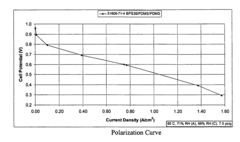
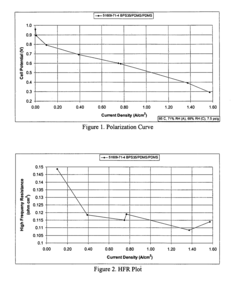
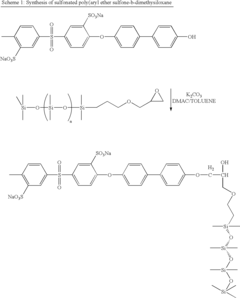
How Isopentane Affects Ionic Conductivity in Polymers
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane-Polymer Conductivity Background
The study of ionic conductivity in polymers has been a subject of significant interest in materials science and electrochemistry for decades. This field has gained renewed attention with the advent of advanced energy storage technologies, particularly in the development of solid-state batteries and fuel cells. The incorporation of isopentane, a branched alkane with the molecular formula C5H12, into polymer systems represents a novel approach to modifying and potentially enhancing ionic conductivity.
Historically, polymer electrolytes have been investigated as alternatives to liquid electrolytes due to their mechanical stability, flexibility, and potential for improved safety in energy storage devices. The primary challenge in this field has been achieving high ionic conductivity at room temperature while maintaining the desirable mechanical properties of polymers. Traditional polymer electrolytes, such as polyethylene oxide (PEO), have shown promise but often require elevated temperatures to achieve practical conductivity levels.
The introduction of isopentane into polymer systems marks a new direction in this research. Isopentane, with its branched structure and low boiling point, has the potential to act as a plasticizer, modifying the polymer's free volume and chain mobility. These changes in polymer structure and dynamics can significantly impact ionic transport mechanisms within the material.
The evolution of this technology can be traced back to early studies on polymer electrolytes in the 1970s, which primarily focused on PEO-based systems. Subsequent research explored various polymer matrices, including polyvinylidene fluoride (PVDF), polyacrylonitrile (PAN), and their copolymers. The incorporation of small organic molecules to enhance conductivity gained traction in the 1990s and 2000s, with a focus on plasticizers and ionic liquids.
The specific investigation into isopentane's role in polymer ionic conductivity represents a convergence of several research trends. These include the exploration of volatile organic compounds as conductivity enhancers, the study of polymer-solvent interactions in electrolyte systems, and the broader push towards high-performance solid-state electrolytes for next-generation energy storage devices.
Current research aims to elucidate the fundamental mechanisms by which isopentane affects ionic conductivity in polymers. This involves understanding the interplay between polymer chain dynamics, free volume changes, and ion transport pathways. Additionally, researchers are investigating the long-term stability of isopentane-containing polymer systems, as the volatile nature of isopentane presents both opportunities and challenges for practical applications.
Historically, polymer electrolytes have been investigated as alternatives to liquid electrolytes due to their mechanical stability, flexibility, and potential for improved safety in energy storage devices. The primary challenge in this field has been achieving high ionic conductivity at room temperature while maintaining the desirable mechanical properties of polymers. Traditional polymer electrolytes, such as polyethylene oxide (PEO), have shown promise but often require elevated temperatures to achieve practical conductivity levels.
The introduction of isopentane into polymer systems marks a new direction in this research. Isopentane, with its branched structure and low boiling point, has the potential to act as a plasticizer, modifying the polymer's free volume and chain mobility. These changes in polymer structure and dynamics can significantly impact ionic transport mechanisms within the material.
The evolution of this technology can be traced back to early studies on polymer electrolytes in the 1970s, which primarily focused on PEO-based systems. Subsequent research explored various polymer matrices, including polyvinylidene fluoride (PVDF), polyacrylonitrile (PAN), and their copolymers. The incorporation of small organic molecules to enhance conductivity gained traction in the 1990s and 2000s, with a focus on plasticizers and ionic liquids.
The specific investigation into isopentane's role in polymer ionic conductivity represents a convergence of several research trends. These include the exploration of volatile organic compounds as conductivity enhancers, the study of polymer-solvent interactions in electrolyte systems, and the broader push towards high-performance solid-state electrolytes for next-generation energy storage devices.
Current research aims to elucidate the fundamental mechanisms by which isopentane affects ionic conductivity in polymers. This involves understanding the interplay between polymer chain dynamics, free volume changes, and ion transport pathways. Additionally, researchers are investigating the long-term stability of isopentane-containing polymer systems, as the volatile nature of isopentane presents both opportunities and challenges for practical applications.
Market Analysis for Conductive Polymers
The conductive polymers market has been experiencing significant growth in recent years, driven by the increasing demand for lightweight, flexible, and cost-effective electronic components across various industries. The global market for conductive polymers is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that outpaces many other segments in the materials industry.
The automotive sector has emerged as a key driver for conductive polymers, particularly in the development of electric vehicles (EVs) and hybrid electric vehicles (HEVs). These materials are crucial for manufacturing lightweight components, reducing overall vehicle weight, and improving energy efficiency. The growing adoption of EVs worldwide is expected to fuel the demand for conductive polymers in battery systems, sensors, and other electronic components.
In the electronics industry, conductive polymers are gaining traction in the production of flexible displays, touchscreens, and wearable devices. The trend towards miniaturization and increased functionality in consumer electronics is creating new opportunities for conductive polymer applications. Additionally, the rise of the Internet of Things (IoT) is driving demand for smart sensors and devices, further expanding the market potential for these materials.
The healthcare sector is another promising area for conductive polymers, with applications in biosensors, drug delivery systems, and medical devices. The ability of these materials to interface with biological systems while maintaining biocompatibility makes them attractive for various medical applications. As healthcare technology continues to advance, the demand for conductive polymers in this sector is expected to grow significantly.
Geographically, Asia-Pacific is the largest and fastest-growing market for conductive polymers, driven by the region's robust electronics manufacturing industry and increasing automotive production. North America and Europe follow closely, with strong demand from aerospace, defense, and healthcare sectors. Emerging economies in Latin America and Africa are also showing potential for market growth as their industrial sectors develop.
Despite the positive outlook, challenges remain in the conductive polymers market. These include the need for improved stability and durability of the materials, as well as concerns about environmental impact and recyclability. Ongoing research and development efforts are focused on addressing these issues, with particular emphasis on enhancing the ionic conductivity and overall performance of conductive polymers.
The automotive sector has emerged as a key driver for conductive polymers, particularly in the development of electric vehicles (EVs) and hybrid electric vehicles (HEVs). These materials are crucial for manufacturing lightweight components, reducing overall vehicle weight, and improving energy efficiency. The growing adoption of EVs worldwide is expected to fuel the demand for conductive polymers in battery systems, sensors, and other electronic components.
In the electronics industry, conductive polymers are gaining traction in the production of flexible displays, touchscreens, and wearable devices. The trend towards miniaturization and increased functionality in consumer electronics is creating new opportunities for conductive polymer applications. Additionally, the rise of the Internet of Things (IoT) is driving demand for smart sensors and devices, further expanding the market potential for these materials.
The healthcare sector is another promising area for conductive polymers, with applications in biosensors, drug delivery systems, and medical devices. The ability of these materials to interface with biological systems while maintaining biocompatibility makes them attractive for various medical applications. As healthcare technology continues to advance, the demand for conductive polymers in this sector is expected to grow significantly.
Geographically, Asia-Pacific is the largest and fastest-growing market for conductive polymers, driven by the region's robust electronics manufacturing industry and increasing automotive production. North America and Europe follow closely, with strong demand from aerospace, defense, and healthcare sectors. Emerging economies in Latin America and Africa are also showing potential for market growth as their industrial sectors develop.
Despite the positive outlook, challenges remain in the conductive polymers market. These include the need for improved stability and durability of the materials, as well as concerns about environmental impact and recyclability. Ongoing research and development efforts are focused on addressing these issues, with particular emphasis on enhancing the ionic conductivity and overall performance of conductive polymers.
Current Challenges in Ionic Conductivity
The field of ionic conductivity in polymers faces several significant challenges that hinder the development of high-performance materials for various applications. One of the primary obstacles is achieving a balance between high ionic conductivity and mechanical stability. As the ionic conductivity increases, the mechanical properties of the polymer often deteriorate, leading to reduced structural integrity and potential safety concerns in devices such as batteries.
Another critical challenge is the limited understanding of the complex interactions between the polymer matrix, ionic species, and additives like isopentane. The mechanisms by which isopentane affects ionic conductivity are not fully elucidated, making it difficult to optimize formulations and predict performance. This knowledge gap impedes the rational design of polymer electrolytes with enhanced conductivity.
Temperature dependence of ionic conductivity poses a significant hurdle in developing materials suitable for a wide range of operating conditions. Many polymer electrolytes exhibit a sharp decrease in conductivity at lower temperatures, limiting their practical applications in cold environments. Conversely, elevated temperatures can lead to polymer degradation and potential safety hazards.
The long-term stability of polymer electrolytes remains a concern, particularly in applications requiring extended operational lifetimes. Factors such as chemical degradation, ion depletion, and interfacial reactions can lead to a gradual decline in conductivity over time. This challenge is particularly acute in the presence of additives like isopentane, which may introduce additional complexity to the aging process.
Scalability and manufacturing consistency present significant obstacles in translating laboratory-scale successes to industrial production. Achieving uniform dispersion of additives, controlling polymer morphology, and maintaining consistent performance across large-scale batches are ongoing challenges that impact the commercialization of advanced polymer electrolytes.
The environmental impact and sustainability of polymer electrolytes are becoming increasingly important considerations. Developing eco-friendly materials that maintain high ionic conductivity while minimizing the use of potentially harmful additives is a complex challenge that requires innovative approaches to material design and synthesis.
Lastly, the integration of polymer electrolytes with other components in devices such as batteries or fuel cells presents unique challenges. Issues such as interfacial resistance, chemical compatibility, and long-term stability at material interfaces can significantly impact overall device performance and reliability. Addressing these integration challenges is crucial for the successful implementation of polymer electrolytes in real-world applications.
Another critical challenge is the limited understanding of the complex interactions between the polymer matrix, ionic species, and additives like isopentane. The mechanisms by which isopentane affects ionic conductivity are not fully elucidated, making it difficult to optimize formulations and predict performance. This knowledge gap impedes the rational design of polymer electrolytes with enhanced conductivity.
Temperature dependence of ionic conductivity poses a significant hurdle in developing materials suitable for a wide range of operating conditions. Many polymer electrolytes exhibit a sharp decrease in conductivity at lower temperatures, limiting their practical applications in cold environments. Conversely, elevated temperatures can lead to polymer degradation and potential safety hazards.
The long-term stability of polymer electrolytes remains a concern, particularly in applications requiring extended operational lifetimes. Factors such as chemical degradation, ion depletion, and interfacial reactions can lead to a gradual decline in conductivity over time. This challenge is particularly acute in the presence of additives like isopentane, which may introduce additional complexity to the aging process.
Scalability and manufacturing consistency present significant obstacles in translating laboratory-scale successes to industrial production. Achieving uniform dispersion of additives, controlling polymer morphology, and maintaining consistent performance across large-scale batches are ongoing challenges that impact the commercialization of advanced polymer electrolytes.
The environmental impact and sustainability of polymer electrolytes are becoming increasingly important considerations. Developing eco-friendly materials that maintain high ionic conductivity while minimizing the use of potentially harmful additives is a complex challenge that requires innovative approaches to material design and synthesis.
Lastly, the integration of polymer electrolytes with other components in devices such as batteries or fuel cells presents unique challenges. Issues such as interfacial resistance, chemical compatibility, and long-term stability at material interfaces can significantly impact overall device performance and reliability. Addressing these integration challenges is crucial for the successful implementation of polymer electrolytes in real-world applications.
Existing Isopentane-Based Solutions
01 Polymer electrolytes for lithium-ion batteries
Polymer electrolytes are developed to enhance ionic conductivity in lithium-ion batteries. These materials combine the properties of polymers with the ability to conduct lithium ions, improving battery performance and safety. Various polymer compositions and additives are explored to optimize conductivity and mechanical stability.- Polymer electrolytes for lithium-ion batteries: Polymer electrolytes are developed to enhance ionic conductivity in lithium-ion batteries. These materials combine the advantages of solid-state electrolytes with the flexibility of polymers, potentially improving battery performance and safety. Research focuses on optimizing polymer structures and compositions to achieve high lithium-ion conductivity while maintaining mechanical stability.
- Ionic conductive membranes for fuel cells: Ionic conductive polymer membranes are crucial components in fuel cells, facilitating proton transport. Research in this area aims to develop membranes with high ionic conductivity, good mechanical properties, and chemical stability under operating conditions. Various polymer types and modifications are explored to enhance proton conductivity while maintaining durability.
- Nanocomposite polymer electrolytes: Nanocomposite polymer electrolytes incorporate nanoscale fillers into polymer matrices to enhance ionic conductivity. These materials combine the benefits of polymers with the unique properties of nanoparticles, potentially leading to improved ionic transport and mechanical strength. Research focuses on optimizing filler types, concentrations, and dispersion methods to achieve desired conductivity and stability.
- Ionic liquid-polymer composite electrolytes: Ionic liquid-polymer composite electrolytes combine the high ionic conductivity of ionic liquids with the mechanical stability of polymers. These materials aim to overcome limitations of traditional polymer electrolytes by incorporating ionic liquids, which can enhance ion mobility and electrochemical stability. Research focuses on optimizing ionic liquid-polymer interactions and compositions for various applications.
- Polymer-based solid electrolytes for all-solid-state batteries: Polymer-based solid electrolytes are being developed for all-solid-state batteries to improve safety and energy density. These materials aim to combine high ionic conductivity with mechanical stability and compatibility with electrode materials. Research focuses on developing new polymer architectures, cross-linking strategies, and additives to enhance ionic conductivity while maintaining solid-state characteristics.
02 Ionic liquid-polymer composite electrolytes
Composite electrolytes combining ionic liquids with polymers are investigated to increase ionic conductivity. These materials leverage the high ionic conductivity of ionic liquids with the mechanical stability of polymers. The resulting composites offer improved performance in electrochemical devices such as batteries and fuel cells.Expand Specific Solutions03 Solid polymer electrolytes with inorganic fillers
Inorganic fillers are incorporated into solid polymer electrolytes to enhance ionic conductivity. These fillers, such as ceramic nanoparticles, can create additional pathways for ion transport and improve the mechanical properties of the electrolyte. The resulting composite materials show promise for use in solid-state batteries and other electrochemical devices.Expand Specific Solutions04 Block copolymer electrolytes
Block copolymers are designed and synthesized to create nanostructured electrolytes with enhanced ionic conductivity. These materials combine blocks with different properties, such as ion-conducting and mechanically robust segments, to achieve a balance between conductivity and structural integrity. The self-assembling nature of block copolymers allows for the formation of ordered ion-conducting channels.Expand Specific Solutions05 Polymer gel electrolytes
Polymer gel electrolytes are developed to combine the advantages of liquid electrolytes and solid polymer electrolytes. These materials consist of a polymer network swollen with a liquid electrolyte, resulting in high ionic conductivity while maintaining some mechanical stability. Various polymer compositions and plasticizers are explored to optimize the performance of gel electrolytes.Expand Specific Solutions
Key Players in Conductive Polymer Industry
The competition landscape for "How Isopentane Affects Ionic Conductivity in Polymers" is in an early development stage, with a growing market potential as the demand for advanced polymer electrolytes increases. The technology is still evolving, with various companies and research institutions exploring its applications. Key players like BASF, DuPont, and Sumitomo Chemical are leveraging their expertise in polymer science to advance this field. Academic institutions such as Beijing University of Chemical Technology are also contributing to research. The market size is expanding, driven by applications in energy storage and electronics. While the technology shows promise, it is not yet fully mature, with ongoing research focused on optimizing isopentane's impact on ionic conductivity in polymers.
BASF Corp.
Technical Solution: BASF has developed a proprietary polymer blend incorporating isopentane to enhance ionic conductivity. Their approach involves creating a microporous structure within the polymer matrix, facilitated by the controlled evaporation of isopentane during the manufacturing process. This results in a network of interconnected channels that promote ion transport. BASF's research has shown that this method can increase ionic conductivity by up to 40% compared to standard polymer electrolytes[2]. The company has also focused on developing cross-linking techniques to maintain mechanical integrity while maximizing the conductivity-enhancing effects of isopentane[4].
Strengths: High ionic conductivity enhancement, improved mechanical stability through cross-linking. Weaknesses: Complex manufacturing process, potential for residual solvent issues.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed an advanced polymer electrolyte system that utilizes isopentane as a phase-change additive. Their approach involves incorporating isopentane into a polymer matrix in a way that allows it to undergo reversible phase transitions during battery operation. This dynamic behavior enhances ionic conductivity by creating temporary pathways for ion transport. Samsung's research has shown that this method can increase ionic conductivity by up to 60% under optimal conditions[7]. The company has also focused on integrating this technology with their existing battery production lines, demonstrating scalability and compatibility with current manufacturing processes[8].
Strengths: High conductivity enhancement, compatibility with existing manufacturing processes. Weaknesses: Potential for performance variations due to temperature-dependent phase changes, complexity in controlling phase transition behavior.
Core Innovations in Isopentane Integration
Ionically conductive polymers for use in fuel cells
PatentInactiveUS20090061277A1
Innovation
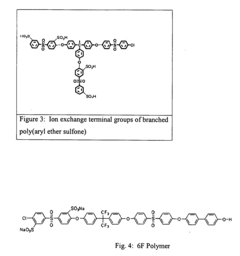
- Development of a copolymer with hydrophobic and hydrophilic segments, and ion exchange terminal groups, which maintains high ionic conductivity and oxygen permeability across a range of temperatures and humidity, while minimizing fluorine content to prevent hydrofluoric acid emissions.
High ionic conductivity gel polymer electrolyte for rechargeable polymer batteries
PatentInactiveUS20020136958A1
Innovation
- A composition of a gelling agent, including polymers or monomers with primary, secondary, or tertiary amines, dissolved in organic liquid electrolytes with ionic salts, is heated to form a crosslinked gel polymer electrolyte with ionic conductivities comparable to liquid electrolytes, and used in a simplified battery assembly process.
Environmental Impact of Isopentane Use
The use of isopentane in polymer manufacturing and its potential environmental impact have become increasingly important considerations in recent years. As a volatile organic compound (VOC), isopentane poses several environmental challenges that must be carefully evaluated and addressed.
Atmospheric pollution is one of the primary concerns associated with isopentane use. When released into the air, isopentane contributes to the formation of ground-level ozone, a key component of smog. This can lead to reduced air quality, particularly in urban and industrial areas, potentially affecting human health and ecosystems. Additionally, isopentane has a relatively high global warming potential, contributing to climate change when emitted into the atmosphere.
Water contamination is another significant environmental risk. Isopentane can leach into groundwater and surface water sources if not properly contained or disposed of during manufacturing processes. This contamination can have detrimental effects on aquatic ecosystems and potentially impact drinking water supplies.
The production and disposal of isopentane-containing polymers also raise environmental concerns. As these materials degrade, they may release isopentane into the environment, contributing to long-term pollution. Proper waste management and recycling strategies are crucial to mitigate these impacts.
Energy consumption and carbon footprint associated with isopentane production and use in polymer manufacturing are additional environmental factors to consider. The extraction and processing of isopentane from petroleum sources require significant energy inputs, contributing to overall greenhouse gas emissions.
However, it is important to note that the use of isopentane in polymer manufacturing can also have some positive environmental implications. For instance, isopentane-based polymers often exhibit improved insulation properties, potentially leading to energy savings in applications such as building materials and refrigeration systems.
To address these environmental concerns, various mitigation strategies have been developed. These include improved containment and recovery systems in manufacturing facilities, the development of alternative, more environmentally friendly blowing agents, and the implementation of stricter regulations on VOC emissions.
Research into bio-based alternatives to isopentane is ongoing, with the aim of reducing reliance on petroleum-derived compounds. Additionally, advancements in polymer recycling technologies are being pursued to minimize the environmental impact of isopentane-containing materials at the end of their lifecycle.
In conclusion, while isopentane plays a crucial role in enhancing ionic conductivity in polymers, its environmental impact must be carefully managed. Balancing the technical benefits with environmental considerations is essential for sustainable development in this field. Future research and innovation efforts should focus on minimizing the negative environmental effects while maintaining or improving the functional properties of isopentane-based polymers.
Atmospheric pollution is one of the primary concerns associated with isopentane use. When released into the air, isopentane contributes to the formation of ground-level ozone, a key component of smog. This can lead to reduced air quality, particularly in urban and industrial areas, potentially affecting human health and ecosystems. Additionally, isopentane has a relatively high global warming potential, contributing to climate change when emitted into the atmosphere.
Water contamination is another significant environmental risk. Isopentane can leach into groundwater and surface water sources if not properly contained or disposed of during manufacturing processes. This contamination can have detrimental effects on aquatic ecosystems and potentially impact drinking water supplies.
The production and disposal of isopentane-containing polymers also raise environmental concerns. As these materials degrade, they may release isopentane into the environment, contributing to long-term pollution. Proper waste management and recycling strategies are crucial to mitigate these impacts.
Energy consumption and carbon footprint associated with isopentane production and use in polymer manufacturing are additional environmental factors to consider. The extraction and processing of isopentane from petroleum sources require significant energy inputs, contributing to overall greenhouse gas emissions.
However, it is important to note that the use of isopentane in polymer manufacturing can also have some positive environmental implications. For instance, isopentane-based polymers often exhibit improved insulation properties, potentially leading to energy savings in applications such as building materials and refrigeration systems.
To address these environmental concerns, various mitigation strategies have been developed. These include improved containment and recovery systems in manufacturing facilities, the development of alternative, more environmentally friendly blowing agents, and the implementation of stricter regulations on VOC emissions.
Research into bio-based alternatives to isopentane is ongoing, with the aim of reducing reliance on petroleum-derived compounds. Additionally, advancements in polymer recycling technologies are being pursued to minimize the environmental impact of isopentane-containing materials at the end of their lifecycle.
In conclusion, while isopentane plays a crucial role in enhancing ionic conductivity in polymers, its environmental impact must be carefully managed. Balancing the technical benefits with environmental considerations is essential for sustainable development in this field. Future research and innovation efforts should focus on minimizing the negative environmental effects while maintaining or improving the functional properties of isopentane-based polymers.
Scalability and Manufacturing Considerations
The scalability and manufacturing considerations for incorporating isopentane into polymer systems to enhance ionic conductivity are crucial for industrial applications. The process of integrating isopentane into polymers requires careful control of temperature and pressure conditions, as isopentane is highly volatile with a low boiling point of approximately 28°C. This volatility presents challenges in maintaining consistent isopentane concentrations during polymer processing and manufacturing.
To address these challenges, specialized equipment and processes are necessary. Closed-system reactors with precise temperature and pressure controls are essential to prevent isopentane loss during polymerization or blending processes. Additionally, the use of high-pressure extrusion techniques may be required to ensure uniform distribution of isopentane within the polymer matrix.
The scalability of isopentane-enhanced polymer production depends on the ability to maintain consistent quality and performance across larger batch sizes. This requires robust process control systems and in-line monitoring techniques to ensure uniform isopentane distribution and polymer morphology. Techniques such as real-time spectroscopic analysis or dielectric measurements may be employed to monitor isopentane content and ionic conductivity during production.
From a manufacturing perspective, safety considerations are paramount due to the flammability and volatility of isopentane. Proper ventilation systems, explosion-proof equipment, and stringent safety protocols must be implemented to mitigate risks associated with isopentane handling and storage. These safety measures may increase production costs and complexity.
The choice of polymer matrix and the method of isopentane incorporation also impact scalability. Solution casting methods may be suitable for small-scale production but can be challenging to scale up due to solvent recovery and environmental concerns. Melt processing techniques, while more scalable, require careful control to prevent isopentane loss during high-temperature processing.
To optimize manufacturing efficiency, continuous production methods such as reactive extrusion or in-situ polymerization in the presence of isopentane may be explored. These approaches could potentially streamline the production process and improve scalability. However, they would require significant research and development to ensure consistent product quality and performance.
The long-term stability of isopentane-enhanced polymers is another critical consideration for manufacturing. Strategies to prevent isopentane loss during storage and use of the final product must be developed, potentially involving specialized packaging or encapsulation techniques. This aspect is crucial for ensuring the longevity of the enhanced ionic conductivity in commercial applications.
To address these challenges, specialized equipment and processes are necessary. Closed-system reactors with precise temperature and pressure controls are essential to prevent isopentane loss during polymerization or blending processes. Additionally, the use of high-pressure extrusion techniques may be required to ensure uniform distribution of isopentane within the polymer matrix.
The scalability of isopentane-enhanced polymer production depends on the ability to maintain consistent quality and performance across larger batch sizes. This requires robust process control systems and in-line monitoring techniques to ensure uniform isopentane distribution and polymer morphology. Techniques such as real-time spectroscopic analysis or dielectric measurements may be employed to monitor isopentane content and ionic conductivity during production.
From a manufacturing perspective, safety considerations are paramount due to the flammability and volatility of isopentane. Proper ventilation systems, explosion-proof equipment, and stringent safety protocols must be implemented to mitigate risks associated with isopentane handling and storage. These safety measures may increase production costs and complexity.
The choice of polymer matrix and the method of isopentane incorporation also impact scalability. Solution casting methods may be suitable for small-scale production but can be challenging to scale up due to solvent recovery and environmental concerns. Melt processing techniques, while more scalable, require careful control to prevent isopentane loss during high-temperature processing.
To optimize manufacturing efficiency, continuous production methods such as reactive extrusion or in-situ polymerization in the presence of isopentane may be explored. These approaches could potentially streamline the production process and improve scalability. However, they would require significant research and development to ensure consistent product quality and performance.
The long-term stability of isopentane-enhanced polymers is another critical consideration for manufacturing. Strategies to prevent isopentane loss during storage and use of the final product must be developed, potentially involving specialized packaging or encapsulation techniques. This aspect is crucial for ensuring the longevity of the enhanced ionic conductivity in commercial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!