties in quantitative ATR-FTIR analysis. Unlike transmission FTIR, where matrix-matched standards are well-established, ATR-FTIR requires standards that not only match the chemical composition but also the physical properties of the sample. The scarcity of certified reference materials specifically designed for ATR-FTIR quantification limits the accuracy and traceability of results.
Spectral preprocessing methods present their own challenges. Techniques such as baseline correction, normalization, and derivative transformations can significantly impact quantitative results. The lack of standardized preprocessing protocols leads to inconsistencies across laboratories and hinders method transferability.
Environmental factors further complicate ATR-FTIR quantitative analysis. Temperature fluctuations can affect both the sample properties and the performance of the ATR crystal, leading to spectral shifts and intensity variations. Humidity changes can alter sample hydration states, particularly for hygroscopic materials, affecting the reliability of quantitative measurements.
Instrument-to-instrument variability represents another significant challenge. Differences in optical configurations, detector sensitivities, and ATR crystal materials between instruments can lead to systematic differences in spectral data, complicating method transfer and standardization efforts across different laboratory settings.
Standard Calibration Methods for Quantitative ATR-FTIR
01 ATR-FTIR spectroscopy for quantitative analysis of chemical compositions
Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) spectroscopy is used for quantitative analysis of chemical compositions in various samples. This technique allows for direct measurement without extensive sample preparation, providing accurate quantification of components based on their characteristic infrared absorption patterns. The method involves calibration models that correlate spectral data with concentration values, enabling precise determination of compound quantities in complex mixtures.- ATR-FTIR techniques for quantitative analysis of chemical compounds: Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) spectroscopy is used for quantitative analysis of various chemical compounds. This technique allows for direct measurement of samples with minimal preparation, providing accurate quantitative results through the analysis of characteristic absorption bands. The method involves creating calibration curves based on reference standards to determine the concentration of target compounds in unknown samples.
- Pharmaceutical applications of ATR-FTIR quantitative analysis: ATR-FTIR spectroscopy is applied in pharmaceutical research and development for quantitative determination of active pharmaceutical ingredients and excipients. The technique enables rapid assessment of drug formulation composition, stability testing, and quality control. It allows for non-destructive analysis of solid dosage forms, detection of polymorphic changes, and quantification of drug content in various pharmaceutical preparations.
- Advanced data processing methods for ATR-FTIR quantitative results: Various data processing methods enhance the accuracy and reliability of ATR-FTIR quantitative results. These include multivariate analysis techniques such as partial least squares regression, principal component analysis, and artificial neural networks. Advanced algorithms for baseline correction, spectral normalization, and interference removal improve the precision of quantitative measurements. Machine learning approaches are increasingly being integrated to handle complex spectral data and extract meaningful quantitative information.
- Environmental and material science applications of ATR-FTIR quantification: ATR-FTIR quantitative analysis is employed in environmental monitoring and material science for determining pollutants, analyzing soil compositions, and characterizing materials. The technique enables quantification of contaminants in water samples, assessment of microplastics in environmental matrices, and determination of chemical components in complex material systems. It provides valuable data for environmental impact assessments and material quality control with high sensitivity and specificity.
- Instrumentation and methodology improvements for ATR-FTIR quantitative analysis: Technological advancements in ATR-FTIR instrumentation and methodology have significantly improved quantitative analysis capabilities. These include development of high-sensitivity detectors, enhanced sampling accessories, automated sample handling systems, and portable devices for field analysis. Innovations in ATR crystal materials, optical designs, and signal processing have led to better detection limits, increased reproducibility, and expanded application range for quantitative measurements across various industries.
02 Pharmaceutical applications of ATR-FTIR quantitative analysis
ATR-FTIR spectroscopy is applied in pharmaceutical research and development for quantitative analysis of drug formulations, active pharmaceutical ingredients, and excipients. The technique enables rapid assessment of drug purity, content uniformity, and stability without destructive testing. It allows for real-time monitoring of pharmaceutical processes and quality control, providing accurate quantification of multiple components simultaneously in complex pharmaceutical matrices.Expand Specific Solutions03 Advanced data processing methods for ATR-FTIR quantitative results
Sophisticated data processing algorithms and chemometric methods enhance the accuracy and reliability of ATR-FTIR quantitative results. These include multivariate analysis techniques such as partial least squares regression, principal component analysis, and artificial neural networks. Such methods improve the extraction of quantitative information from complex spectral data, reduce noise, correct baseline issues, and handle spectral overlaps, ultimately providing more precise concentration measurements from ATR-FTIR spectra.Expand Specific Solutions04 Industrial process monitoring using ATR-FTIR quantitative analysis
ATR-FTIR spectroscopy serves as a powerful tool for real-time quantitative monitoring of industrial processes. The technique enables continuous analysis of reaction progress, product quality, and impurity levels without sampling or preparation steps. This application is particularly valuable in manufacturing environments where immediate feedback on chemical composition is crucial for process control and optimization, allowing for timely adjustments to maintain product specifications.Expand Specific Solutions05 Portable and miniaturized ATR-FTIR systems for field quantitative analysis
Advancements in ATR-FTIR technology have led to the development of portable and miniaturized systems that enable quantitative analysis in field conditions. These compact instruments maintain analytical performance while offering mobility for on-site testing. They incorporate specialized sampling interfaces and robust calibration methods to ensure reliable quantitative results outside laboratory environments, making them valuable tools for environmental monitoring, forensic investigations, and point-of-care diagnostics.Expand Specific Solutions
Leading Manufacturers and Research Institutions in ATR-FTIR
The ATR-FTIR quantitative analysis market is currently in a growth phase, with increasing adoption across pharmaceutical, chemical, and environmental sectors. The global spectroscopy market, which includes ATR-FTIR technology, is estimated at approximately $15 billion with a CAGR of 6-8%. Technologically, ATR-FTIR quantification has reached moderate maturity, with established methodologies for ATR correction and standardization. Key players include instrumentation leaders like Shimadzu, Horiba, and Thermo Scientific, who offer advanced ATR accessories with integrated correction algorithms. Mettler-Toledo and Anton Paar have developed specialized ATR sampling interfaces, while academic institutions such as EPFL and University of California contribute significant research advancing calibration methodologies. The competitive landscape shows a balance between established analytical instrument manufacturers and specialized spectroscopy solution providers focusing on application-specific implementations.
Horiba Ltd.
Technical Solution: Horiba has developed advanced ATR-FTIR systems with proprietary algorithms for quantitative analysis. Their LabRAM series incorporates automatic ATR correction factors that account for wavelength-dependent penetration depth variations. The system calculates correction coefficients based on refractive indices of both the ATR crystal and sample material, applying these dynamically during measurement. Horiba's approach includes a comprehensive calibration protocol using certified reference materials with known concentrations to establish accurate calibration curves. Their software automatically applies matrix-specific correction factors and compensates for potential interference from water vapor and CO2. The system also features patented signal enhancement technology that improves signal-to-noise ratio, enabling detection of trace components down to parts-per-billion levels in complex mixtures.
Strengths: Superior algorithm-based ATR correction with automatic adjustment for different sample matrices; excellent detection limits with high signal-to-noise ratio; comprehensive reference libraries for various applications. Weaknesses: Higher cost compared to basic systems; requires more extensive user training; some proprietary methods limit customization for specialized research applications.
Shimadzu Corp.
Technical Solution: Shimadzu has pioneered quantitative ATR-FTIR technology through their IRTracer-100 and IRAffinity-1S systems with specialized LabSolutions IR software. Their approach implements a multi-layer mathematical model for ATR correction that accounts for both the depth of penetration variations across wavelengths and the optical properties of different crystal materials (diamond, germanium, zinc selenide). The system applies Kramers-Kronig transformation algorithms to correct for anomalous dispersion effects near absorption bands. Shimadzu's quantitative methodology incorporates multivariate calibration techniques including Partial Least Squares (PLS) regression that can handle complex mixture analysis with overlapping spectral features. Their standard development protocol includes the use of matrix-matched reference materials with certified concentrations spanning the expected analytical range, with automatic correction for non-linear responses at higher concentrations. The system also features automatic validation routines that verify calibration stability and provide statistical confidence metrics for quantitative results.
Strengths: Comprehensive ATR correction algorithms that handle multiple crystal types; powerful chemometric tools for complex mixture analysis; excellent reproducibility with automated validation protocols. Weaknesses: Software has steeper learning curve for new users; some advanced features require additional modules at extra cost; calibration transfer between instruments requires additional validation steps.
Key Patents and Literature on ATR Correction Algorithms
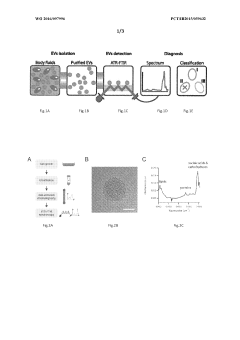
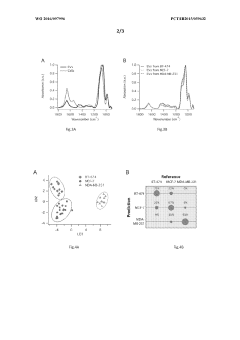
Use of fourier transform infrared spectroscopy analysis of extracellular vesicles isolated from body fluids for diagnosing, prognosing and monitoring pathophysiological states and method therfor
PatentWO2016097996A1
Innovation
- The use of Fourier Transform Infrared Spectroscopy (FTIR) to analyze extracellular vesicles (EVs) isolated from body fluids, combined with multivariate analysis, provides a non-invasive and label-free method for classifying EVs based on their molecular composition, enabling early diagnosis, prognosis, and monitoring of cancer and other proliferative diseases.
Device for multiple ATR analysis
PatentInactiveEP2116839A1
Innovation
- A device with an ATR element featuring a sample compartment with multiple chambers, allowing for the analysis of multiple samples, and an organic compound for grafting the ATR element to stabilize it against hydrolysis, enabling simultaneous analysis of multiple samples and improving surface stability.
Validation Protocols for ATR-FTIR Quantitative Results
Validation protocols for ATR-FTIR quantitative analysis require systematic approaches to ensure reliability and reproducibility of results. These protocols typically begin with instrument qualification, including performance verification tests for wavelength accuracy, photometric precision, and signal-to-noise ratio. Regular calibration using certified reference materials is essential to maintain measurement accuracy across the operational spectrum range.
Method validation must follow established guidelines such as those from regulatory bodies like ICH, FDA, or ISO standards. This includes determining linearity across concentration ranges relevant to the application, with correlation coefficients (R²) exceeding 0.995 considered acceptable for most quantitative work. Precision testing should evaluate both repeatability (intra-day) and intermediate precision (inter-day) with relative standard deviation targets typically below 2%.
Accuracy assessment requires comparison with reference methods or certified reference materials, with recovery rates between 98-102% generally considered acceptable for pharmaceutical applications. Limit of detection (LOD) and limit of quantification (LOQ) determinations are crucial for trace analysis, typically calculated using signal-to-noise ratios or statistical approaches from calibration curves.
Robustness testing evaluates the method's stability under varying conditions, including different analysts, slight changes in sample preparation, or environmental factors. For ATR-FTIR specifically, validation must address the critical aspect of ATR correction factors, which compensate for the wavelength-dependent penetration depth of the evanescent wave.
Sample-specific validation protocols should include evaluation of matrix effects, as complex matrices can significantly impact spectral quality and quantitative accuracy. Reference standards must be characterized for purity, homogeneity, and stability, with traceability to recognized standards when possible.
Ongoing method verification through quality control samples and proficiency testing ensures continued validity of the quantitative method over time. Statistical process control charts can monitor system suitability and detect performance drift before it impacts analytical results.
Documentation requirements include detailed standard operating procedures (SOPs), comprehensive validation reports with raw data, statistical analyses, and clear acceptance criteria. Electronic data integrity measures must comply with regulatory expectations, including audit trails and appropriate data security protocols to prevent unauthorized modifications to results.
Method validation must follow established guidelines such as those from regulatory bodies like ICH, FDA, or ISO standards. This includes determining linearity across concentration ranges relevant to the application, with correlation coefficients (R²) exceeding 0.995 considered acceptable for most quantitative work. Precision testing should evaluate both repeatability (intra-day) and intermediate precision (inter-day) with relative standard deviation targets typically below 2%.
Accuracy assessment requires comparison with reference methods or certified reference materials, with recovery rates between 98-102% generally considered acceptable for pharmaceutical applications. Limit of detection (LOD) and limit of quantification (LOQ) determinations are crucial for trace analysis, typically calculated using signal-to-noise ratios or statistical approaches from calibration curves.
Robustness testing evaluates the method's stability under varying conditions, including different analysts, slight changes in sample preparation, or environmental factors. For ATR-FTIR specifically, validation must address the critical aspect of ATR correction factors, which compensate for the wavelength-dependent penetration depth of the evanescent wave.
Sample-specific validation protocols should include evaluation of matrix effects, as complex matrices can significantly impact spectral quality and quantitative accuracy. Reference standards must be characterized for purity, homogeneity, and stability, with traceability to recognized standards when possible.
Ongoing method verification through quality control samples and proficiency testing ensures continued validity of the quantitative method over time. Statistical process control charts can monitor system suitability and detect performance drift before it impacts analytical results.
Documentation requirements include detailed standard operating procedures (SOPs), comprehensive validation reports with raw data, statistical analyses, and clear acceptance criteria. Electronic data integrity measures must comply with regulatory expectations, including audit trails and appropriate data security protocols to prevent unauthorized modifications to results.
Sample Preparation Techniques for Optimal ATR-FTIR Performance
Sample preparation is a critical determinant of ATR-FTIR spectroscopic analysis quality, directly impacting the accuracy and reliability of quantitative results. Proper sample preparation ensures optimal contact between the sample and the ATR crystal, which is essential for consistent and reproducible spectral data acquisition.
For solid samples, particle size reduction through grinding or milling to dimensions smaller than the penetration depth of the IR beam (typically 0.5-5 μm) significantly enhances spectral quality. This process increases the surface area in contact with the ATR crystal and minimizes void spaces that can lead to signal attenuation. Homogenization techniques such as mortar and pestle grinding or ball milling are recommended for achieving uniform particle distribution.
Liquid samples require careful consideration of viscosity and surface tension properties. For low-viscosity liquids, specialized liquid cells with appropriate path lengths can be employed to ensure consistent sample thickness. High-viscosity samples may benefit from temperature control to maintain consistent flow characteristics during measurement, as viscosity variations can affect the depth of penetration and subsequently the quantitative accuracy.
Powder samples present unique challenges due to potential air gaps between particles and the ATR crystal. Application of consistent pressure using pressure towers or clamps helps establish uniform contact across the sampling area. The pressure applied must be standardized across measurements to ensure comparable penetration depths, as this directly affects the quantitative relationship between spectral intensity and analyte concentration.
For heterogeneous samples, representative sampling protocols must be implemented to capture the true composition. Multiple measurements at different sample locations, followed by spectral averaging, can provide more accurate representations of the overall sample composition. This approach minimizes the impact of local compositional variations on quantitative determinations.
Sample hydration levels must be carefully controlled, particularly for hygroscopic materials. Moisture content can significantly alter spectral features and interfere with quantitative analysis of target compounds. Desiccation chambers or controlled humidity environments may be necessary for sample storage and preparation to maintain consistent moisture levels across measurements.
Temperature stabilization during sample preparation and measurement is essential for reproducible results, as thermal fluctuations can affect molecular vibrations and subsequently alter spectral characteristics. Samples should be equilibrated to the measurement temperature before analysis to prevent drift during data collection, which could compromise quantitative accuracy.
For solid samples, particle size reduction through grinding or milling to dimensions smaller than the penetration depth of the IR beam (typically 0.5-5 μm) significantly enhances spectral quality. This process increases the surface area in contact with the ATR crystal and minimizes void spaces that can lead to signal attenuation. Homogenization techniques such as mortar and pestle grinding or ball milling are recommended for achieving uniform particle distribution.
Liquid samples require careful consideration of viscosity and surface tension properties. For low-viscosity liquids, specialized liquid cells with appropriate path lengths can be employed to ensure consistent sample thickness. High-viscosity samples may benefit from temperature control to maintain consistent flow characteristics during measurement, as viscosity variations can affect the depth of penetration and subsequently the quantitative accuracy.
Powder samples present unique challenges due to potential air gaps between particles and the ATR crystal. Application of consistent pressure using pressure towers or clamps helps establish uniform contact across the sampling area. The pressure applied must be standardized across measurements to ensure comparable penetration depths, as this directly affects the quantitative relationship between spectral intensity and analyte concentration.
For heterogeneous samples, representative sampling protocols must be implemented to capture the true composition. Multiple measurements at different sample locations, followed by spectral averaging, can provide more accurate representations of the overall sample composition. This approach minimizes the impact of local compositional variations on quantitative determinations.
Sample hydration levels must be carefully controlled, particularly for hygroscopic materials. Moisture content can significantly alter spectral features and interfere with quantitative analysis of target compounds. Desiccation chambers or controlled humidity environments may be necessary for sample storage and preparation to maintain consistent moisture levels across measurements.
Temperature stabilization during sample preparation and measurement is essential for reproducible results, as thermal fluctuations can affect molecular vibrations and subsequently alter spectral characteristics. Samples should be equilibrated to the measurement temperature before analysis to prevent drift during data collection, which could compromise quantitative accuracy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!