Isopentane and Zeolite Interactions Structural Implications
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane-Zeolite Interaction Background
The interaction between isopentane and zeolites has been a subject of significant interest in the field of catalysis and materials science for several decades. Zeolites, crystalline aluminosilicate materials with well-defined pore structures, have found extensive applications in various industrial processes, particularly in the petrochemical industry. The study of isopentane-zeolite interactions is crucial for understanding and optimizing processes such as isomerization, cracking, and separation of hydrocarbons.
Isopentane, a branched alkane with five carbon atoms, serves as a model compound for studying the behavior of light hydrocarbons within zeolite frameworks. Its molecular size and shape make it an ideal candidate for probing the structural implications of zeolite-hydrocarbon interactions. The unique pore architecture of zeolites, combined with their tunable acidity and shape selectivity, allows for precise control over molecular transformations and separations.
The historical development of this research area can be traced back to the 1960s when zeolites were first introduced as catalysts in the petroleum industry. Early studies focused on understanding the adsorption and diffusion properties of hydrocarbons in zeolites. As analytical techniques advanced, researchers began to investigate the specific interactions between isopentane and various zeolite structures at a molecular level.
The structural implications of isopentane-zeolite interactions are multifaceted. On one hand, the zeolite framework imposes spatial constraints on the isopentane molecules, influencing their orientation, mobility, and reactivity. On the other hand, the presence of isopentane within the zeolite pores can induce subtle changes in the zeolite structure, affecting its stability and catalytic properties.
Recent advancements in characterization techniques, such as in-situ spectroscopy and high-resolution microscopy, have provided unprecedented insights into the dynamic nature of these interactions. Researchers have been able to observe the behavior of individual isopentane molecules within zeolite channels, revealing complex phenomena such as preferential adsorption sites, diffusion pathways, and the influence of framework flexibility on molecular transport.
Understanding the structural implications of isopentane-zeolite interactions has far-reaching consequences for the design of more efficient catalysts and adsorbents. It enables the rational tailoring of zeolite properties to optimize specific reactions or separation processes involving branched hydrocarbons. Moreover, these studies contribute to the broader field of host-guest chemistry, providing valuable insights into the fundamental principles governing molecular confinement and its effects on reactivity.
Isopentane, a branched alkane with five carbon atoms, serves as a model compound for studying the behavior of light hydrocarbons within zeolite frameworks. Its molecular size and shape make it an ideal candidate for probing the structural implications of zeolite-hydrocarbon interactions. The unique pore architecture of zeolites, combined with their tunable acidity and shape selectivity, allows for precise control over molecular transformations and separations.
The historical development of this research area can be traced back to the 1960s when zeolites were first introduced as catalysts in the petroleum industry. Early studies focused on understanding the adsorption and diffusion properties of hydrocarbons in zeolites. As analytical techniques advanced, researchers began to investigate the specific interactions between isopentane and various zeolite structures at a molecular level.
The structural implications of isopentane-zeolite interactions are multifaceted. On one hand, the zeolite framework imposes spatial constraints on the isopentane molecules, influencing their orientation, mobility, and reactivity. On the other hand, the presence of isopentane within the zeolite pores can induce subtle changes in the zeolite structure, affecting its stability and catalytic properties.
Recent advancements in characterization techniques, such as in-situ spectroscopy and high-resolution microscopy, have provided unprecedented insights into the dynamic nature of these interactions. Researchers have been able to observe the behavior of individual isopentane molecules within zeolite channels, revealing complex phenomena such as preferential adsorption sites, diffusion pathways, and the influence of framework flexibility on molecular transport.
Understanding the structural implications of isopentane-zeolite interactions has far-reaching consequences for the design of more efficient catalysts and adsorbents. It enables the rational tailoring of zeolite properties to optimize specific reactions or separation processes involving branched hydrocarbons. Moreover, these studies contribute to the broader field of host-guest chemistry, providing valuable insights into the fundamental principles governing molecular confinement and its effects on reactivity.
Market Analysis for Zeolite Catalysts
The zeolite catalyst market has experienced significant growth in recent years, driven by increasing demand across various industries. The global zeolite catalyst market size was valued at approximately $5.64 billion in 2020 and is projected to reach $8.12 billion by 2027, growing at a CAGR of 5.3% during the forecast period. This growth is primarily attributed to the rising demand for petroleum products and the increasing focus on environmental sustainability.
The petroleum refining industry remains the largest consumer of zeolite catalysts, accounting for over 40% of the market share. The growing demand for cleaner fuels and stricter environmental regulations have led to increased adoption of zeolite catalysts in fluid catalytic cracking (FCC) units and hydrocracking processes. The automotive industry also plays a crucial role in driving market growth, with zeolite catalysts being extensively used in emission control systems.
Geographically, Asia-Pacific dominates the zeolite catalyst market, holding a market share of approximately 35%. This can be attributed to the rapid industrialization and urbanization in countries like China and India, coupled with the growing automotive and petrochemical industries in the region. North America and Europe follow closely, with significant market shares due to stringent environmental regulations and the presence of major petroleum refineries.
The market for zeolite catalysts is highly competitive, with key players including Honeywell UOP, BASF, Albemarle Corporation, and Clariant. These companies are focusing on research and development activities to enhance the performance of zeolite catalysts and expand their application areas. The increasing emphasis on sustainable practices and the shift towards renewable energy sources present both challenges and opportunities for the zeolite catalyst market.
In the context of isopentane and zeolite interactions, there is growing interest in developing specialized zeolite catalysts for isomerization processes. The demand for high-octane gasoline components, such as isopentane, is driving research into more efficient and selective zeolite catalysts. This niche market segment is expected to witness substantial growth in the coming years, as refineries seek to optimize their production processes and meet evolving fuel quality standards.
The petroleum refining industry remains the largest consumer of zeolite catalysts, accounting for over 40% of the market share. The growing demand for cleaner fuels and stricter environmental regulations have led to increased adoption of zeolite catalysts in fluid catalytic cracking (FCC) units and hydrocracking processes. The automotive industry also plays a crucial role in driving market growth, with zeolite catalysts being extensively used in emission control systems.
Geographically, Asia-Pacific dominates the zeolite catalyst market, holding a market share of approximately 35%. This can be attributed to the rapid industrialization and urbanization in countries like China and India, coupled with the growing automotive and petrochemical industries in the region. North America and Europe follow closely, with significant market shares due to stringent environmental regulations and the presence of major petroleum refineries.
The market for zeolite catalysts is highly competitive, with key players including Honeywell UOP, BASF, Albemarle Corporation, and Clariant. These companies are focusing on research and development activities to enhance the performance of zeolite catalysts and expand their application areas. The increasing emphasis on sustainable practices and the shift towards renewable energy sources present both challenges and opportunities for the zeolite catalyst market.
In the context of isopentane and zeolite interactions, there is growing interest in developing specialized zeolite catalysts for isomerization processes. The demand for high-octane gasoline components, such as isopentane, is driving research into more efficient and selective zeolite catalysts. This niche market segment is expected to witness substantial growth in the coming years, as refineries seek to optimize their production processes and meet evolving fuel quality standards.
Current Challenges in Isopentane-Zeolite Systems
The interaction between isopentane and zeolites presents several significant challenges in current research and industrial applications. One of the primary issues is the complexity of the adsorption and diffusion processes within zeolite pores. The branched structure of isopentane molecules often leads to steric hindrance, affecting their mobility and accessibility to active sites within the zeolite framework.
Another challenge lies in understanding the precise nature of the isopentane-zeolite interactions at the molecular level. The strength and type of these interactions can vary depending on the specific zeolite structure, Si/Al ratio, and the presence of extra-framework cations. This variability makes it difficult to develop a unified model for predicting behavior across different zeolite systems.
The impact of temperature and pressure on isopentane-zeolite interactions poses additional complications. These parameters can significantly alter the adsorption equilibrium, diffusion rates, and even induce structural changes in both the zeolite framework and the isopentane molecules. Researchers struggle to accurately model these effects, particularly under extreme conditions relevant to industrial processes.
Catalyst deactivation is a persistent problem in isopentane-zeolite systems. The formation of coke deposits, resulting from unwanted side reactions or isopentane decomposition, can block pores and active sites, reducing the efficiency and lifespan of zeolite catalysts. Developing strategies to mitigate this deactivation while maintaining high catalytic activity remains a significant challenge.
The heterogeneity of zeolite crystals and the presence of defects further complicate the study of isopentane interactions. These imperfections can create preferential adsorption sites or diffusion pathways, leading to non-uniform behavior across the zeolite structure. Characterizing and accounting for these heterogeneities in experimental and computational studies is an ongoing challenge.
Scaling up laboratory findings to industrial applications presents its own set of difficulties. The behavior of isopentane-zeolite systems observed in small-scale experiments may not directly translate to large-scale processes due to heat and mass transfer limitations, as well as the influence of impurities present in industrial feedstocks.
Lastly, the development of in-situ and operando characterization techniques for studying isopentane-zeolite interactions under realistic conditions remains an active area of research. Current methods often struggle to provide real-time, high-resolution data on molecular interactions and structural changes occurring within the zeolite pores during actual operating conditions.
Another challenge lies in understanding the precise nature of the isopentane-zeolite interactions at the molecular level. The strength and type of these interactions can vary depending on the specific zeolite structure, Si/Al ratio, and the presence of extra-framework cations. This variability makes it difficult to develop a unified model for predicting behavior across different zeolite systems.
The impact of temperature and pressure on isopentane-zeolite interactions poses additional complications. These parameters can significantly alter the adsorption equilibrium, diffusion rates, and even induce structural changes in both the zeolite framework and the isopentane molecules. Researchers struggle to accurately model these effects, particularly under extreme conditions relevant to industrial processes.
Catalyst deactivation is a persistent problem in isopentane-zeolite systems. The formation of coke deposits, resulting from unwanted side reactions or isopentane decomposition, can block pores and active sites, reducing the efficiency and lifespan of zeolite catalysts. Developing strategies to mitigate this deactivation while maintaining high catalytic activity remains a significant challenge.
The heterogeneity of zeolite crystals and the presence of defects further complicate the study of isopentane interactions. These imperfections can create preferential adsorption sites or diffusion pathways, leading to non-uniform behavior across the zeolite structure. Characterizing and accounting for these heterogeneities in experimental and computational studies is an ongoing challenge.
Scaling up laboratory findings to industrial applications presents its own set of difficulties. The behavior of isopentane-zeolite systems observed in small-scale experiments may not directly translate to large-scale processes due to heat and mass transfer limitations, as well as the influence of impurities present in industrial feedstocks.
Lastly, the development of in-situ and operando characterization techniques for studying isopentane-zeolite interactions under realistic conditions remains an active area of research. Current methods often struggle to provide real-time, high-resolution data on molecular interactions and structural changes occurring within the zeolite pores during actual operating conditions.
Existing Isopentane-Zeolite Interaction Models
01 Zeolite structure modification with isopentane
Isopentane can be used to modify the structure of zeolites, potentially altering their pore size, shape, and distribution. This modification can lead to changes in the zeolite's adsorption properties, catalytic activity, and selectivity for specific molecules. The interaction between isopentane and zeolite structures may result in the formation of new active sites or the alteration of existing ones.- Zeolite structure modification with isopentane: Isopentane can be used to modify the structure of zeolites, potentially altering their pore size, shape, and distribution. This modification can lead to changes in the zeolite's adsorption properties, catalytic activity, and selectivity. The interaction between isopentane and zeolite structures may result in the formation of new active sites or the alteration of existing ones.
- Isopentane as a structure-directing agent in zeolite synthesis: Isopentane can be employed as a structure-directing agent during zeolite synthesis, influencing the formation of specific zeolite frameworks. This approach can lead to the creation of zeolites with unique structural features, such as particular channel systems or cage arrangements. The use of isopentane in this context may result in zeolites with enhanced performance in various applications.
- Separation and purification processes using isopentane and zeolites: The combination of isopentane and zeolites can be utilized in separation and purification processes. Zeolites can selectively adsorb isopentane from mixtures, or isopentane can be used to modify zeolites for improved separation of other compounds. This interaction can be applied in various industrial processes, such as the purification of hydrocarbons or the removal of contaminants from gas streams.
- Catalytic applications of isopentane-modified zeolites: Zeolites modified with isopentane can exhibit altered catalytic properties, potentially leading to improved performance in various reactions. The interaction between isopentane and zeolite structures may create new active sites or modify existing ones, resulting in changes in selectivity, activity, or stability of the catalyst. These modified zeolites can find applications in petrochemical processes, fine chemical synthesis, and environmental catalysis.
- Computational modeling of isopentane-zeolite interactions: Computational methods can be employed to study the structural implications of isopentane-zeolite interactions. These simulations can provide insights into the adsorption behavior, diffusion processes, and structural changes that occur when isopentane interacts with zeolite frameworks. Such modeling approaches can aid in the design of new zeolite materials with tailored properties for specific applications.
02 Isopentane as a structure-directing agent in zeolite synthesis
Isopentane can be employed as a structure-directing agent during zeolite synthesis, influencing the formation of specific zeolite frameworks. This approach can lead to the creation of zeolites with unique structural features, such as particular channel systems or cage arrangements. The use of isopentane in this context may result in zeolites with enhanced performance in applications such as catalysis or separation processes.Expand Specific Solutions03 Impact of isopentane on zeolite stability and thermal properties
The presence of isopentane within zeolite structures can affect their thermal stability and other physical properties. This interaction may lead to changes in the zeolite's melting point, thermal expansion coefficient, or resistance to thermal degradation. Understanding these effects is crucial for applications involving high-temperature processes or thermal cycling.Expand Specific Solutions04 Isopentane adsorption and diffusion in zeolite pores
The adsorption and diffusion behavior of isopentane within zeolite pores can provide insights into the structural implications of their interaction. Factors such as pore size, shape, and connectivity influence the adsorption capacity and diffusion rate of isopentane in zeolites. This information is valuable for designing zeolite-based materials for specific applications, such as gas separation or storage.Expand Specific Solutions05 Computational modeling of isopentane-zeolite interactions
Computational methods can be used to model and predict the structural implications of isopentane-zeolite interactions. These simulations can provide insights into the energetics of adsorption, the preferred locations of isopentane molecules within the zeolite framework, and potential structural changes induced by the presence of isopentane. Such modeling approaches can guide experimental work and aid in the design of tailored zeolite materials.Expand Specific Solutions
Key Players in Zeolite Catalyst Industry
The field of isopentane and zeolite interactions is in a mature stage of development, with significant research and industrial applications. The market size for zeolite-based technologies is substantial, driven by their widespread use in petrochemical processes. Technologically, the field is well-established, with companies like China Petroleum & Chemical Corp., UOP LLC, and SABIC leading in research and implementation. These industry giants, along with research institutions such as CNRS and CSIC, continue to refine and optimize zeolite-based processes. The involvement of major oil and chemical companies indicates the technology's commercial viability and ongoing importance in industrial applications, particularly in catalysis and separation processes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced zeolite-based catalysts for isopentane interactions. Their research focuses on optimizing the pore structure and acidity of zeolites to enhance isopentane conversion and selectivity. Sinopec has implemented a hierarchical pore system in their zeolites, combining micropores for shape selectivity with mesopores for improved mass transfer[1]. This approach has led to increased catalytic activity and longer catalyst lifetimes. Additionally, Sinopec has explored the use of modified ZSM-5 zeolites with tailored Si/Al ratios to control acidity and improve isopentane isomerization performance[2]. Their research also extends to the development of bimetallic catalysts supported on zeolites, which have shown enhanced stability and selectivity in isopentane conversion processes[3].
Strengths: Extensive research infrastructure, large-scale industrial application capabilities, and a strong focus on practical implementations. Weaknesses: Potential limitations in rapid adoption of cutting-edge academic research and international collaboration restrictions.
UOP LLC
Technical Solution: UOP LLC, a Honeywell company, has made significant advancements in isopentane and zeolite interactions for refining and petrochemical processes. Their proprietary UOP Penex™ process utilizes specially designed zeolite catalysts for isomerization of light naphtha, including isopentane[4]. The process employs a chlorided alumina catalyst with a platinum metal function, which operates at low temperatures and pressures, resulting in high octane gains and improved yields[5]. UOP has also developed zeolite-based adsorbents for the separation of normal and iso-paraffins, which is crucial for isopentane purification. Their latest generation of zeolite catalysts incorporates nano-sized particles and controlled mesoporosity to enhance diffusion and catalytic performance in isopentane conversion reactions[6].
Strengths: Strong industry presence, proven commercial technologies, and continuous innovation in catalyst design. Weaknesses: Potential dependency on proprietary technologies and higher implementation costs for clients.
Core Innovations in Zeolite Framework Design
Process for xylene and ethylbenzene isomerization using UZM-35hs
PatentActiveUS20110245566A1
Innovation
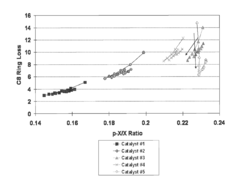
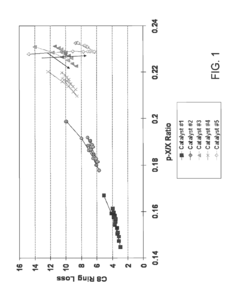
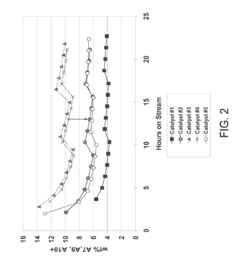
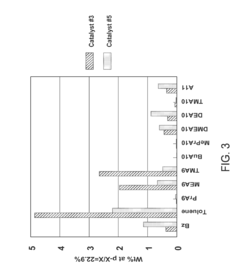
- The use of UZM-35 zeolite, a microporous aluminosilicate with a specific three-dimensional framework and pore geometry, which is synthesized using commercially available structure directing agents, providing high conversion rates and minimizing ring loss through its unique structure and Si/Al ratio, and can be used in various forms including spherical and extruded catalysts.
Method for the direct synthesis of iron-containing AEI-zeolite catalyst
PatentWO2017134006A1
Innovation
- A method for the direct synthesis of an iron-containing AEI zeolite catalyst with reduced alkali content, achieved by preparing a mixture with specific molar compositions and crystallizing it under controlled conditions, followed by removal of occluded organic structure directing agents and alkali metal ions, resulting in a highly stable and selective catalyst.
Environmental Impact of Zeolite Catalysis
The environmental impact of zeolite catalysis in the context of isopentane interactions is a critical aspect to consider in the development and application of this technology. Zeolites, as microporous aluminosilicate materials, have gained significant attention in catalytic processes due to their unique structural properties and high surface area. However, their use in industrial applications, particularly in the petrochemical industry, raises important environmental concerns.
One of the primary environmental benefits of zeolite catalysis is its potential to reduce energy consumption in various chemical processes. The shape-selective properties of zeolites allow for more efficient and targeted reactions, potentially lowering the overall energy requirements of industrial operations. This reduction in energy consumption can lead to decreased greenhouse gas emissions associated with power generation, contributing to global efforts to mitigate climate change.
Furthermore, zeolite catalysts often enable reactions to occur at lower temperatures and pressures compared to traditional catalysts. This not only reduces energy demands but also minimizes the risk of equipment failure and potential environmental hazards associated with high-pressure operations. The ability to conduct reactions under milder conditions can result in safer industrial processes with a lower environmental footprint.
However, the production and disposal of zeolite catalysts present environmental challenges. The synthesis of zeolites typically involves energy-intensive processes and the use of various chemicals, which can have negative environmental impacts if not properly managed. Additionally, the disposal of spent zeolite catalysts requires careful consideration to prevent soil and water contamination.
In the specific case of isopentane interactions with zeolites, the environmental impact is closely tied to the application in fuel production and refining processes. Isopentane is a key component in gasoline blending, and its efficient conversion using zeolite catalysts can lead to improved fuel quality and reduced emissions from vehicles. However, the potential for volatile organic compound (VOC) emissions during the catalytic process must be carefully monitored and controlled to prevent air pollution.
The structural implications of isopentane-zeolite interactions also play a role in the environmental impact. The pore size and structure of zeolites can be tailored to optimize the conversion of isopentane, potentially leading to more efficient use of raw materials and reduced waste generation. This structural optimization can contribute to a more sustainable and environmentally friendly production process.
As research in this field progresses, there is a growing focus on developing more environmentally benign zeolite synthesis methods and improving catalyst regeneration techniques. These advancements aim to reduce the environmental footprint of zeolite catalysis throughout its lifecycle, from production to disposal. Additionally, ongoing studies are exploring the potential of zeolites in environmental remediation applications, such as the removal of pollutants from water and air, further expanding their positive environmental impact.
One of the primary environmental benefits of zeolite catalysis is its potential to reduce energy consumption in various chemical processes. The shape-selective properties of zeolites allow for more efficient and targeted reactions, potentially lowering the overall energy requirements of industrial operations. This reduction in energy consumption can lead to decreased greenhouse gas emissions associated with power generation, contributing to global efforts to mitigate climate change.
Furthermore, zeolite catalysts often enable reactions to occur at lower temperatures and pressures compared to traditional catalysts. This not only reduces energy demands but also minimizes the risk of equipment failure and potential environmental hazards associated with high-pressure operations. The ability to conduct reactions under milder conditions can result in safer industrial processes with a lower environmental footprint.
However, the production and disposal of zeolite catalysts present environmental challenges. The synthesis of zeolites typically involves energy-intensive processes and the use of various chemicals, which can have negative environmental impacts if not properly managed. Additionally, the disposal of spent zeolite catalysts requires careful consideration to prevent soil and water contamination.
In the specific case of isopentane interactions with zeolites, the environmental impact is closely tied to the application in fuel production and refining processes. Isopentane is a key component in gasoline blending, and its efficient conversion using zeolite catalysts can lead to improved fuel quality and reduced emissions from vehicles. However, the potential for volatile organic compound (VOC) emissions during the catalytic process must be carefully monitored and controlled to prevent air pollution.
The structural implications of isopentane-zeolite interactions also play a role in the environmental impact. The pore size and structure of zeolites can be tailored to optimize the conversion of isopentane, potentially leading to more efficient use of raw materials and reduced waste generation. This structural optimization can contribute to a more sustainable and environmentally friendly production process.
As research in this field progresses, there is a growing focus on developing more environmentally benign zeolite synthesis methods and improving catalyst regeneration techniques. These advancements aim to reduce the environmental footprint of zeolite catalysis throughout its lifecycle, from production to disposal. Additionally, ongoing studies are exploring the potential of zeolites in environmental remediation applications, such as the removal of pollutants from water and air, further expanding their positive environmental impact.
Computational Methods for Interaction Studies
Computational methods have become indispensable tools for studying the interactions between isopentane and zeolites, offering valuable insights into the structural implications of these interactions. Molecular dynamics (MD) simulations stand out as a primary technique, allowing researchers to model the dynamic behavior of isopentane molecules within zeolite frameworks over time. These simulations can reveal crucial information about adsorption sites, diffusion pathways, and the influence of temperature and pressure on the system.
Density Functional Theory (DFT) calculations complement MD simulations by providing accurate electronic structure information. DFT methods are particularly useful for investigating the energetics of isopentane-zeolite interactions, including adsorption energies and binding preferences at specific sites within the zeolite structure. The combination of DFT and MD approaches enables a comprehensive understanding of both static and dynamic aspects of the interaction.
Monte Carlo (MC) simulations offer another powerful computational tool for studying isopentane-zeolite systems. Grand Canonical Monte Carlo (GCMC) simulations are especially relevant for investigating adsorption isotherms and the distribution of isopentane molecules within zeolite pores under various conditions. These simulations can predict equilibrium properties and provide insights into the thermodynamics of the adsorption process.
Advanced machine learning techniques, such as neural networks and genetic algorithms, are increasingly being applied to analyze and predict isopentane-zeolite interactions. These methods can process large datasets generated from experimental and computational studies, identifying patterns and correlations that may not be immediately apparent through traditional analysis methods.
Quantum mechanical/molecular mechanical (QM/MM) hybrid methods offer a balanced approach for studying localized interactions between isopentane and specific zeolite sites while considering the broader structural context. This technique is particularly useful for investigating catalytic processes or charge transfer phenomena at the molecular level.
Computational spectroscopy methods, including simulations of infrared, Raman, and NMR spectra, provide a crucial link between theoretical predictions and experimental observations. These techniques help validate computational models and offer detailed insights into the local environment of isopentane molecules within zeolite structures.
As computational power continues to increase, more sophisticated methods such as ab initio molecular dynamics and path integral molecular dynamics are becoming feasible for studying isopentane-zeolite systems. These advanced techniques promise to provide even more accurate and detailed information about the structural implications of isopentane-zeolite interactions, potentially leading to breakthroughs in catalyst design and hydrocarbon processing technologies.
Density Functional Theory (DFT) calculations complement MD simulations by providing accurate electronic structure information. DFT methods are particularly useful for investigating the energetics of isopentane-zeolite interactions, including adsorption energies and binding preferences at specific sites within the zeolite structure. The combination of DFT and MD approaches enables a comprehensive understanding of both static and dynamic aspects of the interaction.
Monte Carlo (MC) simulations offer another powerful computational tool for studying isopentane-zeolite systems. Grand Canonical Monte Carlo (GCMC) simulations are especially relevant for investigating adsorption isotherms and the distribution of isopentane molecules within zeolite pores under various conditions. These simulations can predict equilibrium properties and provide insights into the thermodynamics of the adsorption process.
Advanced machine learning techniques, such as neural networks and genetic algorithms, are increasingly being applied to analyze and predict isopentane-zeolite interactions. These methods can process large datasets generated from experimental and computational studies, identifying patterns and correlations that may not be immediately apparent through traditional analysis methods.
Quantum mechanical/molecular mechanical (QM/MM) hybrid methods offer a balanced approach for studying localized interactions between isopentane and specific zeolite sites while considering the broader structural context. This technique is particularly useful for investigating catalytic processes or charge transfer phenomena at the molecular level.
Computational spectroscopy methods, including simulations of infrared, Raman, and NMR spectra, provide a crucial link between theoretical predictions and experimental observations. These techniques help validate computational models and offer detailed insights into the local environment of isopentane molecules within zeolite structures.
As computational power continues to increase, more sophisticated methods such as ab initio molecular dynamics and path integral molecular dynamics are becoming feasible for studying isopentane-zeolite systems. These advanced techniques promise to provide even more accurate and detailed information about the structural implications of isopentane-zeolite interactions, potentially leading to breakthroughs in catalyst design and hydrocarbon processing technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!