How isotonic solutions are used in plasma volume expanders
AUG 19, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions Background and Objectives
Isotonic solutions have played a crucial role in medical science, particularly in the field of plasma volume expansion. These solutions, which have the same osmotic pressure as blood plasma, have been instrumental in maintaining fluid balance within the body. The development of isotonic solutions for medical use can be traced back to the early 20th century, with significant advancements occurring in the 1930s and 1940s.
The primary objective of using isotonic solutions in plasma volume expanders is to restore and maintain adequate blood volume in patients experiencing hypovolemia, shock, or severe fluid loss. These conditions can arise from various causes, including trauma, burns, severe infections, or major surgeries. By effectively expanding plasma volume, isotonic solutions help improve tissue perfusion, maintain organ function, and ultimately save lives in critical care settings.
Over the years, the composition and formulation of isotonic solutions have evolved to better mimic the electrolyte balance of human plasma. This evolution has led to the development of various types of isotonic solutions, each designed to address specific clinical needs. Common examples include normal saline (0.9% sodium chloride), Ringer's lactate solution, and balanced electrolyte solutions.
The use of isotonic solutions as plasma volume expanders has become a standard practice in emergency medicine, intensive care, and perioperative management. Their effectiveness lies in their ability to rapidly increase intravascular volume without causing significant shifts in fluid between body compartments. This property makes them particularly valuable in acute care situations where quick volume resuscitation is essential.
Recent technological advancements have focused on improving the safety and efficacy of isotonic solutions. Research efforts have been directed towards developing solutions that not only expand plasma volume but also provide additional therapeutic benefits. These include solutions with enhanced oxygen-carrying capacity, improved microcirculatory flow, and reduced inflammatory responses.
As we look towards the future, the field of isotonic solutions in plasma volume expansion continues to evolve. Emerging trends include the development of synthetic colloids, the exploration of novel electrolyte compositions, and the integration of bioengineering principles to create "smart" fluids that can adapt to the patient's physiological state. These advancements aim to further optimize fluid resuscitation strategies and improve patient outcomes in critical care scenarios.
The primary objective of using isotonic solutions in plasma volume expanders is to restore and maintain adequate blood volume in patients experiencing hypovolemia, shock, or severe fluid loss. These conditions can arise from various causes, including trauma, burns, severe infections, or major surgeries. By effectively expanding plasma volume, isotonic solutions help improve tissue perfusion, maintain organ function, and ultimately save lives in critical care settings.
Over the years, the composition and formulation of isotonic solutions have evolved to better mimic the electrolyte balance of human plasma. This evolution has led to the development of various types of isotonic solutions, each designed to address specific clinical needs. Common examples include normal saline (0.9% sodium chloride), Ringer's lactate solution, and balanced electrolyte solutions.
The use of isotonic solutions as plasma volume expanders has become a standard practice in emergency medicine, intensive care, and perioperative management. Their effectiveness lies in their ability to rapidly increase intravascular volume without causing significant shifts in fluid between body compartments. This property makes them particularly valuable in acute care situations where quick volume resuscitation is essential.
Recent technological advancements have focused on improving the safety and efficacy of isotonic solutions. Research efforts have been directed towards developing solutions that not only expand plasma volume but also provide additional therapeutic benefits. These include solutions with enhanced oxygen-carrying capacity, improved microcirculatory flow, and reduced inflammatory responses.
As we look towards the future, the field of isotonic solutions in plasma volume expansion continues to evolve. Emerging trends include the development of synthetic colloids, the exploration of novel electrolyte compositions, and the integration of bioengineering principles to create "smart" fluids that can adapt to the patient's physiological state. These advancements aim to further optimize fluid resuscitation strategies and improve patient outcomes in critical care scenarios.
Market Analysis for Plasma Volume Expanders
The plasma volume expander market has shown significant growth in recent years, driven by an increasing number of surgical procedures, rising incidence of trauma cases, and growing awareness about the importance of maintaining proper blood volume. The global market for plasma volume expanders is expected to continue its upward trajectory, with a compound annual growth rate projected to be in the high single digits over the next five years.
Isotonic solutions, particularly crystalloid solutions like normal saline and Ringer's lactate, dominate the market due to their cost-effectiveness and wide availability. These solutions account for a substantial portion of the overall plasma volume expander market share, with some estimates suggesting they represent over 60% of the total market volume.
The demand for plasma volume expanders is particularly high in regions with advanced healthcare infrastructure, such as North America and Europe. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth in market demand, driven by improving healthcare facilities and increasing healthcare expenditure.
The market is segmented based on product type, with crystalloids, colloids, and combinations forming the main categories. While crystalloids remain the most widely used, there is a growing interest in colloid solutions, especially in critical care settings. This shift is driven by the perceived benefits of colloids in certain clinical scenarios, despite ongoing debates about their efficacy and safety profiles.
Hospital-based usage continues to be the primary driver of market demand, accounting for the largest share of plasma volume expander consumption. Emergency departments, operating rooms, and intensive care units are the key areas of application within hospital settings. However, there is a growing trend towards the use of plasma volume expanders in ambulatory surgical centers and other outpatient facilities, which is expected to contribute to market expansion in the coming years.
The competitive landscape of the plasma volume expander market is characterized by the presence of several large pharmaceutical companies and a number of smaller, specialized manufacturers. Key players are focusing on product innovation, particularly in developing solutions with improved safety profiles and longer shelf lives. Additionally, there is an increasing emphasis on developing plasma volume expanders tailored for specific patient populations or clinical scenarios, such as pediatric patients or those with specific comorbidities.
Regulatory factors play a significant role in shaping the market dynamics. Stringent approval processes for new products and ongoing safety monitoring of existing solutions influence market growth and product development strategies. The market is also affected by healthcare policies and reimbursement scenarios, which vary significantly across different regions and healthcare systems.
Isotonic solutions, particularly crystalloid solutions like normal saline and Ringer's lactate, dominate the market due to their cost-effectiveness and wide availability. These solutions account for a substantial portion of the overall plasma volume expander market share, with some estimates suggesting they represent over 60% of the total market volume.
The demand for plasma volume expanders is particularly high in regions with advanced healthcare infrastructure, such as North America and Europe. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth in market demand, driven by improving healthcare facilities and increasing healthcare expenditure.
The market is segmented based on product type, with crystalloids, colloids, and combinations forming the main categories. While crystalloids remain the most widely used, there is a growing interest in colloid solutions, especially in critical care settings. This shift is driven by the perceived benefits of colloids in certain clinical scenarios, despite ongoing debates about their efficacy and safety profiles.
Hospital-based usage continues to be the primary driver of market demand, accounting for the largest share of plasma volume expander consumption. Emergency departments, operating rooms, and intensive care units are the key areas of application within hospital settings. However, there is a growing trend towards the use of plasma volume expanders in ambulatory surgical centers and other outpatient facilities, which is expected to contribute to market expansion in the coming years.
The competitive landscape of the plasma volume expander market is characterized by the presence of several large pharmaceutical companies and a number of smaller, specialized manufacturers. Key players are focusing on product innovation, particularly in developing solutions with improved safety profiles and longer shelf lives. Additionally, there is an increasing emphasis on developing plasma volume expanders tailored for specific patient populations or clinical scenarios, such as pediatric patients or those with specific comorbidities.
Regulatory factors play a significant role in shaping the market dynamics. Stringent approval processes for new products and ongoing safety monitoring of existing solutions influence market growth and product development strategies. The market is also affected by healthcare policies and reimbursement scenarios, which vary significantly across different regions and healthcare systems.
Current Challenges in Plasma Volume Expansion
Plasma volume expansion remains a critical intervention in various medical scenarios, including hypovolemic shock, severe burns, and major surgeries. However, the field faces several significant challenges that hinder optimal patient outcomes and limit the effectiveness of current solutions.
One of the primary challenges is the short intravascular retention time of many plasma volume expanders. Crystalloid solutions, while widely used, rapidly distribute throughout the extracellular space, leading to a transient effect on plasma volume. This necessitates frequent administration and large volumes, potentially causing fluid overload and tissue edema.
The risk of adverse reactions poses another substantial challenge. Synthetic colloids, such as hydroxyethyl starch (HES), have been associated with increased risks of acute kidney injury and coagulopathy. These safety concerns have led to restrictions on their use in many countries, limiting the available options for plasma volume expansion.
Balancing the oncotic pressure is a complex task that current solutions struggle to address adequately. Natural colloids like albumin, while effective, are expensive and have limited availability. Developing cost-effective alternatives that can maintain appropriate oncotic pressure without compromising safety remains a significant challenge.
The heterogeneity of patient populations further complicates plasma volume expansion strategies. Different patient groups, such as those with pre-existing renal impairment, cardiovascular disease, or sepsis, may respond differently to various expanders. Tailoring solutions to specific patient needs while maintaining broad applicability is an ongoing challenge.
Environmental concerns and sustainability issues are emerging challenges in the field. The production and disposal of large volumes of synthetic solutions have ecological implications that are increasingly coming under scrutiny. Developing eco-friendly alternatives without compromising efficacy is becoming a priority.
Lastly, the lack of standardized protocols for plasma volume expansion across different clinical scenarios creates inconsistencies in patient care. The optimal timing, volume, and choice of expander can vary widely depending on the specific condition and individual patient factors. Establishing evidence-based guidelines that can be universally applied while allowing for personalized approaches remains a significant challenge in the field of plasma volume expansion.
One of the primary challenges is the short intravascular retention time of many plasma volume expanders. Crystalloid solutions, while widely used, rapidly distribute throughout the extracellular space, leading to a transient effect on plasma volume. This necessitates frequent administration and large volumes, potentially causing fluid overload and tissue edema.
The risk of adverse reactions poses another substantial challenge. Synthetic colloids, such as hydroxyethyl starch (HES), have been associated with increased risks of acute kidney injury and coagulopathy. These safety concerns have led to restrictions on their use in many countries, limiting the available options for plasma volume expansion.
Balancing the oncotic pressure is a complex task that current solutions struggle to address adequately. Natural colloids like albumin, while effective, are expensive and have limited availability. Developing cost-effective alternatives that can maintain appropriate oncotic pressure without compromising safety remains a significant challenge.
The heterogeneity of patient populations further complicates plasma volume expansion strategies. Different patient groups, such as those with pre-existing renal impairment, cardiovascular disease, or sepsis, may respond differently to various expanders. Tailoring solutions to specific patient needs while maintaining broad applicability is an ongoing challenge.
Environmental concerns and sustainability issues are emerging challenges in the field. The production and disposal of large volumes of synthetic solutions have ecological implications that are increasingly coming under scrutiny. Developing eco-friendly alternatives without compromising efficacy is becoming a priority.
Lastly, the lack of standardized protocols for plasma volume expansion across different clinical scenarios creates inconsistencies in patient care. The optimal timing, volume, and choice of expander can vary widely depending on the specific condition and individual patient factors. Establishing evidence-based guidelines that can be universally applied while allowing for personalized approaches remains a significant challenge in the field of plasma volume expansion.
Existing Isotonic Solution Formulations
01 Isotonic solutions for maintaining plasma volume
Isotonic solutions are used to maintain plasma volume in various medical situations. These solutions have the same osmotic pressure as blood plasma, preventing fluid shifts between intracellular and extracellular compartments. They are crucial in treating dehydration, blood loss, and maintaining fluid balance during medical procedures.- Isotonic solutions for maintaining plasma volume: Isotonic solutions are used to maintain plasma volume in various medical situations. These solutions have the same osmotic pressure as blood plasma, preventing fluid shifts between intracellular and extracellular compartments. They are crucial in treating dehydration, blood loss, and maintaining fluid balance during medical procedures.
- Composition of isotonic solutions for plasma volume expansion: The composition of isotonic solutions for plasma volume expansion typically includes electrolytes such as sodium, chloride, and sometimes potassium. These solutions may also contain glucose or other osmotically active substances to match the osmolarity of blood. The precise formulation is designed to mimic the electrolyte composition of plasma, ensuring optimal fluid retention and distribution.
- Methods for measuring and monitoring plasma volume: Various techniques are employed to measure and monitor plasma volume, including dilution methods using tracers, bioimpedance analysis, and advanced imaging techniques. These methods are essential for assessing the effectiveness of isotonic solutions in maintaining or expanding plasma volume, particularly in critical care settings.
- Applications of isotonic solutions in medical treatments: Isotonic solutions have wide-ranging applications in medical treatments, including intravenous fluid therapy, perioperative fluid management, and treatment of shock. They are also used in dialysis, as irrigation solutions in surgical procedures, and as a base for delivering medications. The choice of solution depends on the specific clinical scenario and patient needs.
- Development of novel isotonic solutions for specific medical conditions: Research is ongoing to develop specialized isotonic solutions for specific medical conditions. These may include solutions with added components such as antioxidants, energy substrates, or specific ions to address particular physiological needs. The goal is to optimize plasma volume maintenance while providing additional therapeutic benefits tailored to specific patient populations or medical conditions.
02 Composition of isotonic solutions for plasma volume expansion
The composition of isotonic solutions for plasma volume expansion typically includes electrolytes such as sodium, chloride, and sometimes potassium. These solutions may also contain glucose or other osmotically active substances to match the osmolarity of blood. The precise formulation is designed to mimic the electrolyte composition of plasma, ensuring optimal fluid retention and distribution.Expand Specific Solutions03 Methods for measuring and monitoring plasma volume
Various techniques are employed to measure and monitor plasma volume, including dilution methods, radioisotope labeling, and bioimpedance analysis. These methods help in assessing the effectiveness of isotonic solutions in maintaining or expanding plasma volume, allowing for precise fluid management in clinical settings.Expand Specific Solutions04 Applications of isotonic solutions in medical treatments
Isotonic solutions have wide-ranging applications in medical treatments, including intravenous fluid therapy, perioperative fluid management, and treatment of shock. They are also used in dialysis, as irrigation solutions in surgical procedures, and as vehicles for drug delivery. The choice of solution depends on the specific clinical scenario and patient needs.Expand Specific Solutions05 Development of novel isotonic solutions for plasma volume maintenance
Research is ongoing to develop improved isotonic solutions for maintaining plasma volume. This includes the exploration of balanced electrolyte solutions, the incorporation of colloids for prolonged volume expansion, and the addition of specific compounds to enhance oxygen delivery or provide other therapeutic benefits while maintaining isotonicity.Expand Specific Solutions
Key Players in Plasma Expander Industry
The market for isotonic solutions in plasma volume expanders is in a mature stage, with a stable global market size estimated in the billions of dollars. The technology is well-established, with major players like Novo Nordisk A/S, Novartis AG, and Fresenius Kabi Deutschland GmbH leading the field. These companies have extensive experience in developing and manufacturing pharmaceutical products, including plasma volume expanders. The competitive landscape is characterized by ongoing research and development efforts to improve efficacy and safety profiles, as well as to explore new applications in critical care and emergency medicine. Smaller biotechnology firms like Vivacelle Bio, Inc. are also entering the market with innovative approaches, potentially disrupting the established players.
Fresenius Kabi Deutschland GmbH
Technical Solution: Fresenius Kabi is a leading provider of plasma volume expanders, offering a comprehensive portfolio of isotonic solutions. Their products include both crystalloid and colloid-based expanders, such as balanced electrolyte solutions and hydroxyethyl starch (HES) preparations[7]. Fresenius Kabi's isotonic solutions are designed to rapidly expand intravascular volume while maintaining physiological electrolyte balance. The company has conducted extensive research on the efficacy and safety of their plasma volume expanders, particularly in critical care and perioperative settings[8]. Fresenius Kabi also focuses on developing innovative packaging and delivery systems to enhance the usability and safety of their isotonic solutions in clinical practice[9].
Strengths: Comprehensive product portfolio, strong research and development capabilities, global market presence. Weaknesses: Ongoing debates about the safety of certain colloid solutions (e.g., HES) in specific patient populations.
Sichuan Kelun Pharmaceutical Co., Ltd.
Technical Solution: Sichuan Kelun Pharmaceutical has developed a range of isotonic solutions for use as plasma volume expanders. Their products include balanced electrolyte solutions and colloid-based expanders[4]. The company's isotonic solutions are formulated to closely match the osmolality and electrolyte composition of human plasma, minimizing the risk of fluid shifts between compartments[5]. Kelun's plasma volume expanders are used in various clinical settings, including the treatment of hypovolemia, shock, and burn injuries. The company has invested in advanced manufacturing processes to ensure high-quality, sterile production of large-volume parenteral solutions[6].
Strengths: Wide range of isotonic solutions, established manufacturing capabilities for large-volume parenterals. Weaknesses: Limited global market presence compared to multinational pharmaceutical companies.
Core Innovations in Isotonic Expanders
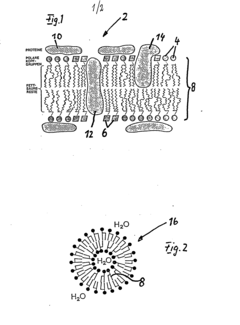
Lipid vesicles containing drugs, method of preparation and introduction in the living creatures' bodies thereof, and liberation of the drugs contained in the lipid vesicles
PatentInactiveEP0244557A1
Innovation
- Focused sonication of a target volume temporarily opens lipid vesicles or liposomes, allowing controlled and localized release of pharmaceuticals, enhanced by surface-active modifications for specific attachment and replenishment via the bloodstream, and supported by methods like hyperthermia or gas injection to facilitate targeted delivery.
Starch based plasma extenders prepn.
PatentInactiveES8700674A3
Innovation
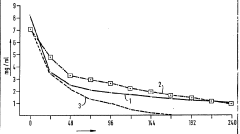
- A process involving enzymatic hydrolysis of starch rich in amylopectin, followed by partial etherization, using enzymes like β-amylase and pullulanase, and controlled etherization with alkylene oxides, followed by purification to achieve rapid concentration decrease and short organic half-periods without allergic reactions.
Regulatory Framework for Plasma Expanders
The regulatory framework for plasma expanders is a critical aspect of their development, approval, and use in clinical settings. In the United States, the Food and Drug Administration (FDA) oversees the regulation of plasma expanders as medical devices under the Center for Biologics Evaluation and Research (CBER). These products are classified as Class III devices, requiring the highest level of regulatory control due to their potential risks and importance in medical interventions.
The approval process for plasma expanders involves rigorous clinical trials to demonstrate safety and efficacy. Manufacturers must submit a Premarket Approval (PMA) application, which includes comprehensive data on the product's composition, manufacturing process, and clinical performance. The FDA evaluates this information to ensure that the benefits of the plasma expander outweigh any potential risks.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) in the European Union and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan have similar stringent requirements for plasma expanders. These agencies often collaborate to harmonize regulatory standards, facilitating global development and distribution of these critical medical products.
Quality control and post-market surveillance are integral components of the regulatory framework. Manufacturers are required to implement robust quality management systems and conduct ongoing monitoring of their products' performance and safety profiles. Any adverse events or product defects must be promptly reported to the relevant regulatory authorities.
The regulatory landscape for plasma expanders also addresses specific formulation requirements, including the use of isotonic solutions. Regulations typically specify acceptable osmolality ranges and electrolyte compositions to ensure physiological compatibility and minimize potential side effects. These specifications are crucial for maintaining the safety and efficacy of plasma expanders in clinical use.
Labeling and packaging regulations for plasma expanders are designed to provide healthcare professionals with clear and accurate information about the product's composition, indications, contraindications, and proper administration. This includes specific guidelines on storage conditions, shelf life, and handling instructions to maintain product integrity.
As medical knowledge and technology advance, regulatory frameworks for plasma expanders continue to evolve. Agencies periodically review and update their guidelines to incorporate new scientific evidence and address emerging safety concerns. This dynamic regulatory environment ensures that plasma expanders remain safe, effective, and aligned with current medical standards.
The approval process for plasma expanders involves rigorous clinical trials to demonstrate safety and efficacy. Manufacturers must submit a Premarket Approval (PMA) application, which includes comprehensive data on the product's composition, manufacturing process, and clinical performance. The FDA evaluates this information to ensure that the benefits of the plasma expander outweigh any potential risks.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) in the European Union and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan have similar stringent requirements for plasma expanders. These agencies often collaborate to harmonize regulatory standards, facilitating global development and distribution of these critical medical products.
Quality control and post-market surveillance are integral components of the regulatory framework. Manufacturers are required to implement robust quality management systems and conduct ongoing monitoring of their products' performance and safety profiles. Any adverse events or product defects must be promptly reported to the relevant regulatory authorities.
The regulatory landscape for plasma expanders also addresses specific formulation requirements, including the use of isotonic solutions. Regulations typically specify acceptable osmolality ranges and electrolyte compositions to ensure physiological compatibility and minimize potential side effects. These specifications are crucial for maintaining the safety and efficacy of plasma expanders in clinical use.
Labeling and packaging regulations for plasma expanders are designed to provide healthcare professionals with clear and accurate information about the product's composition, indications, contraindications, and proper administration. This includes specific guidelines on storage conditions, shelf life, and handling instructions to maintain product integrity.
As medical knowledge and technology advance, regulatory frameworks for plasma expanders continue to evolve. Agencies periodically review and update their guidelines to incorporate new scientific evidence and address emerging safety concerns. This dynamic regulatory environment ensures that plasma expanders remain safe, effective, and aligned with current medical standards.
Safety and Efficacy Considerations
The safety and efficacy of isotonic solutions used as plasma volume expanders are critical considerations in clinical practice. These solutions, typically containing electrolytes in concentrations similar to human plasma, are designed to maintain intravascular volume without causing significant fluid shifts between compartments.
Safety considerations primarily focus on the potential adverse effects of rapid infusion and excessive administration. Overuse of isotonic solutions can lead to fluid overload, potentially causing pulmonary edema, especially in patients with compromised cardiac or renal function. Careful monitoring of fluid balance, hemodynamic parameters, and electrolyte levels is essential to prevent these complications.
Another safety concern is the risk of electrolyte imbalances. While isotonic solutions aim to mimic plasma composition, prolonged or excessive use may still alter electrolyte concentrations, particularly sodium and chloride levels. This can potentially lead to hyperchloremic metabolic acidosis, which may have adverse effects on organ function and patient outcomes.
Efficacy considerations revolve around the ability of isotonic solutions to effectively expand plasma volume and improve tissue perfusion. The duration of volume expansion is a key factor, as these solutions tend to redistribute into the interstitial space over time. This necessitates ongoing assessment of the patient's fluid status and may require repeated administration to maintain the desired effect.
The choice of specific isotonic solution can impact efficacy. For instance, balanced solutions like Ringer's lactate may offer advantages over normal saline in certain clinical scenarios, potentially reducing the risk of hyperchloremic acidosis and providing a more physiological electrolyte profile.
Patient-specific factors also influence the efficacy of plasma volume expansion. Age, underlying medical conditions, and the cause of volume depletion all play roles in determining the optimal approach to fluid resuscitation. Tailoring the choice and volume of isotonic solutions to individual patient needs is crucial for maximizing efficacy while minimizing risks.
Monitoring tools and protocols are essential for ensuring both safety and efficacy. Regular assessment of vital signs, urine output, and laboratory parameters helps guide fluid therapy and allows for timely adjustments. Advanced hemodynamic monitoring techniques may be employed in critical care settings to optimize fluid management and assess the response to volume expansion.
Safety considerations primarily focus on the potential adverse effects of rapid infusion and excessive administration. Overuse of isotonic solutions can lead to fluid overload, potentially causing pulmonary edema, especially in patients with compromised cardiac or renal function. Careful monitoring of fluid balance, hemodynamic parameters, and electrolyte levels is essential to prevent these complications.
Another safety concern is the risk of electrolyte imbalances. While isotonic solutions aim to mimic plasma composition, prolonged or excessive use may still alter electrolyte concentrations, particularly sodium and chloride levels. This can potentially lead to hyperchloremic metabolic acidosis, which may have adverse effects on organ function and patient outcomes.
Efficacy considerations revolve around the ability of isotonic solutions to effectively expand plasma volume and improve tissue perfusion. The duration of volume expansion is a key factor, as these solutions tend to redistribute into the interstitial space over time. This necessitates ongoing assessment of the patient's fluid status and may require repeated administration to maintain the desired effect.
The choice of specific isotonic solution can impact efficacy. For instance, balanced solutions like Ringer's lactate may offer advantages over normal saline in certain clinical scenarios, potentially reducing the risk of hyperchloremic acidosis and providing a more physiological electrolyte profile.
Patient-specific factors also influence the efficacy of plasma volume expansion. Age, underlying medical conditions, and the cause of volume depletion all play roles in determining the optimal approach to fluid resuscitation. Tailoring the choice and volume of isotonic solutions to individual patient needs is crucial for maximizing efficacy while minimizing risks.
Monitoring tools and protocols are essential for ensuring both safety and efficacy. Regular assessment of vital signs, urine output, and laboratory parameters helps guide fluid therapy and allows for timely adjustments. Advanced hemodynamic monitoring techniques may be employed in critical care settings to optimize fluid management and assess the response to volume expansion.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!