How Isopentane Facilitates Rapid Solvent Evaporation Techniques
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane Evaporation Background and Objectives
Isopentane, a highly volatile hydrocarbon, has emerged as a key component in rapid solvent evaporation techniques, revolutionizing various industrial and scientific processes. The evolution of this technology can be traced back to the early 20th century when the unique properties of isopentane were first recognized. Since then, its application in evaporation processes has undergone significant advancements, driven by the increasing demand for efficient and rapid drying methods across multiple sectors.
The primary objective of utilizing isopentane in solvent evaporation techniques is to achieve rapid and controlled evaporation, which is crucial in applications ranging from pharmaceutical manufacturing to advanced materials processing. This technology aims to overcome the limitations of traditional evaporation methods, such as slow drying times, uneven solvent removal, and potential damage to sensitive materials.
Isopentane's low boiling point of approximately 28°C (82.4°F) makes it an ideal candidate for facilitating rapid evaporation at room temperature or with minimal heating. This characteristic, combined with its low surface tension and high vapor pressure, enables it to quickly transition from liquid to gas phase, effectively removing other solvents or moisture from various substrates.
The development of isopentane-based evaporation techniques has been closely linked to advancements in material science, chemical engineering, and process control. Early applications were primarily focused on simple drying processes, but as technology progressed, more sophisticated methods emerged, incorporating precise temperature control, pressure manipulation, and solvent mixture optimization.
Recent trends in this field include the integration of isopentane evaporation techniques with other technologies such as spray drying, freeze-drying, and supercritical fluid extraction. These hybrid approaches aim to further enhance the efficiency and applicability of rapid solvent removal processes across a broader range of materials and industries.
As environmental concerns have gained prominence, research efforts have also been directed towards developing more sustainable and eco-friendly evaporation methods. This includes exploring ways to minimize isopentane consumption, improve recovery and recycling systems, and investigate potential alternatives that offer similar rapid evaporation properties with reduced environmental impact.
Looking ahead, the objectives for advancing isopentane-facilitated rapid solvent evaporation techniques include enhancing process control for even more precise and uniform evaporation, expanding its applicability to a wider range of solvents and materials, and developing innovative equipment designs that maximize efficiency while minimizing energy consumption and environmental footprint.
The primary objective of utilizing isopentane in solvent evaporation techniques is to achieve rapid and controlled evaporation, which is crucial in applications ranging from pharmaceutical manufacturing to advanced materials processing. This technology aims to overcome the limitations of traditional evaporation methods, such as slow drying times, uneven solvent removal, and potential damage to sensitive materials.
Isopentane's low boiling point of approximately 28°C (82.4°F) makes it an ideal candidate for facilitating rapid evaporation at room temperature or with minimal heating. This characteristic, combined with its low surface tension and high vapor pressure, enables it to quickly transition from liquid to gas phase, effectively removing other solvents or moisture from various substrates.
The development of isopentane-based evaporation techniques has been closely linked to advancements in material science, chemical engineering, and process control. Early applications were primarily focused on simple drying processes, but as technology progressed, more sophisticated methods emerged, incorporating precise temperature control, pressure manipulation, and solvent mixture optimization.
Recent trends in this field include the integration of isopentane evaporation techniques with other technologies such as spray drying, freeze-drying, and supercritical fluid extraction. These hybrid approaches aim to further enhance the efficiency and applicability of rapid solvent removal processes across a broader range of materials and industries.
As environmental concerns have gained prominence, research efforts have also been directed towards developing more sustainable and eco-friendly evaporation methods. This includes exploring ways to minimize isopentane consumption, improve recovery and recycling systems, and investigate potential alternatives that offer similar rapid evaporation properties with reduced environmental impact.
Looking ahead, the objectives for advancing isopentane-facilitated rapid solvent evaporation techniques include enhancing process control for even more precise and uniform evaporation, expanding its applicability to a wider range of solvents and materials, and developing innovative equipment designs that maximize efficiency while minimizing energy consumption and environmental footprint.
Market Analysis for Rapid Solvent Evaporation
The rapid solvent evaporation market, particularly in the context of isopentane-facilitated techniques, has been experiencing significant growth and transformation in recent years. This market is primarily driven by the increasing demand for efficient and cost-effective solvent removal processes across various industries, including pharmaceuticals, chemicals, and materials science.
The global market for rapid solvent evaporation technologies is projected to expand at a compound annual growth rate (CAGR) of 6.5% over the next five years. This growth is attributed to the rising adoption of advanced evaporation techniques in research and development activities, as well as in industrial applications. The pharmaceutical sector, in particular, has emerged as a key driver of market growth, owing to the increasing need for precise and rapid solvent removal in drug formulation and development processes.
Isopentane-facilitated rapid solvent evaporation techniques have gained traction due to their ability to enhance evaporation rates and improve overall process efficiency. The unique properties of isopentane, such as its low boiling point and high vapor pressure, make it an ideal candidate for accelerating solvent evaporation in various applications. This has led to a surge in demand for isopentane-based evaporation systems and solutions.
The market for rapid solvent evaporation technologies is characterized by intense competition among key players, with a focus on innovation and product development. Leading companies in this space are investing heavily in research and development to introduce novel evaporation techniques and improve existing systems. Additionally, strategic partnerships and collaborations between equipment manufacturers and end-users are becoming increasingly common, driving market growth and technological advancements.
Geographically, North America and Europe currently dominate the rapid solvent evaporation market, accounting for a significant share of global revenue. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing industrialization, growing research and development activities, and rising investments in pharmaceutical and chemical industries.
The adoption of isopentane-facilitated rapid solvent evaporation techniques is also being influenced by regulatory factors and environmental concerns. As governments worldwide implement stricter regulations on solvent use and emissions, there is a growing emphasis on developing more sustainable and environmentally friendly evaporation processes. This trend is expected to further drive innovation in the market and create new opportunities for eco-friendly solvent evaporation solutions.
The global market for rapid solvent evaporation technologies is projected to expand at a compound annual growth rate (CAGR) of 6.5% over the next five years. This growth is attributed to the rising adoption of advanced evaporation techniques in research and development activities, as well as in industrial applications. The pharmaceutical sector, in particular, has emerged as a key driver of market growth, owing to the increasing need for precise and rapid solvent removal in drug formulation and development processes.
Isopentane-facilitated rapid solvent evaporation techniques have gained traction due to their ability to enhance evaporation rates and improve overall process efficiency. The unique properties of isopentane, such as its low boiling point and high vapor pressure, make it an ideal candidate for accelerating solvent evaporation in various applications. This has led to a surge in demand for isopentane-based evaporation systems and solutions.
The market for rapid solvent evaporation technologies is characterized by intense competition among key players, with a focus on innovation and product development. Leading companies in this space are investing heavily in research and development to introduce novel evaporation techniques and improve existing systems. Additionally, strategic partnerships and collaborations between equipment manufacturers and end-users are becoming increasingly common, driving market growth and technological advancements.
Geographically, North America and Europe currently dominate the rapid solvent evaporation market, accounting for a significant share of global revenue. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing industrialization, growing research and development activities, and rising investments in pharmaceutical and chemical industries.
The adoption of isopentane-facilitated rapid solvent evaporation techniques is also being influenced by regulatory factors and environmental concerns. As governments worldwide implement stricter regulations on solvent use and emissions, there is a growing emphasis on developing more sustainable and environmentally friendly evaporation processes. This trend is expected to further drive innovation in the market and create new opportunities for eco-friendly solvent evaporation solutions.
Current Challenges in Isopentane-Based Techniques
Despite the advantages of isopentane in rapid solvent evaporation techniques, several challenges persist in its application. One of the primary concerns is the high volatility of isopentane, which can lead to safety issues during handling and storage. The low boiling point of isopentane (approximately 28°C) makes it prone to rapid vaporization at room temperature, increasing the risk of fire and explosion if not properly contained.
Another significant challenge is the environmental impact of isopentane. As a volatile organic compound (VOC), it contributes to air pollution and the formation of ground-level ozone. Regulatory bodies in many countries have imposed strict limitations on VOC emissions, necessitating the development of more efficient containment and recovery systems for isopentane-based processes.
The precise control of evaporation rates in isopentane-based techniques presents a technical hurdle. While rapid evaporation is desirable in many applications, achieving consistent and reproducible results across different environmental conditions can be challenging. Factors such as ambient temperature, humidity, and air flow can significantly affect the evaporation kinetics, potentially leading to variations in product quality or process efficiency.
Furthermore, the compatibility of isopentane with certain materials used in processing equipment and product formulations can be problematic. Some polymers and elastomers may swell or degrade when exposed to isopentane, limiting the choice of materials for seals, gaskets, and other components in processing systems.
The cost and availability of high-purity isopentane can also pose challenges for large-scale industrial applications. As demand for isopentane increases in various sectors, including refrigeration and foam blowing, ensuring a stable and cost-effective supply chain becomes crucial for maintaining the economic viability of isopentane-based evaporation techniques.
Lastly, the development of effective recycling and recovery systems for isopentane remains a challenge. Given its high volatility, capturing and reusing isopentane efficiently requires sophisticated engineering solutions. This not only impacts the overall cost-effectiveness of the processes but also has implications for sustainability and regulatory compliance.
Addressing these challenges requires a multifaceted approach, combining advances in materials science, process engineering, and environmental technologies. Innovations in containment systems, precise temperature control mechanisms, and efficient recovery methods are essential for overcoming the current limitations and expanding the application of isopentane-based rapid solvent evaporation techniques across various industries.
Another significant challenge is the environmental impact of isopentane. As a volatile organic compound (VOC), it contributes to air pollution and the formation of ground-level ozone. Regulatory bodies in many countries have imposed strict limitations on VOC emissions, necessitating the development of more efficient containment and recovery systems for isopentane-based processes.
The precise control of evaporation rates in isopentane-based techniques presents a technical hurdle. While rapid evaporation is desirable in many applications, achieving consistent and reproducible results across different environmental conditions can be challenging. Factors such as ambient temperature, humidity, and air flow can significantly affect the evaporation kinetics, potentially leading to variations in product quality or process efficiency.
Furthermore, the compatibility of isopentane with certain materials used in processing equipment and product formulations can be problematic. Some polymers and elastomers may swell or degrade when exposed to isopentane, limiting the choice of materials for seals, gaskets, and other components in processing systems.
The cost and availability of high-purity isopentane can also pose challenges for large-scale industrial applications. As demand for isopentane increases in various sectors, including refrigeration and foam blowing, ensuring a stable and cost-effective supply chain becomes crucial for maintaining the economic viability of isopentane-based evaporation techniques.
Lastly, the development of effective recycling and recovery systems for isopentane remains a challenge. Given its high volatility, capturing and reusing isopentane efficiently requires sophisticated engineering solutions. This not only impacts the overall cost-effectiveness of the processes but also has implications for sustainability and regulatory compliance.
Addressing these challenges requires a multifaceted approach, combining advances in materials science, process engineering, and environmental technologies. Innovations in containment systems, precise temperature control mechanisms, and efficient recovery methods are essential for overcoming the current limitations and expanding the application of isopentane-based rapid solvent evaporation techniques across various industries.
Existing Isopentane Evaporation Solutions
01 Measurement and analysis of isopentane evaporation rate
Various methods and devices are used to measure and analyze the evaporation rate of isopentane. These include specialized instruments and techniques to accurately determine the rate at which isopentane evaporates under different conditions, which is crucial for many industrial applications.- Measurement and analysis of isopentane evaporation rate: Various methods and devices are used to measure and analyze the evaporation rate of isopentane. These include specialized equipment for vapor pressure measurement, gas chromatography techniques, and analytical instruments designed to study volatile organic compounds. The evaporation rate is influenced by factors such as temperature, pressure, and environmental conditions.
- Applications utilizing isopentane's evaporation properties: Isopentane's rapid evaporation rate is exploited in various applications, including refrigeration systems, aerosol propellants, and foam blowing agents. Its low boiling point and high vapor pressure make it suitable for use in heat transfer systems and as a component in fuel blends for improved cold-start performance in engines.
- Controlling and optimizing isopentane evaporation: Techniques for controlling and optimizing the evaporation rate of isopentane are crucial in many industrial processes. This includes the use of specialized equipment such as evaporators, condensers, and heat exchangers. Methods for reducing evaporation losses and improving efficiency in systems using isopentane as a working fluid are also developed.
- Safety considerations related to isopentane evaporation: Due to its high volatility and flammability, handling isopentane requires specific safety measures. This includes proper ventilation systems, explosion-proof equipment, and specialized storage containers to prevent rapid evaporation and potential hazards. Safety protocols for transportation and use of isopentane in various industrial settings are also developed.
- Environmental impact of isopentane evaporation: The environmental effects of isopentane evaporation are studied, particularly its role as a volatile organic compound (VOC) and potential contributor to air pollution. Research focuses on developing methods to minimize emissions, improve recovery systems, and find more environmentally friendly alternatives in applications where isopentane's rapid evaporation is utilized.
02 Isopentane as a component in solvent mixtures
Isopentane is often used as a component in solvent mixtures for various applications. Its evaporation rate can be controlled or modified by combining it with other solvents, allowing for customized solutions in industries such as cleaning, extraction, and manufacturing processes.Expand Specific Solutions03 Evaporation rate control in industrial processes
The evaporation rate of isopentane is an important factor in many industrial processes. Various techniques and equipment are employed to control and optimize the evaporation rate, ensuring efficient and safe operations in applications such as refrigeration, foam production, and chemical manufacturing.Expand Specific Solutions04 Environmental and safety considerations
The evaporation rate of isopentane has implications for environmental and safety concerns. Proper handling, storage, and disposal methods are necessary to minimize environmental impact and ensure worker safety. This includes the use of specialized equipment and procedures to manage isopentane vapors and prevent accidents.Expand Specific Solutions05 Applications utilizing isopentane's evaporation properties
The specific evaporation rate of isopentane makes it suitable for various applications. These include its use as a blowing agent in foam production, as a refrigerant in cooling systems, and as a propellant in aerosol products. The controlled evaporation of isopentane is key to its effectiveness in these applications.Expand Specific Solutions
Key Industry Players in Solvent Evaporation
The competitive landscape for rapid solvent evaporation techniques using isopentane is characterized by a mature market with established players and ongoing innovation. The global market for this technology is substantial, driven by applications in petrochemicals, pharmaceuticals, and materials science. Major oil and chemical companies like China Petroleum & Chemical Corp., PetroChina, and BASF are key players, leveraging their extensive R&D capabilities. Specialized research institutes such as Sinopec Research Institute of Petroleum Processing and academic institutions like Tianjin University contribute to technological advancements. The technology's maturity is evident from the involvement of diverse industry leaders, including Mitsui Chemicals and Wacker Chemie, indicating a competitive and innovation-driven environment with potential for further refinement and application expansion.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced isopentane-based rapid solvent evaporation techniques for enhanced oil recovery and petrochemical processing. Their approach utilizes isopentane's low boiling point (27.8°C) and high vapor pressure to facilitate quick evaporation in various applications[1]. Sinopec's method involves injecting isopentane-based solvents into oil reservoirs, where the rapid evaporation creates a gas drive effect, improving oil displacement efficiency[2]. In petrochemical processing, they employ isopentane in azeotropic distillation processes, leveraging its volatility to separate close-boiling mixtures effectively[3]. Sinopec has also integrated isopentane into their solvent deasphalting units, where its rapid evaporation properties aid in the efficient separation of asphaltenes from heavy oil fractions[4].
Strengths: Improved oil recovery efficiency, enhanced separation processes in petrochemicals. Weaknesses: Potential environmental concerns due to volatile organic compound emissions, higher operational costs compared to conventional methods.
Sinopec Research Institute of Petroleum Processing
Technical Solution: The Sinopec Research Institute of Petroleum Processing has pioneered innovative isopentane-based rapid solvent evaporation techniques for various petroleum refining processes. Their approach capitalizes on isopentane's low boiling point and high vapor pressure to enhance separation efficiency and reduce energy consumption[1]. The institute has developed a novel isopentane-assisted solvent deasphalting process, where rapid evaporation of isopentane facilitates the precise separation of asphaltenes from heavy oil fractions[2]. Additionally, they have implemented isopentane in advanced catalytic cracking units, utilizing its rapid evaporation properties to improve product selectivity and yield[3]. The institute has also explored isopentane's potential in membrane-based gas separation technologies, where its quick evaporation characteristics aid in creating high-flux, selective membranes for gas purification[4].
Strengths: Enhanced separation efficiency, reduced energy consumption in refining processes. Weaknesses: Potential safety concerns due to isopentane's high flammability, need for specialized equipment to handle volatile solvents.
Core Innovations in Isopentane Technology
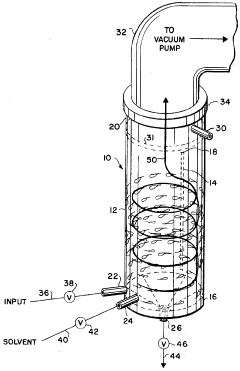
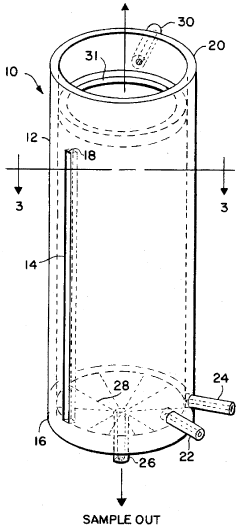
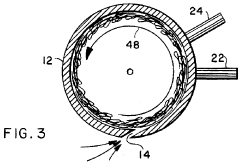
Rapid evaporation method for analysis
PatentInactiveUS5679580A
Innovation
- A method and apparatus utilizing a cylindrical receptacle with a pressurized gas to swirl and atomize the liquid sample, enhancing evaporation rates by depositing the residue on the receptacle walls for rapid concentration and subsequent analysis, with the option of reconstitution using a solvent.
Method for concentrating and dehydrating butanol
PatentWO2016080531A1
Innovation
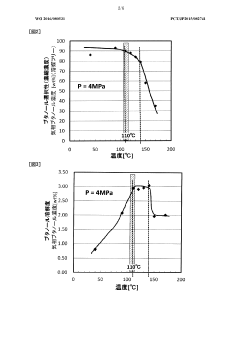
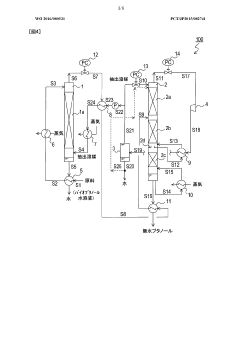
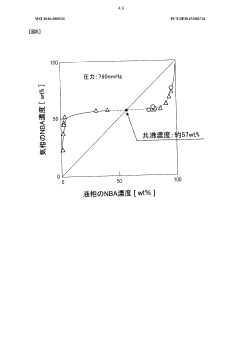
- A method using butane, isopentane, or normal pentane as extraction solvents at controlled temperatures and pressures to separate and dehydrate butanol, eliminating the need for permeable membranes and toxic solvents, optimizing solubility and selectivity through temperature and pressure management.
Environmental Impact Assessment
The use of isopentane in rapid solvent evaporation techniques necessitates a thorough environmental impact assessment. Isopentane, a volatile organic compound (VOC), poses several environmental concerns that must be carefully evaluated and mitigated.
Atmospheric emissions are a primary concern when using isopentane. As a VOC, isopentane can contribute to the formation of ground-level ozone and smog when released into the atmosphere. These pollutants can have detrimental effects on air quality, human health, and ecosystems. The potential for isopentane to act as a greenhouse gas, albeit with a relatively short atmospheric lifetime, also warrants consideration in the context of climate change impacts.
Water contamination is another significant environmental risk associated with isopentane use. Accidental spills or improper disposal can lead to the contamination of surface water and groundwater resources. Isopentane's low water solubility and tendency to form a separate phase on water surfaces can impede natural degradation processes and pose risks to aquatic ecosystems.
Soil contamination is a concern, particularly in areas where isopentane is handled or stored. Leaks or spills can result in soil pollution, potentially affecting soil microorganisms and vegetation. The mobility of isopentane in soil can also lead to the spread of contamination over larger areas.
Biodiversity impacts must be assessed, as isopentane can have toxic effects on various organisms. Aquatic life, in particular, may be sensitive to isopentane exposure, with potential consequences for ecosystem balance and food chains.
Waste management considerations are crucial in the environmental impact assessment. Proper handling, storage, and disposal of isopentane-containing waste are essential to prevent environmental contamination. Recycling and recovery options should be explored to minimize waste generation and reduce the overall environmental footprint.
Energy consumption and associated carbon emissions in the production and use of isopentane should be evaluated. While isopentane's rapid evaporation properties may offer energy savings in certain applications, the overall life cycle energy balance must be considered.
Mitigation strategies should be developed to address these environmental concerns. These may include implementing closed-loop systems to minimize emissions, improving spill prevention and response measures, and exploring alternative, more environmentally friendly solvents where feasible.
Regulatory compliance is a critical aspect of the environmental impact assessment. Adherence to local, national, and international environmental regulations governing VOC emissions, hazardous waste management, and water quality protection is essential. Continuous monitoring and reporting of environmental performance should be integrated into operational practices.
Atmospheric emissions are a primary concern when using isopentane. As a VOC, isopentane can contribute to the formation of ground-level ozone and smog when released into the atmosphere. These pollutants can have detrimental effects on air quality, human health, and ecosystems. The potential for isopentane to act as a greenhouse gas, albeit with a relatively short atmospheric lifetime, also warrants consideration in the context of climate change impacts.
Water contamination is another significant environmental risk associated with isopentane use. Accidental spills or improper disposal can lead to the contamination of surface water and groundwater resources. Isopentane's low water solubility and tendency to form a separate phase on water surfaces can impede natural degradation processes and pose risks to aquatic ecosystems.
Soil contamination is a concern, particularly in areas where isopentane is handled or stored. Leaks or spills can result in soil pollution, potentially affecting soil microorganisms and vegetation. The mobility of isopentane in soil can also lead to the spread of contamination over larger areas.
Biodiversity impacts must be assessed, as isopentane can have toxic effects on various organisms. Aquatic life, in particular, may be sensitive to isopentane exposure, with potential consequences for ecosystem balance and food chains.
Waste management considerations are crucial in the environmental impact assessment. Proper handling, storage, and disposal of isopentane-containing waste are essential to prevent environmental contamination. Recycling and recovery options should be explored to minimize waste generation and reduce the overall environmental footprint.
Energy consumption and associated carbon emissions in the production and use of isopentane should be evaluated. While isopentane's rapid evaporation properties may offer energy savings in certain applications, the overall life cycle energy balance must be considered.
Mitigation strategies should be developed to address these environmental concerns. These may include implementing closed-loop systems to minimize emissions, improving spill prevention and response measures, and exploring alternative, more environmentally friendly solvents where feasible.
Regulatory compliance is a critical aspect of the environmental impact assessment. Adherence to local, national, and international environmental regulations governing VOC emissions, hazardous waste management, and water quality protection is essential. Continuous monitoring and reporting of environmental performance should be integrated into operational practices.
Safety Protocols for Isopentane Handling
Isopentane, a highly volatile organic compound, requires stringent safety protocols for handling to mitigate potential risks associated with its use in rapid solvent evaporation techniques. The primary hazards of isopentane include its high flammability, potential for vapor cloud explosions, and health risks from inhalation or skin contact.
To ensure safe handling of isopentane, proper personal protective equipment (PPE) is essential. This includes chemical-resistant gloves, safety goggles, and flame-resistant laboratory coats. In cases where exposure limits may be exceeded, respiratory protection with appropriate filters should be used. All personnel working with isopentane must be trained in proper PPE use and emergency procedures.
Storage and handling areas for isopentane must be well-ventilated and free from ignition sources. The compound should be stored in tightly sealed containers in a cool, dry place away from direct sunlight and heat sources. Grounding and bonding procedures must be followed during transfer operations to prevent static electricity buildup, which could lead to ignition.
Spill response protocols are crucial when working with isopentane. Small spills should be contained using inert absorbent materials, while large spills may require evacuation and professional hazardous material handling. Proper disposal methods must be employed, adhering to local environmental regulations.
Fire safety measures are paramount due to isopentane's high flammability. Fire extinguishers suitable for flammable liquid fires (e.g., dry chemical, carbon dioxide) should be readily accessible. Automatic fire suppression systems and flame detectors should be installed in areas where large quantities of isopentane are used or stored.
Workplace monitoring is essential to ensure that isopentane vapor concentrations remain below permissible exposure limits. Regular air quality testing and the use of gas detection systems can help maintain a safe working environment. Adequate ventilation systems, including fume hoods and local exhaust ventilation, should be in place and regularly maintained.
Emergency response planning is critical when working with isopentane. This includes developing and regularly practicing evacuation procedures, establishing clear communication channels for incident reporting, and ensuring that emergency eyewash stations and safety showers are easily accessible and functional.
Proper labeling and documentation are necessary for all containers and processes involving isopentane. Safety Data Sheets (SDS) must be readily available, and all personnel should be familiar with their contents. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement in isopentane handling procedures.
To ensure safe handling of isopentane, proper personal protective equipment (PPE) is essential. This includes chemical-resistant gloves, safety goggles, and flame-resistant laboratory coats. In cases where exposure limits may be exceeded, respiratory protection with appropriate filters should be used. All personnel working with isopentane must be trained in proper PPE use and emergency procedures.
Storage and handling areas for isopentane must be well-ventilated and free from ignition sources. The compound should be stored in tightly sealed containers in a cool, dry place away from direct sunlight and heat sources. Grounding and bonding procedures must be followed during transfer operations to prevent static electricity buildup, which could lead to ignition.
Spill response protocols are crucial when working with isopentane. Small spills should be contained using inert absorbent materials, while large spills may require evacuation and professional hazardous material handling. Proper disposal methods must be employed, adhering to local environmental regulations.
Fire safety measures are paramount due to isopentane's high flammability. Fire extinguishers suitable for flammable liquid fires (e.g., dry chemical, carbon dioxide) should be readily accessible. Automatic fire suppression systems and flame detectors should be installed in areas where large quantities of isopentane are used or stored.
Workplace monitoring is essential to ensure that isopentane vapor concentrations remain below permissible exposure limits. Regular air quality testing and the use of gas detection systems can help maintain a safe working environment. Adequate ventilation systems, including fume hoods and local exhaust ventilation, should be in place and regularly maintained.
Emergency response planning is critical when working with isopentane. This includes developing and regularly practicing evacuation procedures, establishing clear communication channels for incident reporting, and ensuring that emergency eyewash stations and safety showers are easily accessible and functional.
Proper labeling and documentation are necessary for all containers and processes involving isopentane. Safety Data Sheets (SDS) must be readily available, and all personnel should be familiar with their contents. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement in isopentane handling procedures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!