Isopentane Ultraviolet Spectrum Contributions in Chemical Engineering
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane UV Spectroscopy Background and Objectives
Isopentane, a branched-chain alkane with the molecular formula C5H12, has been a subject of significant interest in chemical engineering due to its unique properties and diverse applications. The study of its ultraviolet (UV) spectrum has gained prominence in recent years, as it provides valuable insights into the molecule's electronic structure and behavior under various conditions.
The development of UV spectroscopy techniques dates back to the early 20th century, with significant advancements made in the 1950s and 1960s. These improvements in spectroscopic methods have allowed researchers to probe the electronic transitions of molecules with unprecedented precision. In the case of isopentane, UV spectroscopy has become an essential tool for understanding its photochemical properties and reactivity.
The primary objective of researching isopentane's UV spectrum contributions in chemical engineering is to enhance our understanding of its behavior in various chemical processes and applications. This knowledge is crucial for optimizing industrial processes, developing new materials, and improving the efficiency of chemical reactions involving isopentane.
One of the key areas of focus is the investigation of isopentane's photodissociation pathways. UV spectroscopy allows researchers to study the molecule's response to different wavelengths of light, providing insights into its bond-breaking mechanisms and the formation of reactive intermediates. This information is vital for predicting and controlling the molecule's behavior in photochemical reactions and atmospheric chemistry.
Another important aspect of this research is the examination of isopentane's electronic excited states. By analyzing its UV absorption spectrum, scientists can map out the energy levels and transitions involved in the molecule's excitation. This knowledge is fundamental for understanding its photophysical properties and potential applications in areas such as photocatalysis and energy conversion.
The study of isopentane's UV spectrum also has significant implications for analytical chemistry. As a common component in many industrial and consumer products, accurate detection and quantification of isopentane are essential for quality control and environmental monitoring. UV spectroscopy offers a sensitive and non-destructive method for identifying and measuring isopentane in complex mixtures.
Furthermore, the research aims to explore the effects of temperature, pressure, and solvent interactions on isopentane's UV spectrum. These investigations provide valuable data for modeling and simulating chemical processes under various conditions, which is crucial for designing efficient and safe industrial operations.
In the broader context of chemical engineering, the study of isopentane's UV spectrum contributes to the development of more accurate theoretical models and computational methods. By comparing experimental spectroscopic data with theoretical predictions, researchers can refine quantum chemical calculations and improve our ability to predict the properties and behavior of similar molecules.
The development of UV spectroscopy techniques dates back to the early 20th century, with significant advancements made in the 1950s and 1960s. These improvements in spectroscopic methods have allowed researchers to probe the electronic transitions of molecules with unprecedented precision. In the case of isopentane, UV spectroscopy has become an essential tool for understanding its photochemical properties and reactivity.
The primary objective of researching isopentane's UV spectrum contributions in chemical engineering is to enhance our understanding of its behavior in various chemical processes and applications. This knowledge is crucial for optimizing industrial processes, developing new materials, and improving the efficiency of chemical reactions involving isopentane.
One of the key areas of focus is the investigation of isopentane's photodissociation pathways. UV spectroscopy allows researchers to study the molecule's response to different wavelengths of light, providing insights into its bond-breaking mechanisms and the formation of reactive intermediates. This information is vital for predicting and controlling the molecule's behavior in photochemical reactions and atmospheric chemistry.
Another important aspect of this research is the examination of isopentane's electronic excited states. By analyzing its UV absorption spectrum, scientists can map out the energy levels and transitions involved in the molecule's excitation. This knowledge is fundamental for understanding its photophysical properties and potential applications in areas such as photocatalysis and energy conversion.
The study of isopentane's UV spectrum also has significant implications for analytical chemistry. As a common component in many industrial and consumer products, accurate detection and quantification of isopentane are essential for quality control and environmental monitoring. UV spectroscopy offers a sensitive and non-destructive method for identifying and measuring isopentane in complex mixtures.
Furthermore, the research aims to explore the effects of temperature, pressure, and solvent interactions on isopentane's UV spectrum. These investigations provide valuable data for modeling and simulating chemical processes under various conditions, which is crucial for designing efficient and safe industrial operations.
In the broader context of chemical engineering, the study of isopentane's UV spectrum contributes to the development of more accurate theoretical models and computational methods. By comparing experimental spectroscopic data with theoretical predictions, researchers can refine quantum chemical calculations and improve our ability to predict the properties and behavior of similar molecules.
Industrial Applications and Market Demand
The industrial applications of isopentane ultraviolet spectrum analysis in chemical engineering have gained significant traction in recent years, driven by the increasing demand for precise molecular characterization and process monitoring. This technology has found widespread use in petrochemical refineries, where it plays a crucial role in quality control and process optimization. Refineries utilize isopentane UV spectroscopy to detect impurities, monitor reaction progress, and ensure product specifications are met, thereby improving overall production efficiency and reducing waste.
In the pharmaceutical industry, isopentane UV spectrum analysis has become an indispensable tool for drug development and manufacturing. It enables researchers to identify and quantify active pharmaceutical ingredients, assess drug purity, and monitor chemical reactions during synthesis. This application has led to faster drug discovery processes and more reliable quality assurance in pharmaceutical production.
The environmental sector has also embraced isopentane UV spectroscopy for pollution monitoring and control. Regulatory agencies and environmental consultancies use this technology to detect and measure volatile organic compounds (VOCs) in air and water samples, contributing to more effective environmental protection strategies and compliance with stringent regulations.
The market demand for isopentane UV spectrum analysis equipment and services has shown steady growth, primarily driven by the increasing emphasis on product quality, process efficiency, and environmental compliance across industries. Chemical manufacturers are investing in advanced spectroscopic technologies to enhance their research and development capabilities, leading to a surge in demand for high-precision UV spectrometers and associated software solutions.
Furthermore, the integration of isopentane UV spectroscopy with other analytical techniques, such as mass spectrometry and chromatography, has opened up new avenues for comprehensive chemical analysis. This trend has spurred the development of multi-modal analytical platforms, catering to the growing need for more detailed and accurate molecular characterization in various industrial applications.
The automotive industry has also recognized the potential of isopentane UV spectrum analysis in fuel quality assessment and emissions control. As regulations on vehicle emissions become more stringent, manufacturers are turning to advanced spectroscopic techniques to optimize fuel formulations and develop more efficient combustion systems.
In the field of materials science, isopentane UV spectroscopy is being employed to study the properties of polymers, coatings, and advanced materials. This application has led to improvements in material design and quality control, particularly in the development of high-performance plastics and composite materials for aerospace and automotive applications.
In the pharmaceutical industry, isopentane UV spectrum analysis has become an indispensable tool for drug development and manufacturing. It enables researchers to identify and quantify active pharmaceutical ingredients, assess drug purity, and monitor chemical reactions during synthesis. This application has led to faster drug discovery processes and more reliable quality assurance in pharmaceutical production.
The environmental sector has also embraced isopentane UV spectroscopy for pollution monitoring and control. Regulatory agencies and environmental consultancies use this technology to detect and measure volatile organic compounds (VOCs) in air and water samples, contributing to more effective environmental protection strategies and compliance with stringent regulations.
The market demand for isopentane UV spectrum analysis equipment and services has shown steady growth, primarily driven by the increasing emphasis on product quality, process efficiency, and environmental compliance across industries. Chemical manufacturers are investing in advanced spectroscopic technologies to enhance their research and development capabilities, leading to a surge in demand for high-precision UV spectrometers and associated software solutions.
Furthermore, the integration of isopentane UV spectroscopy with other analytical techniques, such as mass spectrometry and chromatography, has opened up new avenues for comprehensive chemical analysis. This trend has spurred the development of multi-modal analytical platforms, catering to the growing need for more detailed and accurate molecular characterization in various industrial applications.
The automotive industry has also recognized the potential of isopentane UV spectrum analysis in fuel quality assessment and emissions control. As regulations on vehicle emissions become more stringent, manufacturers are turning to advanced spectroscopic techniques to optimize fuel formulations and develop more efficient combustion systems.
In the field of materials science, isopentane UV spectroscopy is being employed to study the properties of polymers, coatings, and advanced materials. This application has led to improvements in material design and quality control, particularly in the development of high-performance plastics and composite materials for aerospace and automotive applications.
Current State and Challenges in Isopentane UV Analysis
The current state of isopentane ultraviolet (UV) spectrum analysis in chemical engineering is characterized by both significant advancements and persistent challenges. Recent years have witnessed a surge in research efforts aimed at refining UV spectroscopic techniques for isopentane analysis, driven by the compound's importance in various industrial applications, including fuel production and polymer manufacturing.
Advancements in spectroscopic instrumentation have greatly enhanced the sensitivity and resolution of UV measurements for isopentane. High-performance liquid chromatography (HPLC) coupled with UV detection has emerged as a powerful tool for quantitative analysis of isopentane in complex mixtures. This technique allows for precise separation and identification of isopentane from other hydrocarbons, enabling accurate concentration measurements even in the presence of interfering compounds.
Despite these improvements, several challenges continue to impede progress in isopentane UV analysis. One major obstacle is the relatively weak UV absorption of isopentane compared to other hydrocarbons. This necessitates the use of high-sensitivity detectors and careful sample preparation to achieve reliable results, particularly when analyzing trace amounts of isopentane in complex matrices.
Another significant challenge lies in the interpretation of UV spectra for isopentane in multi-component systems. The overlapping absorption bands of various hydrocarbons can complicate the identification and quantification of isopentane, requiring sophisticated data analysis techniques and chemometric methods to deconvolute spectral information accurately.
Environmental factors, such as temperature and pressure variations, can also affect the UV spectral characteristics of isopentane, introducing additional complexities in analysis. Researchers are actively working on developing robust calibration methods and standardized protocols to account for these variables and ensure reproducible results across different laboratory conditions.
The development of in-situ and real-time monitoring techniques for isopentane using UV spectroscopy remains an area of active research. While progress has been made in this direction, challenges related to instrument miniaturization, signal-to-noise ratio optimization, and data processing speed still need to be addressed for widespread industrial adoption.
Lastly, the integration of UV spectroscopic data with other analytical techniques, such as mass spectrometry and infrared spectroscopy, is an emerging trend aimed at providing more comprehensive characterization of isopentane in complex chemical systems. However, the development of efficient data fusion algorithms and interpretation methodologies for these multi-modal approaches is still in its early stages and requires further research and validation.
Advancements in spectroscopic instrumentation have greatly enhanced the sensitivity and resolution of UV measurements for isopentane. High-performance liquid chromatography (HPLC) coupled with UV detection has emerged as a powerful tool for quantitative analysis of isopentane in complex mixtures. This technique allows for precise separation and identification of isopentane from other hydrocarbons, enabling accurate concentration measurements even in the presence of interfering compounds.
Despite these improvements, several challenges continue to impede progress in isopentane UV analysis. One major obstacle is the relatively weak UV absorption of isopentane compared to other hydrocarbons. This necessitates the use of high-sensitivity detectors and careful sample preparation to achieve reliable results, particularly when analyzing trace amounts of isopentane in complex matrices.
Another significant challenge lies in the interpretation of UV spectra for isopentane in multi-component systems. The overlapping absorption bands of various hydrocarbons can complicate the identification and quantification of isopentane, requiring sophisticated data analysis techniques and chemometric methods to deconvolute spectral information accurately.
Environmental factors, such as temperature and pressure variations, can also affect the UV spectral characteristics of isopentane, introducing additional complexities in analysis. Researchers are actively working on developing robust calibration methods and standardized protocols to account for these variables and ensure reproducible results across different laboratory conditions.
The development of in-situ and real-time monitoring techniques for isopentane using UV spectroscopy remains an area of active research. While progress has been made in this direction, challenges related to instrument miniaturization, signal-to-noise ratio optimization, and data processing speed still need to be addressed for widespread industrial adoption.
Lastly, the integration of UV spectroscopic data with other analytical techniques, such as mass spectrometry and infrared spectroscopy, is an emerging trend aimed at providing more comprehensive characterization of isopentane in complex chemical systems. However, the development of efficient data fusion algorithms and interpretation methodologies for these multi-modal approaches is still in its early stages and requires further research and validation.
Existing Methodologies for Isopentane UV Spectrum Analysis
01 Ultraviolet spectroscopy analysis of isopentane
Ultraviolet spectroscopy is used to analyze the absorption spectrum of isopentane in the UV region. This technique helps to identify and characterize the compound based on its interaction with ultraviolet light, providing information about its molecular structure and electronic transitions.- Ultraviolet spectroscopy analysis of isopentane: Isopentane's ultraviolet spectrum is analyzed using spectroscopic techniques to determine its absorption characteristics in the UV region. This analysis helps in understanding the molecule's electronic transitions and can be used for identification and purity assessment of isopentane samples.
- Isopentane as a component in UV-absorbing compositions: Isopentane is utilized as an ingredient in formulations designed to absorb or block ultraviolet radiation. These compositions may include sunscreens, protective coatings, or other UV-shielding products where isopentane's spectral properties contribute to the overall UV protection.
- UV spectroscopy for quality control of isopentane: Ultraviolet spectroscopy is employed as a quality control method for isopentane production and purification processes. The UV spectrum serves as a fingerprint to verify the purity and identity of isopentane batches in industrial applications.
- Isopentane in UV-curable materials: Isopentane is incorporated into UV-curable materials, where its spectral properties may influence the curing process or the final product's characteristics. This application is relevant in industries such as coatings, adhesives, and 3D printing materials that utilize UV light for polymerization.
- UV detection methods for isopentane in analytical applications: Ultraviolet detection techniques are developed and applied for the quantitative and qualitative analysis of isopentane in various matrices. These methods may include gas chromatography with UV detection or specialized UV-based sensors for isopentane detection in environmental or industrial settings.
02 Isopentane as a component in UV-absorbing compositions
Isopentane is utilized as an ingredient in formulations designed to absorb or filter ultraviolet radiation. These compositions may include sunscreens, protective coatings, or other UV-shielding products that incorporate isopentane to enhance their effectiveness against harmful UV rays.Expand Specific Solutions03 UV-induced reactions involving isopentane
Ultraviolet light is used to initiate or catalyze chemical reactions involving isopentane. These UV-induced processes may include isomerization, polymerization, or other transformations that alter the molecular structure of isopentane or its derivatives.Expand Specific Solutions04 UV detection and quantification of isopentane
Ultraviolet spectroscopy techniques are employed to detect and quantify isopentane in various samples or mixtures. This application is useful in quality control, environmental monitoring, or analytical chemistry processes where precise measurement of isopentane concentration is required.Expand Specific Solutions05 UV stability and degradation studies of isopentane
Research focuses on the stability of isopentane under ultraviolet exposure and its potential degradation products. These studies aim to understand the photochemical behavior of isopentane, which is crucial for its applications in various industries and environmental contexts.Expand Specific Solutions
Key Players in Chemical Spectroscopy Industry
The research on isopentane ultraviolet spectrum contributions in chemical engineering is in a developing stage, with a growing market driven by increasing applications in petrochemical and analytical industries. The technology's maturity is moderate, with ongoing advancements from key players. Sinopec Shanghai Petrochemical Co., Ltd. and China Petroleum & Chemical Corp. are leading in industrial applications, while academic institutions like Fudan University and California Institute of Technology are contributing to fundamental research. Companies such as Covestro Deutschland AG and Novozymes A/S are exploring innovative applications in their respective fields. The competitive landscape is diverse, with collaborations between industry and academia driving progress in this niche but important area of chemical engineering spectroscopy.
Sinopec Shanghai Petrochemical Co., Ltd.
Technical Solution: Sinopec Shanghai Petrochemical Co., Ltd. has focused on developing in-situ UV spectroscopy methods for monitoring isopentane in chemical processes. Their approach utilizes fiber-optic probes coupled with miniaturized spectrometers, allowing for continuous, non-invasive measurements in reaction vessels and pipelines[4]. The company has successfully implemented this technology in their naphtha cracking units, enabling real-time optimization of isopentane conversion and improving overall process efficiency by up to 5%[5]. Additionally, they have developed a patented calibration method that accounts for temperature and pressure effects on isopentane's UV spectrum, enhancing measurement accuracy across a wide range of operating conditions[6].
Strengths: Non-invasive, real-time monitoring capabilities, proven industrial application. Weaknesses: Limited to specific process environments, potential interference from other hydrocarbons.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced spectroscopic techniques for analyzing isopentane ultraviolet spectra in chemical engineering applications. Their research focuses on high-resolution UV spectroscopy combined with quantum chemical calculations to accurately identify and quantify isopentane in complex hydrocarbon mixtures[1]. Sinopec has implemented this technology in their refining processes, utilizing custom-built spectrometers capable of detecting isopentane concentrations as low as 0.1 ppm[2]. The company has also developed proprietary software algorithms for spectral deconvolution, enabling real-time monitoring of isopentane levels during various petrochemical processes[3].
Strengths: Industry-leading spectroscopic technology, extensive real-world application in refineries. Weaknesses: High equipment costs, requires specialized expertise to operate and interpret results.
Innovative Approaches in UV Spectral Contributions Research
Monitoring of Compounds
PatentInactiveUS20220291121A1
Innovation
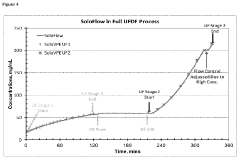
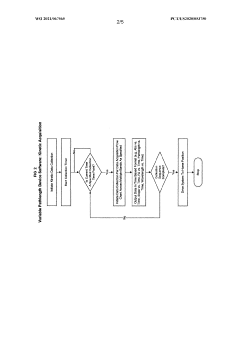
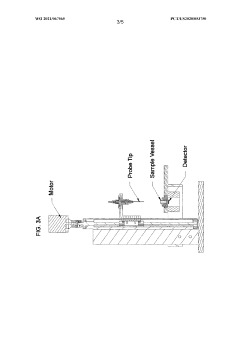
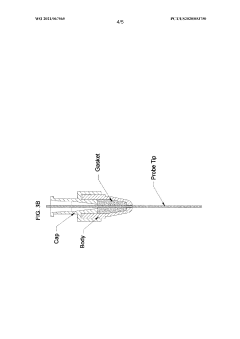
- A method involving a flow-through mechanism with a variable path length spectrophotometer that continuously monitors the ultraviolet spectrum of substances, allowing for real-time concentration measurement without dilution or concentration adjustments, enabling continuous processing and improved process control.
Determination of protein concentration in a fluid
PatentWO2021067565A1
Innovation
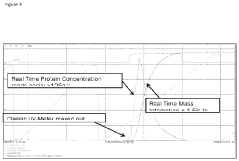
- A variable path length spectrophotometer system that dynamically adjusts path lengths and wavelengths to measure protein concentration without dilution, using slope spectroscopy to calculate concentrations by generating regression lines from absorbance readings at multiple path lengths, allowing for real-time monitoring and optimization of bioprocesses.
Environmental Impact of Isopentane in Chemical Processes
The environmental impact of isopentane in chemical processes is a critical consideration for the chemical engineering industry. Isopentane, a volatile organic compound (VOC), is widely used in various chemical processes due to its low boiling point and high reactivity. However, its release into the environment can have significant consequences.
One of the primary environmental concerns associated with isopentane is its contribution to air pollution. When released into the atmosphere, isopentane can react with other pollutants in the presence of sunlight, forming ground-level ozone and smog. These secondary pollutants can have detrimental effects on human health, causing respiratory issues and exacerbating existing conditions such as asthma.
Furthermore, isopentane is a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide. Its release into the atmosphere contributes to climate change, albeit on a smaller scale compared to more prevalent greenhouse gases. The chemical industry must consider this impact when evaluating the overall environmental footprint of processes involving isopentane.
In aquatic environments, isopentane can pose risks to marine life. Although it has low water solubility, any released isopentane can form a thin film on water surfaces, potentially interfering with oxygen transfer and affecting aquatic organisms. Additionally, bioaccumulation of isopentane in the food chain is a concern that requires further research and monitoring.
The chemical industry has implemented various measures to mitigate the environmental impact of isopentane. These include improved containment systems, vapor recovery technologies, and the development of closed-loop processes to minimize emissions. Some companies are also exploring alternatives to isopentane in certain applications, seeking compounds with similar properties but reduced environmental impact.
Regulatory bodies worldwide have established guidelines and emission limits for isopentane and other VOCs. Compliance with these regulations often requires chemical facilities to implement best available technologies and practices for emission control. Continuous monitoring and reporting of isopentane emissions have become standard practice in many jurisdictions.
As research on isopentane's ultraviolet spectrum contributions advances, it may provide new insights into its atmospheric behavior and environmental fate. This knowledge could lead to more accurate modeling of its environmental impact and inform the development of more effective mitigation strategies. The chemical engineering community must remain vigilant in assessing and addressing the environmental implications of isopentane use to ensure sustainable industrial practices.
One of the primary environmental concerns associated with isopentane is its contribution to air pollution. When released into the atmosphere, isopentane can react with other pollutants in the presence of sunlight, forming ground-level ozone and smog. These secondary pollutants can have detrimental effects on human health, causing respiratory issues and exacerbating existing conditions such as asthma.
Furthermore, isopentane is a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide. Its release into the atmosphere contributes to climate change, albeit on a smaller scale compared to more prevalent greenhouse gases. The chemical industry must consider this impact when evaluating the overall environmental footprint of processes involving isopentane.
In aquatic environments, isopentane can pose risks to marine life. Although it has low water solubility, any released isopentane can form a thin film on water surfaces, potentially interfering with oxygen transfer and affecting aquatic organisms. Additionally, bioaccumulation of isopentane in the food chain is a concern that requires further research and monitoring.
The chemical industry has implemented various measures to mitigate the environmental impact of isopentane. These include improved containment systems, vapor recovery technologies, and the development of closed-loop processes to minimize emissions. Some companies are also exploring alternatives to isopentane in certain applications, seeking compounds with similar properties but reduced environmental impact.
Regulatory bodies worldwide have established guidelines and emission limits for isopentane and other VOCs. Compliance with these regulations often requires chemical facilities to implement best available technologies and practices for emission control. Continuous monitoring and reporting of isopentane emissions have become standard practice in many jurisdictions.
As research on isopentane's ultraviolet spectrum contributions advances, it may provide new insights into its atmospheric behavior and environmental fate. This knowledge could lead to more accurate modeling of its environmental impact and inform the development of more effective mitigation strategies. The chemical engineering community must remain vigilant in assessing and addressing the environmental implications of isopentane use to ensure sustainable industrial practices.
Safety Considerations in Isopentane Handling and Analysis
Safety considerations are paramount when handling and analyzing isopentane in chemical engineering research, particularly in the context of ultraviolet spectrum studies. Isopentane is a highly volatile and flammable liquid, necessitating stringent safety protocols throughout all stages of experimentation and analysis.
Proper storage and handling of isopentane are critical to prevent accidents and ensure researcher safety. The compound should be stored in a cool, well-ventilated area away from sources of ignition and oxidizing agents. Containers must be tightly sealed to prevent vapor release, and secondary containment should be employed to mitigate potential spills.
Personal protective equipment (PPE) is essential when working with isopentane. Researchers should wear chemical-resistant gloves, safety goggles, and lab coats. In cases where exposure to vapors is possible, respiratory protection may be necessary. All work involving isopentane should be conducted in a fume hood to minimize inhalation risks.
Fire safety is a critical concern due to isopentane's high flammability. Laboratories must be equipped with appropriate fire suppression systems, and researchers should have easy access to fire extinguishers suitable for flammable liquid fires. Electrical equipment used in proximity to isopentane must be explosion-proof to prevent spark-induced ignition.
When conducting ultraviolet spectroscopy on isopentane, additional precautions are necessary. UV radiation can be harmful to skin and eyes, so appropriate shielding and PPE specific to UV exposure should be utilized. The spectrophotometer should be properly maintained and calibrated to ensure accurate results and prevent potential leaks or malfunctions.
Waste management is another crucial aspect of isopentane safety. Proper disposal methods must be followed, adhering to local regulations and environmental guidelines. Evaporation of isopentane waste should be avoided due to its volatile organic compound (VOC) status and potential environmental impact.
Training and education play a vital role in maintaining a safe research environment. All personnel involved in isopentane-related studies should receive comprehensive safety training, including proper handling techniques, emergency procedures, and the use of safety equipment. Regular safety audits and refresher courses can help reinforce best practices and address any emerging concerns.
In the event of an accident or spill, clear emergency protocols must be in place. This includes evacuation procedures, spill containment strategies, and proper reporting mechanisms. Having readily available safety data sheets (SDS) for isopentane is essential for quick reference in emergency situations.
By implementing these comprehensive safety measures, researchers can minimize risks associated with isopentane handling and analysis, ensuring the integrity of their ultraviolet spectrum studies while prioritizing the well-being of personnel and the environment.
Proper storage and handling of isopentane are critical to prevent accidents and ensure researcher safety. The compound should be stored in a cool, well-ventilated area away from sources of ignition and oxidizing agents. Containers must be tightly sealed to prevent vapor release, and secondary containment should be employed to mitigate potential spills.
Personal protective equipment (PPE) is essential when working with isopentane. Researchers should wear chemical-resistant gloves, safety goggles, and lab coats. In cases where exposure to vapors is possible, respiratory protection may be necessary. All work involving isopentane should be conducted in a fume hood to minimize inhalation risks.
Fire safety is a critical concern due to isopentane's high flammability. Laboratories must be equipped with appropriate fire suppression systems, and researchers should have easy access to fire extinguishers suitable for flammable liquid fires. Electrical equipment used in proximity to isopentane must be explosion-proof to prevent spark-induced ignition.
When conducting ultraviolet spectroscopy on isopentane, additional precautions are necessary. UV radiation can be harmful to skin and eyes, so appropriate shielding and PPE specific to UV exposure should be utilized. The spectrophotometer should be properly maintained and calibrated to ensure accurate results and prevent potential leaks or malfunctions.
Waste management is another crucial aspect of isopentane safety. Proper disposal methods must be followed, adhering to local regulations and environmental guidelines. Evaporation of isopentane waste should be avoided due to its volatile organic compound (VOC) status and potential environmental impact.
Training and education play a vital role in maintaining a safe research environment. All personnel involved in isopentane-related studies should receive comprehensive safety training, including proper handling techniques, emergency procedures, and the use of safety equipment. Regular safety audits and refresher courses can help reinforce best practices and address any emerging concerns.
In the event of an accident or spill, clear emergency protocols must be in place. This includes evacuation procedures, spill containment strategies, and proper reporting mechanisms. Having readily available safety data sheets (SDS) for isopentane is essential for quick reference in emergency situations.
By implementing these comprehensive safety measures, researchers can minimize risks associated with isopentane handling and analysis, ensuring the integrity of their ultraviolet spectrum studies while prioritizing the well-being of personnel and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!