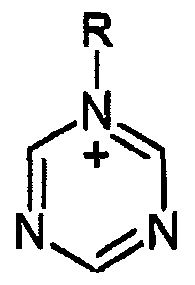
Isotonic solutions role in enhancing enzyme cascade reactions
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Enzyme Cascade Background
Enzyme cascade reactions have emerged as a powerful tool in biotechnology, offering enhanced efficiency and control over complex biochemical processes. These reactions involve a series of enzymes working in sequence, where the product of one enzyme serves as the substrate for the next. This sequential nature allows for the amplification of signals and the production of desired compounds with high specificity and yield.
The concept of enzyme cascades draws inspiration from natural metabolic pathways, where multiple enzymes work in concert to carry out complex biochemical transformations. In recent years, researchers have harnessed this principle to design artificial enzyme cascades for various applications, including biosensing, biocatalysis, and drug delivery.
One of the key challenges in optimizing enzyme cascade reactions is maintaining the stability and activity of each enzyme in the sequence. Enzymes are sensitive to their environment, and changes in pH, temperature, or ionic strength can significantly affect their performance. This is where isotonic solutions come into play, offering a potential means to enhance the overall efficiency of enzyme cascades.
Isotonic solutions are those that have the same osmotic pressure as the cellular environment, typically achieved by carefully balancing the concentration of solutes. In the context of enzyme cascades, isotonic conditions can provide a stable and physiologically relevant environment for the enzymes to operate. This is particularly important when working with enzymes derived from different sources or when attempting to mimic intracellular conditions.
The use of isotonic solutions in enzyme cascade reactions has gained attention due to several potential benefits. Firstly, isotonic conditions can help maintain the native conformation of enzymes, preserving their catalytic activity and stability over extended periods. This is crucial for multi-step reactions that may take hours or even days to complete.
Secondly, isotonic solutions can facilitate the co-localization of enzymes and substrates, potentially enhancing the overall reaction kinetics. By providing a uniform environment, isotonic conditions may promote more efficient substrate channeling between consecutive enzymes in the cascade, reducing diffusion limitations and improving yield.
Furthermore, isotonic solutions can mitigate the risk of osmotic stress on enzymes, which is particularly relevant when working with immobilized enzyme systems or when incorporating enzyme cascades into cellular or subcellular environments. This protection against osmotic imbalances can lead to more robust and reproducible reaction systems.
As research in this field progresses, the role of isotonic solutions in enhancing enzyme cascade reactions continues to evolve. Scientists are exploring various formulations and additives to fine-tune the isotonic environment, aiming to optimize the performance of specific enzyme combinations and reaction pathways.
The concept of enzyme cascades draws inspiration from natural metabolic pathways, where multiple enzymes work in concert to carry out complex biochemical transformations. In recent years, researchers have harnessed this principle to design artificial enzyme cascades for various applications, including biosensing, biocatalysis, and drug delivery.
One of the key challenges in optimizing enzyme cascade reactions is maintaining the stability and activity of each enzyme in the sequence. Enzymes are sensitive to their environment, and changes in pH, temperature, or ionic strength can significantly affect their performance. This is where isotonic solutions come into play, offering a potential means to enhance the overall efficiency of enzyme cascades.
Isotonic solutions are those that have the same osmotic pressure as the cellular environment, typically achieved by carefully balancing the concentration of solutes. In the context of enzyme cascades, isotonic conditions can provide a stable and physiologically relevant environment for the enzymes to operate. This is particularly important when working with enzymes derived from different sources or when attempting to mimic intracellular conditions.
The use of isotonic solutions in enzyme cascade reactions has gained attention due to several potential benefits. Firstly, isotonic conditions can help maintain the native conformation of enzymes, preserving their catalytic activity and stability over extended periods. This is crucial for multi-step reactions that may take hours or even days to complete.
Secondly, isotonic solutions can facilitate the co-localization of enzymes and substrates, potentially enhancing the overall reaction kinetics. By providing a uniform environment, isotonic conditions may promote more efficient substrate channeling between consecutive enzymes in the cascade, reducing diffusion limitations and improving yield.
Furthermore, isotonic solutions can mitigate the risk of osmotic stress on enzymes, which is particularly relevant when working with immobilized enzyme systems or when incorporating enzyme cascades into cellular or subcellular environments. This protection against osmotic imbalances can lead to more robust and reproducible reaction systems.
As research in this field progresses, the role of isotonic solutions in enhancing enzyme cascade reactions continues to evolve. Scientists are exploring various formulations and additives to fine-tune the isotonic environment, aiming to optimize the performance of specific enzyme combinations and reaction pathways.
Market Analysis
The market for isotonic solutions in enzyme cascade reactions is experiencing significant growth, driven by the increasing demand for efficient and sustainable biocatalytic processes across various industries. The pharmaceutical sector, in particular, has shown a strong interest in leveraging enzyme cascade reactions for drug synthesis, leading to a surge in research and development activities focused on optimizing reaction conditions.
Isotonic solutions play a crucial role in maintaining the stability and activity of enzymes during cascade reactions, thereby enhancing overall process efficiency. This has led to a growing market for specialized isotonic formulations tailored to specific enzyme systems. The biotechnology industry has also recognized the potential of isotonic solutions in improving the performance of enzyme-based diagnostic tools and biosensors, further expanding the market opportunities.
The food and beverage industry represents another significant market segment for isotonic solutions in enzyme cascade reactions. As consumers increasingly demand natural and clean-label products, manufacturers are turning to enzymatic processes for food production and modification. Isotonic solutions that can enhance the efficiency and yield of these processes are gaining traction in this sector.
Environmental applications, such as bioremediation and waste treatment, are emerging as promising markets for isotonic solutions in enzyme cascade reactions. The ability to maintain enzyme activity in complex environmental matrices is crucial for these applications, driving the demand for advanced isotonic formulations.
The global market for enzyme stabilizers, including isotonic solutions, is projected to grow steadily over the next five years. This growth is attributed to the expanding applications of enzyme cascade reactions in various industries and the continuous efforts to improve process efficiency and sustainability.
Geographically, North America and Europe currently dominate the market for isotonic solutions in enzyme cascade reactions, owing to their well-established biotechnology and pharmaceutical industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in biotechnology research and the rapid expansion of the pharmaceutical and food processing sectors in countries like China and India.
Key market players are focusing on developing customized isotonic solutions for specific enzyme systems and applications. This trend is expected to lead to a more diversified and specialized market landscape in the near future. Additionally, collaborations between academic institutions and industry partners are likely to accelerate innovation in this field, potentially opening up new market opportunities.
Isotonic solutions play a crucial role in maintaining the stability and activity of enzymes during cascade reactions, thereby enhancing overall process efficiency. This has led to a growing market for specialized isotonic formulations tailored to specific enzyme systems. The biotechnology industry has also recognized the potential of isotonic solutions in improving the performance of enzyme-based diagnostic tools and biosensors, further expanding the market opportunities.
The food and beverage industry represents another significant market segment for isotonic solutions in enzyme cascade reactions. As consumers increasingly demand natural and clean-label products, manufacturers are turning to enzymatic processes for food production and modification. Isotonic solutions that can enhance the efficiency and yield of these processes are gaining traction in this sector.
Environmental applications, such as bioremediation and waste treatment, are emerging as promising markets for isotonic solutions in enzyme cascade reactions. The ability to maintain enzyme activity in complex environmental matrices is crucial for these applications, driving the demand for advanced isotonic formulations.
The global market for enzyme stabilizers, including isotonic solutions, is projected to grow steadily over the next five years. This growth is attributed to the expanding applications of enzyme cascade reactions in various industries and the continuous efforts to improve process efficiency and sustainability.
Geographically, North America and Europe currently dominate the market for isotonic solutions in enzyme cascade reactions, owing to their well-established biotechnology and pharmaceutical industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in biotechnology research and the rapid expansion of the pharmaceutical and food processing sectors in countries like China and India.
Key market players are focusing on developing customized isotonic solutions for specific enzyme systems and applications. This trend is expected to lead to a more diversified and specialized market landscape in the near future. Additionally, collaborations between academic institutions and industry partners are likely to accelerate innovation in this field, potentially opening up new market opportunities.
Isotonic Solutions Status
Isotonic solutions have emerged as a crucial component in enhancing enzyme cascade reactions, playing a significant role in maintaining optimal conditions for enzymatic activity. These solutions, which have the same osmotic pressure as the surrounding cellular environment, provide a stable and conducive medium for enzyme-catalyzed reactions to occur efficiently.
The current status of isotonic solutions in enzyme cascade reactions reflects a growing understanding of their importance in biotechnology and biomedical applications. Researchers have made substantial progress in developing and optimizing isotonic formulations that can significantly improve the performance and stability of enzyme systems. These advancements have led to increased reaction rates, higher product yields, and enhanced overall efficiency of enzyme cascade reactions.
One of the key developments in this field is the customization of isotonic solutions for specific enzyme systems. Scientists have recognized that different enzyme cascades may require tailored isotonic environments to achieve optimal performance. This has resulted in the creation of a diverse range of isotonic formulations, each designed to meet the unique requirements of particular enzyme combinations and reaction conditions.
The use of isotonic solutions has also addressed several challenges previously faced in enzyme cascade reactions. By maintaining a consistent osmotic pressure, these solutions help prevent enzyme denaturation and loss of activity that can occur due to osmotic stress. This has been particularly beneficial in multi-step enzyme reactions, where maintaining the stability and activity of all enzymes involved is crucial for the overall success of the cascade.
Recent studies have demonstrated the potential of isotonic solutions in improving the spatial organization of enzymes in cascade reactions. By providing a suitable medium, these solutions facilitate the formation of enzyme complexes and the co-localization of reaction intermediates, leading to enhanced substrate channeling and improved reaction kinetics.
Furthermore, the integration of isotonic solutions with other advanced technologies, such as microfluidics and immobilization techniques, has opened up new possibilities for enzyme cascade reactions. These combinations have enabled the development of more efficient and controllable reaction systems, with potential applications in biosensors, biofuel production, and pharmaceutical synthesis.
Despite these advancements, challenges remain in fully harnessing the potential of isotonic solutions in enzyme cascade reactions. Ongoing research is focused on further optimizing solution compositions, exploring novel additives to enhance enzyme stability, and developing strategies to maintain isotonic conditions over extended reaction periods. Additionally, efforts are being made to scale up these systems for industrial applications while maintaining their effectiveness and efficiency.
The current status of isotonic solutions in enzyme cascade reactions reflects a growing understanding of their importance in biotechnology and biomedical applications. Researchers have made substantial progress in developing and optimizing isotonic formulations that can significantly improve the performance and stability of enzyme systems. These advancements have led to increased reaction rates, higher product yields, and enhanced overall efficiency of enzyme cascade reactions.
One of the key developments in this field is the customization of isotonic solutions for specific enzyme systems. Scientists have recognized that different enzyme cascades may require tailored isotonic environments to achieve optimal performance. This has resulted in the creation of a diverse range of isotonic formulations, each designed to meet the unique requirements of particular enzyme combinations and reaction conditions.
The use of isotonic solutions has also addressed several challenges previously faced in enzyme cascade reactions. By maintaining a consistent osmotic pressure, these solutions help prevent enzyme denaturation and loss of activity that can occur due to osmotic stress. This has been particularly beneficial in multi-step enzyme reactions, where maintaining the stability and activity of all enzymes involved is crucial for the overall success of the cascade.
Recent studies have demonstrated the potential of isotonic solutions in improving the spatial organization of enzymes in cascade reactions. By providing a suitable medium, these solutions facilitate the formation of enzyme complexes and the co-localization of reaction intermediates, leading to enhanced substrate channeling and improved reaction kinetics.
Furthermore, the integration of isotonic solutions with other advanced technologies, such as microfluidics and immobilization techniques, has opened up new possibilities for enzyme cascade reactions. These combinations have enabled the development of more efficient and controllable reaction systems, with potential applications in biosensors, biofuel production, and pharmaceutical synthesis.
Despite these advancements, challenges remain in fully harnessing the potential of isotonic solutions in enzyme cascade reactions. Ongoing research is focused on further optimizing solution compositions, exploring novel additives to enhance enzyme stability, and developing strategies to maintain isotonic conditions over extended reaction periods. Additionally, efforts are being made to scale up these systems for industrial applications while maintaining their effectiveness and efficiency.
Current Enhancement Methods
01 Isotonic solutions for enzyme cascade reactions
Isotonic solutions are used to maintain optimal conditions for enzyme cascade reactions. These solutions help preserve enzyme activity and stability by matching the osmotic pressure of the cellular environment. This is crucial for maintaining the efficiency and accuracy of multi-step enzymatic processes in biotechnology and diagnostic applications.- Isotonic solutions for enzyme cascade reactions: Isotonic solutions are used to maintain optimal conditions for enzyme cascade reactions. These solutions help preserve enzyme activity and stability by matching the osmotic pressure of the cellular environment. This approach is crucial for maintaining the efficiency and reproducibility of multi-step enzymatic processes in various biotechnological applications.
- Enzyme immobilization techniques for cascade reactions: Immobilization of enzymes involved in cascade reactions can enhance their stability and reusability. Various methods, such as covalent binding, entrapment, or adsorption, are employed to immobilize enzymes on solid supports. This approach allows for the creation of more efficient and cost-effective biocatalytic systems for industrial applications.
- Microfluidic systems for enzyme cascade reactions: Microfluidic devices are utilized to perform enzyme cascade reactions in a controlled and miniaturized environment. These systems offer advantages such as reduced reagent consumption, improved reaction kinetics, and the ability to integrate multiple reaction steps. Microfluidic platforms enable high-throughput screening and optimization of enzyme cascade reactions for various applications.
- Synthetic enzyme cascades for biocatalysis: Synthetic biology approaches are employed to design and construct artificial enzyme cascades. These engineered pathways can be used for the production of valuable compounds or for the development of novel biosensors. By combining enzymes from different organisms or creating de novo enzymes, researchers can create more efficient and versatile biocatalytic systems.
- Optimization of reaction conditions for enzyme cascades: Various strategies are employed to optimize reaction conditions for enzyme cascade reactions. These include the use of cofactor regeneration systems, pH and temperature control, and the addition of stabilizing agents. Optimization techniques aim to enhance the overall efficiency, yield, and stability of multi-step enzymatic processes in both research and industrial settings.
02 Enzyme cascade reactions in biosensors
Enzyme cascade reactions are utilized in biosensor development for enhanced sensitivity and specificity. Multiple enzymes are arranged in a sequential manner, where the product of one reaction serves as the substrate for the next. This amplifies the signal and allows for the detection of low concentration analytes in complex biological samples.Expand Specific Solutions03 Optimization of enzyme cascade reactions
Techniques for optimizing enzyme cascade reactions include adjusting enzyme ratios, controlling reaction conditions, and engineering enzyme properties. These approaches aim to improve reaction rates, yield, and overall efficiency of the cascade. Computational modeling and high-throughput screening methods are often employed to identify optimal conditions.Expand Specific Solutions04 Immobilization strategies for enzyme cascades
Immobilization of enzymes involved in cascade reactions on various supports enhances their stability and reusability. Techniques such as covalent binding, entrapment, and cross-linking are used to immobilize multiple enzymes in close proximity, facilitating substrate channeling and improving overall reaction efficiency.Expand Specific Solutions05 Applications of isotonic enzyme cascade reactions
Isotonic enzyme cascade reactions find applications in various fields, including biocatalysis, biofuel production, and biomedical diagnostics. These systems are used for the synthesis of complex molecules, energy production from renewable resources, and the development of highly sensitive diagnostic assays for disease detection and monitoring.Expand Specific Solutions
Key Industry Players
The field of isotonic solutions in enhancing enzyme cascade reactions is in a growth phase, with increasing market size and technological advancements. The competitive landscape is characterized by a mix of established pharmaceutical companies and specialized biotechnology firms. Key players like Novartis AG, F. Hoffmann-La Roche Ltd., and Pfizer Inc. are investing in research and development to leverage this technology for drug delivery and therapeutic applications. Smaller, innovative companies such as Chr. Hansen A/S and Rigel Pharmaceuticals, Inc. are also making significant contributions, focusing on niche applications and novel enzyme formulations. The technology's maturity is progressing, with academic institutions like The University of Chicago and Carnegie Mellon University collaborating with industry partners to push the boundaries of enzyme cascade reactions in isotonic environments.
Novartis AG
Technical Solution: Novartis AG has made significant strides in leveraging isotonic solutions to enhance enzyme cascade reactions, particularly in the context of pharmaceutical manufacturing and drug discovery. Their approach focuses on developing specialized isotonic formulations that not only stabilize enzymes but also optimize their catalytic efficiency. Novartis has demonstrated that these tailored isotonic environments can significantly reduce side reactions and improve the overall yield of desired products in complex multi-enzyme systems[5]. Their research also extends to the use of isotonic solutions in maintaining the activity of immobilized enzyme cascades, which has potential applications in continuous flow biocatalysis and biosensors[6].
Strengths: Strong focus on pharmaceutical applications and potential for integration with drug discovery pipelines. Weaknesses: May be less adaptable to non-pharmaceutical industrial processes.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche Ltd. has developed innovative approaches to enhance enzyme cascade reactions using isotonic solutions, particularly in the context of diagnostic assays and biopharmaceutical production. Their research focuses on creating optimized isotonic environments that not only stabilize enzymes but also enhance their catalytic efficiency and specificity. Roche has demonstrated that carefully designed isotonic solutions can significantly improve the performance of enzyme cascades in complex biological matrices, leading to more sensitive and reliable diagnostic tests[7]. Additionally, they have explored the use of isotonic solutions in conjunction with enzyme engineering techniques to develop more robust and efficient biocatalysts for pharmaceutical synthesis[8].
Strengths: Strong integration with diagnostic and biopharmaceutical applications. Weaknesses: May face challenges in adapting technologies to non-medical industrial processes.
Isotonic Solution Innovations
Enhancing coupled catalytic activity of multi-enzyme cascades with liquid-liquid phase separation using peptide-based condensates
PatentWO2024163309A1
Innovation
- The method involves configuring a plurality of enzymes as an enzymatic cascade and contacting them with a substrate and a peptide to form peptide-driven coacervates, where at least one enzyme has multiple polyhistidine tags to cross-link nanoparticles, thereby increasing reaction efficiency.
Enzyme catalysis in the presence of ionic liquids
PatentWO2002038784A1
Innovation
- The use of ionic liquids as a reaction medium, which can be miscible or immiscible with water, improves solubility, selectivity, and enzyme stability, and suppresses side reactions, allowing for increased yields and selectivity in enzyme-catalyzed reactions without adverse effects on enzyme stability.
Regulatory Considerations
The regulatory landscape surrounding isotonic solutions in enzyme cascade reactions is complex and multifaceted, requiring careful consideration from researchers and manufacturers. Regulatory bodies such as the FDA and EMA have established guidelines for the use of isotonic solutions in various applications, including their role in enhancing enzyme cascade reactions.
One key regulatory aspect is the classification of isotonic solutions used in enzyme cascade reactions. Depending on their intended use and composition, these solutions may be categorized as medical devices, pharmaceuticals, or laboratory reagents. Each classification carries distinct regulatory requirements, impacting the development, manufacturing, and marketing processes.
Safety considerations are paramount in regulatory frameworks. Manufacturers must demonstrate that their isotonic solutions do not introduce harmful contaminants or interfere with the accuracy of enzyme cascade reactions. This often involves rigorous testing protocols and quality control measures to ensure consistency and reliability across batches.
Efficacy claims related to the enhancement of enzyme cascade reactions using isotonic solutions are subject to scrutiny by regulatory agencies. Manufacturers must provide substantial scientific evidence to support any performance-related claims, typically through well-designed studies and clinical trials where applicable.
Labeling and packaging regulations play a crucial role in ensuring proper use and handling of isotonic solutions. Clear instructions, storage requirements, and expiration dates must be prominently displayed, adhering to specific guidelines set forth by regulatory bodies.
For isotonic solutions used in diagnostic applications involving enzyme cascade reactions, additional regulatory considerations come into play. These may include compliance with standards such as ISO 13485 for quality management systems in medical devices or specific performance criteria outlined in regulatory guidelines.
International harmonization efforts, such as those led by the International Conference on Harmonisation (ICH), aim to streamline regulatory requirements across different regions. This can facilitate global market access for manufacturers of isotonic solutions used in enzyme cascade reactions, but also requires careful navigation of varying regional requirements.
Regulatory compliance in this field extends beyond initial approval. Ongoing post-market surveillance and reporting of adverse events are typically mandated to ensure continued safety and efficacy of isotonic solutions in enhancing enzyme cascade reactions.
As the field of enzyme cascade reactions continues to evolve, regulatory frameworks are likely to adapt. Manufacturers and researchers must stay abreast of these changes, engaging proactively with regulatory bodies to ensure compliance and facilitate innovation in this promising area of biotechnology.
One key regulatory aspect is the classification of isotonic solutions used in enzyme cascade reactions. Depending on their intended use and composition, these solutions may be categorized as medical devices, pharmaceuticals, or laboratory reagents. Each classification carries distinct regulatory requirements, impacting the development, manufacturing, and marketing processes.
Safety considerations are paramount in regulatory frameworks. Manufacturers must demonstrate that their isotonic solutions do not introduce harmful contaminants or interfere with the accuracy of enzyme cascade reactions. This often involves rigorous testing protocols and quality control measures to ensure consistency and reliability across batches.
Efficacy claims related to the enhancement of enzyme cascade reactions using isotonic solutions are subject to scrutiny by regulatory agencies. Manufacturers must provide substantial scientific evidence to support any performance-related claims, typically through well-designed studies and clinical trials where applicable.
Labeling and packaging regulations play a crucial role in ensuring proper use and handling of isotonic solutions. Clear instructions, storage requirements, and expiration dates must be prominently displayed, adhering to specific guidelines set forth by regulatory bodies.
For isotonic solutions used in diagnostic applications involving enzyme cascade reactions, additional regulatory considerations come into play. These may include compliance with standards such as ISO 13485 for quality management systems in medical devices or specific performance criteria outlined in regulatory guidelines.
International harmonization efforts, such as those led by the International Conference on Harmonisation (ICH), aim to streamline regulatory requirements across different regions. This can facilitate global market access for manufacturers of isotonic solutions used in enzyme cascade reactions, but also requires careful navigation of varying regional requirements.
Regulatory compliance in this field extends beyond initial approval. Ongoing post-market surveillance and reporting of adverse events are typically mandated to ensure continued safety and efficacy of isotonic solutions in enhancing enzyme cascade reactions.
As the field of enzyme cascade reactions continues to evolve, regulatory frameworks are likely to adapt. Manufacturers and researchers must stay abreast of these changes, engaging proactively with regulatory bodies to ensure compliance and facilitate innovation in this promising area of biotechnology.
Environmental Impact
The use of isotonic solutions in enzyme cascade reactions has significant environmental implications that warrant careful consideration. These solutions, designed to maintain osmotic balance, play a crucial role in optimizing enzymatic processes but also present potential environmental challenges.
One of the primary environmental concerns is the disposal of isotonic solutions after their use in enzyme cascade reactions. These solutions often contain a mixture of salts, buffers, and other additives that, if released untreated into the environment, could disrupt local ecosystems. Proper waste management protocols are essential to mitigate this risk, including appropriate treatment and disposal methods that comply with environmental regulations.
The production of isotonic solutions also has environmental ramifications. The manufacturing process requires various chemical components, some of which may have substantial environmental footprints in terms of resource extraction, energy consumption, and greenhouse gas emissions. As the demand for these solutions increases with the growing application of enzyme cascade reactions, the cumulative environmental impact of their production becomes more significant.
Water usage is another critical environmental factor to consider. Isotonic solutions typically require high-purity water, and the purification processes can be resource-intensive. In regions facing water scarcity, the production of these solutions may compete with other essential water needs, potentially exacerbating local water stress.
On the positive side, the use of isotonic solutions in enhancing enzyme cascade reactions can lead to more efficient and environmentally friendly industrial processes. By optimizing enzymatic reactions, these solutions can reduce the need for harsh chemicals, minimize waste production, and lower energy requirements in various applications, from biofuel production to pharmaceutical manufacturing.
The potential for biodegradable or bio-based isotonic solutions presents an opportunity for reducing environmental impact. Research into developing these alternatives could lead to more sustainable options that maintain the effectiveness of enzyme cascade reactions while minimizing ecological harm.
Lifecycle assessment studies are crucial for fully understanding the environmental impact of isotonic solutions in enzyme cascade reactions. These assessments should consider all stages, from raw material extraction to end-of-life disposal, to identify areas for improvement and guide the development of more sustainable practices.
As the field advances, there is a growing emphasis on developing closed-loop systems for isotonic solutions. These systems aim to recycle and reuse the solutions, significantly reducing waste and resource consumption. While technically challenging, such approaches hold promise for dramatically improving the environmental profile of enzyme cascade reactions.
One of the primary environmental concerns is the disposal of isotonic solutions after their use in enzyme cascade reactions. These solutions often contain a mixture of salts, buffers, and other additives that, if released untreated into the environment, could disrupt local ecosystems. Proper waste management protocols are essential to mitigate this risk, including appropriate treatment and disposal methods that comply with environmental regulations.
The production of isotonic solutions also has environmental ramifications. The manufacturing process requires various chemical components, some of which may have substantial environmental footprints in terms of resource extraction, energy consumption, and greenhouse gas emissions. As the demand for these solutions increases with the growing application of enzyme cascade reactions, the cumulative environmental impact of their production becomes more significant.
Water usage is another critical environmental factor to consider. Isotonic solutions typically require high-purity water, and the purification processes can be resource-intensive. In regions facing water scarcity, the production of these solutions may compete with other essential water needs, potentially exacerbating local water stress.
On the positive side, the use of isotonic solutions in enhancing enzyme cascade reactions can lead to more efficient and environmentally friendly industrial processes. By optimizing enzymatic reactions, these solutions can reduce the need for harsh chemicals, minimize waste production, and lower energy requirements in various applications, from biofuel production to pharmaceutical manufacturing.
The potential for biodegradable or bio-based isotonic solutions presents an opportunity for reducing environmental impact. Research into developing these alternatives could lead to more sustainable options that maintain the effectiveness of enzyme cascade reactions while minimizing ecological harm.
Lifecycle assessment studies are crucial for fully understanding the environmental impact of isotonic solutions in enzyme cascade reactions. These assessments should consider all stages, from raw material extraction to end-of-life disposal, to identify areas for improvement and guide the development of more sustainable practices.
As the field advances, there is a growing emphasis on developing closed-loop systems for isotonic solutions. These systems aim to recycle and reuse the solutions, significantly reducing waste and resource consumption. While technically challenging, such approaches hold promise for dramatically improving the environmental profile of enzyme cascade reactions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!