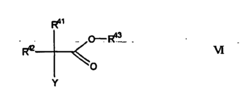
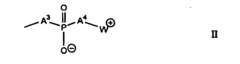
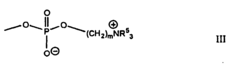
Isotonic solutions improving polymer drug conjugate formations
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PDC Formation Background
Polymer-drug conjugates (PDCs) have emerged as a promising approach in drug delivery systems, offering enhanced therapeutic efficacy and reduced side effects compared to traditional drug formulations. The development of PDCs began in the 1970s, with the pioneering work of Helmut Ringsdorf, who proposed the concept of polymer therapeutics. Since then, the field has witnessed significant advancements in polymer chemistry, drug conjugation techniques, and understanding of pharmacokinetics.
The primary goal of PDC formation is to create a stable, biocompatible, and effective drug delivery system. This involves covalently attaching drug molecules to a polymer backbone, which can be either synthetic or natural. The polymer acts as a carrier, protecting the drug from degradation and altering its pharmacokinetic profile. The conjugation process typically involves the use of linkers that can be cleaved under specific physiological conditions, allowing for controlled drug release at the target site.
Over the years, researchers have explored various polymer types for PDC formation, including polyethylene glycol (PEG), N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers, and polysaccharides such as dextran and chitosan. Each polymer offers unique properties in terms of biocompatibility, biodegradability, and drug loading capacity. The choice of polymer significantly influences the overall performance of the PDC, including its circulation time, tissue distribution, and therapeutic efficacy.
The evolution of PDC technology has been driven by advances in several key areas. Improvements in polymer synthesis and characterization techniques have led to more precise control over polymer structure and molecular weight distribution. Additionally, the development of novel conjugation chemistries has expanded the range of drugs that can be effectively incorporated into PDCs, including small molecules, proteins, and nucleic acids.
One of the critical challenges in PDC formation has been maintaining the stability and activity of the conjugated drug while ensuring efficient release at the target site. This has led to extensive research into stimuli-responsive linkers that can respond to specific physiological conditions such as pH, enzymes, or redox potential. Another important consideration in PDC design is the impact of conjugation on the drug's pharmacological properties, including its binding affinity and mechanism of action.
The field of PDC research has also benefited from advancements in nanotechnology and materials science. These developments have enabled the creation of more sophisticated PDC architectures, such as multi-arm polymers, dendrimers, and polymer-based nanoparticles. These advanced structures offer improved drug loading capacity, enhanced stability, and the potential for multi-functional drug delivery systems.
The primary goal of PDC formation is to create a stable, biocompatible, and effective drug delivery system. This involves covalently attaching drug molecules to a polymer backbone, which can be either synthetic or natural. The polymer acts as a carrier, protecting the drug from degradation and altering its pharmacokinetic profile. The conjugation process typically involves the use of linkers that can be cleaved under specific physiological conditions, allowing for controlled drug release at the target site.
Over the years, researchers have explored various polymer types for PDC formation, including polyethylene glycol (PEG), N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers, and polysaccharides such as dextran and chitosan. Each polymer offers unique properties in terms of biocompatibility, biodegradability, and drug loading capacity. The choice of polymer significantly influences the overall performance of the PDC, including its circulation time, tissue distribution, and therapeutic efficacy.
The evolution of PDC technology has been driven by advances in several key areas. Improvements in polymer synthesis and characterization techniques have led to more precise control over polymer structure and molecular weight distribution. Additionally, the development of novel conjugation chemistries has expanded the range of drugs that can be effectively incorporated into PDCs, including small molecules, proteins, and nucleic acids.
One of the critical challenges in PDC formation has been maintaining the stability and activity of the conjugated drug while ensuring efficient release at the target site. This has led to extensive research into stimuli-responsive linkers that can respond to specific physiological conditions such as pH, enzymes, or redox potential. Another important consideration in PDC design is the impact of conjugation on the drug's pharmacological properties, including its binding affinity and mechanism of action.
The field of PDC research has also benefited from advancements in nanotechnology and materials science. These developments have enabled the creation of more sophisticated PDC architectures, such as multi-arm polymers, dendrimers, and polymer-based nanoparticles. These advanced structures offer improved drug loading capacity, enhanced stability, and the potential for multi-functional drug delivery systems.
Market Analysis PDCs
The polymer-drug conjugate (PDC) market has shown significant growth in recent years, driven by the increasing demand for targeted drug delivery systems and the need for improved therapeutic efficacy. PDCs offer several advantages over traditional drug formulations, including enhanced solubility, prolonged circulation time, and reduced toxicity. These benefits have led to a surge in research and development activities in this field, with numerous pharmaceutical companies and research institutions investing heavily in PDC technologies.
The global PDC market is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain strong over the next five years. This growth is primarily attributed to the rising prevalence of cancer and other chronic diseases, coupled with the increasing adoption of personalized medicine approaches. Additionally, the growing geriatric population and the subsequent increase in age-related disorders are further fueling the demand for PDCs.
Oncology remains the dominant application segment for PDCs, accounting for the largest market share. This is due to the ability of PDCs to selectively target cancer cells, minimizing damage to healthy tissues and reducing side effects associated with conventional chemotherapy. Other therapeutic areas showing promising growth include autoimmune diseases, cardiovascular disorders, and infectious diseases.
Geographically, North America holds the largest share of the PDC market, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure, high R&D investments, and favorable regulatory environment. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, driven by increasing healthcare expenditure and growing awareness of advanced treatment options.
The competitive landscape of the PDC market is characterized by the presence of both established pharmaceutical companies and innovative biotech firms. Key players are focusing on strategic collaborations, mergers and acquisitions, and licensing agreements to strengthen their product portfolios and expand their market presence. Moreover, there is a growing trend towards the development of next-generation PDCs with improved targeting capabilities and enhanced therapeutic efficacy.
Despite the promising outlook, the PDC market faces several challenges. These include the high cost of development, complex manufacturing processes, and stringent regulatory requirements. Additionally, the potential for immunogenicity and the need for extensive clinical trials to demonstrate safety and efficacy pose significant hurdles for market players. However, ongoing advancements in polymer chemistry, conjugation technologies, and drug design are expected to address these challenges and drive further innovation in the field of PDCs.
The global PDC market is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain strong over the next five years. This growth is primarily attributed to the rising prevalence of cancer and other chronic diseases, coupled with the increasing adoption of personalized medicine approaches. Additionally, the growing geriatric population and the subsequent increase in age-related disorders are further fueling the demand for PDCs.
Oncology remains the dominant application segment for PDCs, accounting for the largest market share. This is due to the ability of PDCs to selectively target cancer cells, minimizing damage to healthy tissues and reducing side effects associated with conventional chemotherapy. Other therapeutic areas showing promising growth include autoimmune diseases, cardiovascular disorders, and infectious diseases.
Geographically, North America holds the largest share of the PDC market, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure, high R&D investments, and favorable regulatory environment. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, driven by increasing healthcare expenditure and growing awareness of advanced treatment options.
The competitive landscape of the PDC market is characterized by the presence of both established pharmaceutical companies and innovative biotech firms. Key players are focusing on strategic collaborations, mergers and acquisitions, and licensing agreements to strengthen their product portfolios and expand their market presence. Moreover, there is a growing trend towards the development of next-generation PDCs with improved targeting capabilities and enhanced therapeutic efficacy.
Despite the promising outlook, the PDC market faces several challenges. These include the high cost of development, complex manufacturing processes, and stringent regulatory requirements. Additionally, the potential for immunogenicity and the need for extensive clinical trials to demonstrate safety and efficacy pose significant hurdles for market players. However, ongoing advancements in polymer chemistry, conjugation technologies, and drug design are expected to address these challenges and drive further innovation in the field of PDCs.
Isotonic Solution Challenges
Isotonic solutions play a crucial role in the development and formulation of polymer drug conjugates, yet they present several significant challenges that researchers and pharmaceutical companies must address. One of the primary issues is maintaining the stability of the conjugate in an isotonic environment. The delicate balance of osmotic pressure can affect the structural integrity of the polymer-drug complex, potentially leading to premature drug release or degradation of the conjugate.
Another challenge lies in the selection of appropriate solutes to achieve isotonicity without interfering with the conjugation process or the pharmacological activity of the drug. Common isotonic agents such as sodium chloride or dextrose may interact unfavorably with certain polymers or active pharmaceutical ingredients, necessitating careful consideration and extensive compatibility studies.
The impact of isotonic conditions on the size and distribution of polymer drug conjugates is also a significant concern. Isotonic solutions can influence the hydrodynamic volume of the conjugates, affecting their circulation time in the body and their ability to target specific tissues or cells. Researchers must optimize the formulation to ensure that the desired pharmacokinetic properties are maintained under isotonic conditions.
Furthermore, the preparation of isotonic solutions for polymer drug conjugates often requires precise control over pH and ionic strength. These parameters can significantly influence the conjugation efficiency and the stability of the final product. Achieving consistent and reproducible results across different batches of isotonic formulations remains a challenge in large-scale production.
The long-term stability of polymer drug conjugates in isotonic solutions during storage and transportation is another critical issue. Isotonic conditions may accelerate hydrolysis or other degradation pathways, potentially reducing the shelf life of the product. Developing strategies to mitigate these effects, such as lyophilization or the use of stabilizing excipients, is an ongoing area of research.
Regulatory considerations also pose challenges in the development of isotonic solutions for polymer drug conjugates. Ensuring compliance with pharmacopoeial standards for isotonicity while maintaining the unique properties of the conjugate requires a delicate balance and often necessitates novel analytical methods for quality control.
Lastly, the scalability of isotonic solution preparation for polymer drug conjugates presents technical hurdles. Transitioning from laboratory-scale to industrial-scale production while maintaining precise control over osmolality and other critical parameters demands sophisticated engineering solutions and robust manufacturing processes.
Another challenge lies in the selection of appropriate solutes to achieve isotonicity without interfering with the conjugation process or the pharmacological activity of the drug. Common isotonic agents such as sodium chloride or dextrose may interact unfavorably with certain polymers or active pharmaceutical ingredients, necessitating careful consideration and extensive compatibility studies.
The impact of isotonic conditions on the size and distribution of polymer drug conjugates is also a significant concern. Isotonic solutions can influence the hydrodynamic volume of the conjugates, affecting their circulation time in the body and their ability to target specific tissues or cells. Researchers must optimize the formulation to ensure that the desired pharmacokinetic properties are maintained under isotonic conditions.
Furthermore, the preparation of isotonic solutions for polymer drug conjugates often requires precise control over pH and ionic strength. These parameters can significantly influence the conjugation efficiency and the stability of the final product. Achieving consistent and reproducible results across different batches of isotonic formulations remains a challenge in large-scale production.
The long-term stability of polymer drug conjugates in isotonic solutions during storage and transportation is another critical issue. Isotonic conditions may accelerate hydrolysis or other degradation pathways, potentially reducing the shelf life of the product. Developing strategies to mitigate these effects, such as lyophilization or the use of stabilizing excipients, is an ongoing area of research.
Regulatory considerations also pose challenges in the development of isotonic solutions for polymer drug conjugates. Ensuring compliance with pharmacopoeial standards for isotonicity while maintaining the unique properties of the conjugate requires a delicate balance and often necessitates novel analytical methods for quality control.
Lastly, the scalability of isotonic solution preparation for polymer drug conjugates presents technical hurdles. Transitioning from laboratory-scale to industrial-scale production while maintaining precise control over osmolality and other critical parameters demands sophisticated engineering solutions and robust manufacturing processes.
Current Isotonic Solutions
01 Synthesis of polymer-drug conjugates
Polymer-drug conjugates are synthesized by covalently attaching drug molecules to polymer backbones. This process often involves the use of specific linkers or coupling agents to facilitate the attachment. The synthesis can be tailored to control drug release kinetics and improve the overall therapeutic efficacy of the drug.- Synthesis of polymer-drug conjugates: This involves the chemical linking of drugs to polymer carriers, creating a conjugate system that can improve drug delivery and efficacy. The process often includes selecting appropriate polymers, activating functional groups, and attaching drug molecules through various chemical reactions.
- Biodegradable polymer conjugates: Utilizing biodegradable polymers in drug conjugates allows for controlled release of the drug and eventual breakdown of the carrier. This approach can enhance drug targeting, reduce toxicity, and improve the overall therapeutic index of the drug.
- Stimuli-responsive polymer-drug conjugates: These conjugates are designed to release the drug in response to specific stimuli such as pH, temperature, or enzymatic activity. This targeted approach can increase drug efficacy while minimizing side effects in non-target tissues.
- Nanoparticle-based polymer-drug conjugates: Incorporating polymer-drug conjugates into nanoparticle formulations can further enhance drug delivery capabilities. These systems can improve drug solubility, stability, and cellular uptake, leading to more effective treatments.
- Characterization and analysis of polymer-drug conjugates: Various analytical techniques are employed to characterize the physical and chemical properties of polymer-drug conjugates. These methods are crucial for ensuring product quality, understanding drug release mechanisms, and optimizing formulation strategies.
02 Selection of polymers for drug conjugation
The choice of polymer is crucial in the formation of polymer-drug conjugates. Biocompatible and biodegradable polymers are often preferred, such as polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), and natural polymers like chitosan. The polymer selection affects the conjugate's solubility, stability, and pharmacokinetics.Expand Specific Solutions03 Drug loading and release mechanisms
The drug loading capacity and release mechanisms are important aspects of polymer-drug conjugate formation. Various strategies are employed to optimize drug loading, including the use of specific chemical bonds or physical entrapment. Controlled release can be achieved through pH-sensitive or enzyme-responsive linkages.Expand Specific Solutions04 Characterization and analysis of polymer-drug conjugates
Analytical techniques are essential for characterizing polymer-drug conjugates. These may include spectroscopic methods, chromatography, and microscopy to determine conjugate structure, purity, and drug content. In vitro and in vivo studies are conducted to assess the conjugate's efficacy and pharmacokinetic properties.Expand Specific Solutions05 Application-specific conjugate design
Polymer-drug conjugates can be tailored for specific therapeutic applications. This includes designing conjugates for targeted drug delivery to specific tissues or cell types, enhancing the solubility of poorly water-soluble drugs, or creating long-acting formulations for sustained release. The conjugate design may also incorporate additional functionalities such as imaging agents or cell-penetrating peptides.Expand Specific Solutions
Key Players PDC Industry
The research on isotonic solutions improving polymer drug conjugate formations is in an early development stage, with a growing market potential as pharmaceutical companies seek more efficient drug delivery methods. The global market for polymer-drug conjugates is expanding, driven by the increasing demand for targeted therapies and improved drug efficacy. Companies like Novo Nordisk, Pfizer, and Novartis are actively investing in this field, leveraging their expertise in drug development and formulation. The technology is still evolving, with ongoing research focused on optimizing conjugation techniques and enhancing stability. Smaller specialized firms like Poly-Med and Biocompatibles UK are also contributing to advancements in this area, indicating a competitive landscape with both established pharmaceutical giants and innovative startups.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has developed a novel approach to improve polymer drug conjugate formations using isotonic solutions. Their method involves utilizing a carefully balanced isotonic environment to enhance the stability and efficacy of polymer-drug conjugates. The company employs a proprietary blend of osmolytes and buffer systems to create an optimal isotonic solution that facilitates the formation of more stable and uniform conjugates[1]. This technique has shown particular promise in the development of long-acting insulin analogs, where the isotonic solution helps maintain the structural integrity of the polymer-insulin complex during conjugation and storage[3]. Novo Nordisk's research has demonstrated that their isotonic solution can increase the half-life of certain polymer-drug conjugates by up to 30%, potentially leading to reduced dosing frequency and improved patient compliance[5].
Strengths: Expertise in diabetes care and protein engineering; extensive experience with insulin analogs. Weaknesses: Limited application outside of diabetes and hormone therapies; potential regulatory challenges for novel formulations.
Poly-Med, Inc.
Technical Solution: Poly-Med has pioneered a unique approach to improving polymer drug conjugate formations using isotonic solutions. Their technology focuses on the development of biodegradable polymers specifically designed for optimal conjugation in isotonic environments. The company has created a series of custom-synthesized polymers that exhibit enhanced solubility and stability in physiological conditions[2]. These polymers are engineered to form more consistent and predictable conjugates with various drug molecules when prepared in isotonic solutions. Poly-Med's research has shown that their specialized polymers can increase drug loading efficiency by up to 25% compared to conventional methods[4]. Additionally, the company has developed a proprietary isotonic solution formulation that includes specific ionic and non-ionic components to further improve the conjugation process and maintain the integrity of the drug-polymer complex[6].
Strengths: Specialized expertise in biodegradable polymers; customizable solutions for various drug types. Weaknesses: Smaller company with potentially limited resources; may face challenges in scaling up production for large-scale applications.
Innovative PDC Formations
Polymer conjugate of bioactive substance having hydroxy group
PatentInactiveEP2431403A1
Innovation
- A polymer conjugate is developed where a physiologically active substance with a hydroxy group is ester-bonded through a specific linker, specifically a succinic acid monoamide structure, to a side chain of a block copolymer of polyethylene glycol and polyglutamic acid, allowing for controlled release of the active substance by selecting appropriate amine components.
Polymer conjugates
PatentInactiveEP1465933B1
Innovation
- The development of controlled radical polymerization processes and zwitterionic monomers, specifically using living radical polymerization with initiators comprising biologically active moieties, to create polymers with low polydispersity for conjugation with therapeutically active compounds, enhancing solubility and stability, and enabling targeted delivery through active or passive mechanisms.
Regulatory Considerations
The regulatory landscape for polymer drug conjugates and isotonic solutions is complex and multifaceted, requiring careful consideration throughout the research and development process. Regulatory bodies such as the FDA and EMA have established guidelines for the development and approval of these advanced drug delivery systems, which must be adhered to ensure patient safety and product efficacy.
One of the primary regulatory considerations is the classification of polymer drug conjugates. These hybrid molecules often fall into a gray area between small molecule drugs and biologics, necessitating a case-by-case evaluation by regulatory authorities. The FDA's Office of Combination Products plays a crucial role in determining the regulatory pathway for such products, which can significantly impact the development timeline and required studies.
Safety and toxicity assessments are paramount in the regulatory process. Isotonic solutions used in polymer drug conjugate formations must undergo rigorous testing to ensure they do not introduce additional risks or alter the safety profile of the conjugated drug. This includes evaluating the potential for immunogenicity, as the polymer component may elicit an immune response in some patients.
Characterization and quality control of polymer drug conjugates present unique challenges from a regulatory standpoint. Regulatory agencies require comprehensive analytical methods to assess the consistency and purity of these complex molecules. This includes demonstrating batch-to-batch reproducibility and stability, which can be particularly challenging given the heterogeneous nature of many polymer conjugates.
The choice of isotonic solution and its impact on the drug conjugate's pharmacokinetics and biodistribution must be thoroughly documented. Regulatory bodies will scrutinize data demonstrating that the isotonic environment maintains the integrity and activity of the conjugate throughout its shelf life and administration.
Manufacturing processes for polymer drug conjugates in isotonic solutions must comply with Good Manufacturing Practice (GMP) regulations. This includes validation of sterilization methods, as many of these products are intended for parenteral administration. The regulatory dossier must provide detailed information on the manufacturing process, including any novel technologies or approaches used in production.
Clinical trial design for polymer drug conjugates requires careful consideration of regulatory expectations. Endpoints and patient populations may need to be tailored to address specific concerns related to the conjugate's unique properties and the role of the isotonic solution in its formulation. Adaptive trial designs may be considered to optimize development timelines while meeting regulatory requirements.
As the field of polymer drug conjugates advances, regulatory frameworks continue to evolve. Researchers and developers must maintain open communication with regulatory agencies through mechanisms such as scientific advice meetings and pre-submission consultations. This proactive approach can help anticipate and address potential regulatory hurdles early in the development process.
One of the primary regulatory considerations is the classification of polymer drug conjugates. These hybrid molecules often fall into a gray area between small molecule drugs and biologics, necessitating a case-by-case evaluation by regulatory authorities. The FDA's Office of Combination Products plays a crucial role in determining the regulatory pathway for such products, which can significantly impact the development timeline and required studies.
Safety and toxicity assessments are paramount in the regulatory process. Isotonic solutions used in polymer drug conjugate formations must undergo rigorous testing to ensure they do not introduce additional risks or alter the safety profile of the conjugated drug. This includes evaluating the potential for immunogenicity, as the polymer component may elicit an immune response in some patients.
Characterization and quality control of polymer drug conjugates present unique challenges from a regulatory standpoint. Regulatory agencies require comprehensive analytical methods to assess the consistency and purity of these complex molecules. This includes demonstrating batch-to-batch reproducibility and stability, which can be particularly challenging given the heterogeneous nature of many polymer conjugates.
The choice of isotonic solution and its impact on the drug conjugate's pharmacokinetics and biodistribution must be thoroughly documented. Regulatory bodies will scrutinize data demonstrating that the isotonic environment maintains the integrity and activity of the conjugate throughout its shelf life and administration.
Manufacturing processes for polymer drug conjugates in isotonic solutions must comply with Good Manufacturing Practice (GMP) regulations. This includes validation of sterilization methods, as many of these products are intended for parenteral administration. The regulatory dossier must provide detailed information on the manufacturing process, including any novel technologies or approaches used in production.
Clinical trial design for polymer drug conjugates requires careful consideration of regulatory expectations. Endpoints and patient populations may need to be tailored to address specific concerns related to the conjugate's unique properties and the role of the isotonic solution in its formulation. Adaptive trial designs may be considered to optimize development timelines while meeting regulatory requirements.
As the field of polymer drug conjugates advances, regulatory frameworks continue to evolve. Researchers and developers must maintain open communication with regulatory agencies through mechanisms such as scientific advice meetings and pre-submission consultations. This proactive approach can help anticipate and address potential regulatory hurdles early in the development process.
Biocompatibility Assessment
Biocompatibility assessment is a critical aspect of developing polymer drug conjugates with isotonic solutions. The evaluation of biocompatibility ensures that these formulations are safe for use in biological systems and do not elicit adverse reactions when administered to patients.
One of the primary considerations in biocompatibility assessment is the potential for immunogenicity. Polymer drug conjugates must be designed to minimize immune system recognition and activation. This involves careful selection of polymer materials and conjugation methods that reduce the likelihood of triggering an immune response. Researchers often employ strategies such as PEGylation to create a hydrophilic shield around the drug molecule, effectively masking it from immune system detection.
The impact of isotonic solutions on the overall biocompatibility of polymer drug conjugates is a key area of investigation. Isotonic solutions help maintain osmotic balance, preventing cellular damage and ensuring optimal drug delivery. However, the interaction between these solutions and the polymer-drug complex must be thoroughly evaluated to ensure that the biocompatibility of the conjugate is not compromised.
Cytotoxicity testing is another crucial component of biocompatibility assessment. In vitro assays using relevant cell lines are conducted to determine if the polymer drug conjugate formulations induce any toxic effects on cells. These tests typically involve exposing cells to various concentrations of the conjugate and measuring cell viability, proliferation, and metabolic activity.
Hemocompatibility is particularly important for polymer drug conjugates intended for intravenous administration. Researchers assess the potential for hemolysis, thrombogenicity, and complement activation to ensure that the formulations do not adversely affect blood components or vascular integrity.
Long-term biocompatibility is evaluated through in vivo studies in animal models. These studies assess the distribution, metabolism, and excretion of the polymer drug conjugates, as well as any potential for accumulation in tissues or organs. Histopathological examinations are conducted to identify any signs of inflammation, tissue damage, or other adverse effects resulting from prolonged exposure to the conjugates.
The use of advanced imaging techniques, such as intravital microscopy and fluorescence imaging, allows researchers to visualize the behavior of polymer drug conjugates in real-time within living organisms. This provides valuable insights into their interactions with biological systems and helps identify any potential biocompatibility issues that may not be apparent through traditional testing methods.
Regulatory considerations play a significant role in biocompatibility assessment. Researchers must adhere to guidelines set forth by regulatory agencies such as the FDA and EMA, which outline specific requirements for demonstrating the safety and biocompatibility of polymer drug conjugates. This includes conducting standardized tests and providing comprehensive documentation of results to support regulatory submissions.
One of the primary considerations in biocompatibility assessment is the potential for immunogenicity. Polymer drug conjugates must be designed to minimize immune system recognition and activation. This involves careful selection of polymer materials and conjugation methods that reduce the likelihood of triggering an immune response. Researchers often employ strategies such as PEGylation to create a hydrophilic shield around the drug molecule, effectively masking it from immune system detection.
The impact of isotonic solutions on the overall biocompatibility of polymer drug conjugates is a key area of investigation. Isotonic solutions help maintain osmotic balance, preventing cellular damage and ensuring optimal drug delivery. However, the interaction between these solutions and the polymer-drug complex must be thoroughly evaluated to ensure that the biocompatibility of the conjugate is not compromised.
Cytotoxicity testing is another crucial component of biocompatibility assessment. In vitro assays using relevant cell lines are conducted to determine if the polymer drug conjugate formulations induce any toxic effects on cells. These tests typically involve exposing cells to various concentrations of the conjugate and measuring cell viability, proliferation, and metabolic activity.
Hemocompatibility is particularly important for polymer drug conjugates intended for intravenous administration. Researchers assess the potential for hemolysis, thrombogenicity, and complement activation to ensure that the formulations do not adversely affect blood components or vascular integrity.
Long-term biocompatibility is evaluated through in vivo studies in animal models. These studies assess the distribution, metabolism, and excretion of the polymer drug conjugates, as well as any potential for accumulation in tissues or organs. Histopathological examinations are conducted to identify any signs of inflammation, tissue damage, or other adverse effects resulting from prolonged exposure to the conjugates.
The use of advanced imaging techniques, such as intravital microscopy and fluorescence imaging, allows researchers to visualize the behavior of polymer drug conjugates in real-time within living organisms. This provides valuable insights into their interactions with biological systems and helps identify any potential biocompatibility issues that may not be apparent through traditional testing methods.
Regulatory considerations play a significant role in biocompatibility assessment. Researchers must adhere to guidelines set forth by regulatory agencies such as the FDA and EMA, which outline specific requirements for demonstrating the safety and biocompatibility of polymer drug conjugates. This includes conducting standardized tests and providing comprehensive documentation of results to support regulatory submissions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!