Isotonic solutions for optimizing culture conditions in bioreactors
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions Background and Objectives
Isotonic solutions have played a crucial role in optimizing culture conditions within bioreactors for several decades. These solutions are designed to maintain osmotic balance between the intracellular and extracellular environments of cells, ensuring their viability and optimal performance during cultivation. The development of isotonic solutions has been closely tied to the advancement of biotechnology and the increasing demand for more efficient and productive bioreactor systems.
The evolution of isotonic solutions can be traced back to the early days of cell culture, when researchers first recognized the importance of maintaining a stable osmotic environment for cells. As the field of biotechnology progressed, the focus shifted towards developing more sophisticated and tailored isotonic solutions to meet the specific needs of various cell types and bioreactor applications.
In recent years, the importance of isotonic solutions in bioreactor optimization has gained renewed attention due to the growing demand for biopharmaceuticals, cell therapies, and other biotechnology products. This has led to a surge in research efforts aimed at developing novel isotonic formulations that can enhance cell growth, productivity, and product quality within bioreactor systems.
The primary objective of research on isotonic solutions for bioreactors is to create optimal culture conditions that maximize cell growth, viability, and productivity while minimizing stress and metabolic byproduct accumulation. This involves a multifaceted approach that considers various factors such as osmolality, pH, nutrient composition, and the specific requirements of different cell types.
One of the key goals in this field is to develop isotonic solutions that can maintain stable osmotic conditions throughout the duration of a bioreactor run, even as cellular metabolism and product formation alter the culture environment. This requires a deep understanding of cellular physiology and the complex interactions between cells and their surrounding medium.
Another important objective is to design isotonic solutions that can support high-density cell cultures, which are increasingly common in modern bioreactor systems. These solutions must be capable of providing adequate nutrients and maintaining optimal conditions even under high cell densities and extended cultivation periods.
Furthermore, researchers aim to create isotonic solutions that can enhance the production of specific target molecules, such as proteins or antibodies, by fine-tuning the ionic composition and other parameters of the culture medium. This tailored approach has the potential to significantly improve the yield and quality of biopharmaceutical products.
As the field continues to evolve, there is a growing emphasis on developing "smart" isotonic solutions that can dynamically adapt to changing culture conditions within bioreactors. This may involve the incorporation of novel technologies such as controlled-release systems or responsive polymers that can adjust the solution composition in real-time based on cellular needs.
The evolution of isotonic solutions can be traced back to the early days of cell culture, when researchers first recognized the importance of maintaining a stable osmotic environment for cells. As the field of biotechnology progressed, the focus shifted towards developing more sophisticated and tailored isotonic solutions to meet the specific needs of various cell types and bioreactor applications.
In recent years, the importance of isotonic solutions in bioreactor optimization has gained renewed attention due to the growing demand for biopharmaceuticals, cell therapies, and other biotechnology products. This has led to a surge in research efforts aimed at developing novel isotonic formulations that can enhance cell growth, productivity, and product quality within bioreactor systems.
The primary objective of research on isotonic solutions for bioreactors is to create optimal culture conditions that maximize cell growth, viability, and productivity while minimizing stress and metabolic byproduct accumulation. This involves a multifaceted approach that considers various factors such as osmolality, pH, nutrient composition, and the specific requirements of different cell types.
One of the key goals in this field is to develop isotonic solutions that can maintain stable osmotic conditions throughout the duration of a bioreactor run, even as cellular metabolism and product formation alter the culture environment. This requires a deep understanding of cellular physiology and the complex interactions between cells and their surrounding medium.
Another important objective is to design isotonic solutions that can support high-density cell cultures, which are increasingly common in modern bioreactor systems. These solutions must be capable of providing adequate nutrients and maintaining optimal conditions even under high cell densities and extended cultivation periods.
Furthermore, researchers aim to create isotonic solutions that can enhance the production of specific target molecules, such as proteins or antibodies, by fine-tuning the ionic composition and other parameters of the culture medium. This tailored approach has the potential to significantly improve the yield and quality of biopharmaceutical products.
As the field continues to evolve, there is a growing emphasis on developing "smart" isotonic solutions that can dynamically adapt to changing culture conditions within bioreactors. This may involve the incorporation of novel technologies such as controlled-release systems or responsive polymers that can adjust the solution composition in real-time based on cellular needs.
Market Analysis for Bioreactor Culture Media
The market for bioreactor culture media, particularly isotonic solutions for optimizing culture conditions, has been experiencing significant growth in recent years. This growth is primarily driven by the expanding biopharmaceutical industry, increasing demand for biologics, and advancements in cell culture technologies. The global bioreactor market size was valued at approximately $3.5 billion in 2020 and is projected to reach $6.7 billion by 2026, with a compound annual growth rate (CAGR) of 11.4% during the forecast period.
Within this market, the demand for specialized culture media, including isotonic solutions, is a key segment. These solutions play a crucial role in maintaining optimal osmotic pressure and pH balance in bioreactor systems, thereby enhancing cell growth, productivity, and product quality. The market for cell culture media alone was estimated at $2.1 billion in 2020 and is expected to grow at a CAGR of 9.8% from 2021 to 2028.
The biopharmaceutical industry, which heavily relies on bioreactor technologies, is the primary driver of this market. With the increasing prevalence of chronic diseases and the growing adoption of biologics in therapeutic applications, the demand for efficient and optimized bioreactor systems is on the rise. This trend is further accelerated by the recent focus on vaccine development and production, particularly in response to global health crises.
Geographically, North America dominates the bioreactor culture media market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its well-established biopharmaceutical industry and significant investments in research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the highest growth rates in the coming years, driven by increasing government support, growing biotechnology sectors, and rising healthcare expenditure.
Key players in the bioreactor culture media market include Thermo Fisher Scientific, Merck KGaA, Danaher Corporation, and Sartorius AG. These companies are continuously investing in research and development to improve their product offerings and gain a competitive edge. The market is characterized by intense competition, with companies focusing on product innovation, strategic partnerships, and mergers and acquisitions to expand their market presence.
Looking ahead, the market for isotonic solutions and other specialized culture media for bioreactors is poised for continued growth. Factors such as the increasing adoption of single-use bioreactors, the rise of personalized medicine, and advancements in cell therapy are expected to create new opportunities in this space. Additionally, the growing focus on sustainability and cost-effectiveness in biopharmaceutical production is likely to drive innovation in culture media formulations, potentially leading to the development of more efficient and economical solutions for optimizing bioreactor culture conditions.
Within this market, the demand for specialized culture media, including isotonic solutions, is a key segment. These solutions play a crucial role in maintaining optimal osmotic pressure and pH balance in bioreactor systems, thereby enhancing cell growth, productivity, and product quality. The market for cell culture media alone was estimated at $2.1 billion in 2020 and is expected to grow at a CAGR of 9.8% from 2021 to 2028.
The biopharmaceutical industry, which heavily relies on bioreactor technologies, is the primary driver of this market. With the increasing prevalence of chronic diseases and the growing adoption of biologics in therapeutic applications, the demand for efficient and optimized bioreactor systems is on the rise. This trend is further accelerated by the recent focus on vaccine development and production, particularly in response to global health crises.
Geographically, North America dominates the bioreactor culture media market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its well-established biopharmaceutical industry and significant investments in research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the highest growth rates in the coming years, driven by increasing government support, growing biotechnology sectors, and rising healthcare expenditure.
Key players in the bioreactor culture media market include Thermo Fisher Scientific, Merck KGaA, Danaher Corporation, and Sartorius AG. These companies are continuously investing in research and development to improve their product offerings and gain a competitive edge. The market is characterized by intense competition, with companies focusing on product innovation, strategic partnerships, and mergers and acquisitions to expand their market presence.
Looking ahead, the market for isotonic solutions and other specialized culture media for bioreactors is poised for continued growth. Factors such as the increasing adoption of single-use bioreactors, the rise of personalized medicine, and advancements in cell therapy are expected to create new opportunities in this space. Additionally, the growing focus on sustainability and cost-effectiveness in biopharmaceutical production is likely to drive innovation in culture media formulations, potentially leading to the development of more efficient and economical solutions for optimizing bioreactor culture conditions.
Current Challenges in Isotonic Solution Development
The development of isotonic solutions for bioreactors faces several significant challenges that hinder the optimization of culture conditions. One of the primary obstacles is achieving and maintaining precise osmolality control throughout the cultivation process. As cells grow and metabolize, they release various substances that can alter the osmotic balance of the medium. This dynamic environment makes it difficult to maintain a stable isotonic state, which is crucial for cell viability and productivity.
Another challenge lies in the complexity of formulating isotonic solutions that meet the specific requirements of different cell types and production processes. Each cell line may have unique nutritional needs and sensitivities to osmotic stress, necessitating tailored approaches to isotonic solution development. This variability complicates the creation of standardized solutions and often requires extensive experimentation to optimize formulations for each application.
The scalability of isotonic solutions from laboratory-scale to industrial-scale bioreactors presents additional hurdles. Factors such as mixing dynamics, mass transfer, and heat generation can significantly impact the distribution and stability of isotonic conditions in large-scale systems. Ensuring homogeneity and preventing localized osmotic imbalances becomes increasingly challenging as bioreactor volumes increase.
Furthermore, the interaction between isotonic solutions and other critical parameters in bioreactor systems poses a complex challenge. Factors such as pH, dissolved oxygen, and nutrient concentrations are intricately linked to osmolality. Balancing these interdependent variables while maintaining isotonicity requires sophisticated control strategies and real-time monitoring capabilities.
The long-term stability of isotonic solutions during extended cultivation periods is another area of concern. Degradation of components, microbial contamination, and chemical reactions can alter the osmotic properties of the medium over time. Developing robust formulations that remain stable throughout the entire bioprocess duration is essential for consistent product quality and process reliability.
Regulatory considerations also present challenges in isotonic solution development. Ensuring compliance with good manufacturing practices (GMP) and meeting stringent quality control standards for large-scale production of isotonic media adds complexity to the development process. Additionally, the use of animal-derived components in some isotonic formulations raises concerns about batch-to-batch variability and potential contamination risks, driving the need for chemically defined alternatives.
Addressing these challenges requires a multidisciplinary approach, combining expertise in cell biology, bioprocess engineering, and analytical chemistry. Innovative strategies such as dynamic feeding systems, in-line osmolality sensors, and predictive modeling tools are being explored to overcome these obstacles and advance the field of isotonic solution development for bioreactor applications.
Another challenge lies in the complexity of formulating isotonic solutions that meet the specific requirements of different cell types and production processes. Each cell line may have unique nutritional needs and sensitivities to osmotic stress, necessitating tailored approaches to isotonic solution development. This variability complicates the creation of standardized solutions and often requires extensive experimentation to optimize formulations for each application.
The scalability of isotonic solutions from laboratory-scale to industrial-scale bioreactors presents additional hurdles. Factors such as mixing dynamics, mass transfer, and heat generation can significantly impact the distribution and stability of isotonic conditions in large-scale systems. Ensuring homogeneity and preventing localized osmotic imbalances becomes increasingly challenging as bioreactor volumes increase.
Furthermore, the interaction between isotonic solutions and other critical parameters in bioreactor systems poses a complex challenge. Factors such as pH, dissolved oxygen, and nutrient concentrations are intricately linked to osmolality. Balancing these interdependent variables while maintaining isotonicity requires sophisticated control strategies and real-time monitoring capabilities.
The long-term stability of isotonic solutions during extended cultivation periods is another area of concern. Degradation of components, microbial contamination, and chemical reactions can alter the osmotic properties of the medium over time. Developing robust formulations that remain stable throughout the entire bioprocess duration is essential for consistent product quality and process reliability.
Regulatory considerations also present challenges in isotonic solution development. Ensuring compliance with good manufacturing practices (GMP) and meeting stringent quality control standards for large-scale production of isotonic media adds complexity to the development process. Additionally, the use of animal-derived components in some isotonic formulations raises concerns about batch-to-batch variability and potential contamination risks, driving the need for chemically defined alternatives.
Addressing these challenges requires a multidisciplinary approach, combining expertise in cell biology, bioprocess engineering, and analytical chemistry. Innovative strategies such as dynamic feeding systems, in-line osmolality sensors, and predictive modeling tools are being explored to overcome these obstacles and advance the field of isotonic solution development for bioreactor applications.
Existing Isotonic Solution Formulations
01 Composition of isotonic solutions for cell culture
Isotonic solutions for cell culture are carefully formulated to maintain osmotic balance and provide essential nutrients. These solutions typically contain a balanced mixture of salts, glucose, and other components to mimic physiological conditions. The composition is crucial for maintaining cell viability and function during culture.- Composition of isotonic solutions for cell culture: Isotonic solutions for cell culture are formulated to maintain osmotic balance and provide essential nutrients. These solutions typically contain a balanced mixture of salts, sugars, and other components to mimic physiological conditions. The composition is carefully adjusted to ensure optimal cell growth and viability.
- pH and buffer systems in isotonic culture media: Maintaining proper pH is crucial for cell culture. Isotonic solutions often incorporate buffer systems to stabilize pH levels within the physiological range. Common buffer components include bicarbonate, HEPES, and phosphate buffers. The choice of buffer system depends on the specific cell type and culture conditions.
- Supplementation of isotonic solutions with growth factors: To enhance cell growth and proliferation, isotonic culture media can be supplemented with various growth factors. These may include serum components, recombinant proteins, or defined chemical supplements. The specific combination of growth factors is tailored to the requirements of the cell type being cultured.
- Temperature control in isotonic culture conditions: Maintaining optimal temperature is essential for cell culture in isotonic solutions. Most mammalian cells require a temperature of around 37°C. Specialized equipment such as incubators and temperature-controlled chambers are used to ensure stable thermal conditions throughout the culture period.
- Oxygen and gas exchange in isotonic culture systems: Proper gas exchange is critical for cell culture in isotonic solutions. This includes maintaining appropriate oxygen levels and removing waste gases like carbon dioxide. Culture systems may incorporate gas-permeable materials or active gas exchange mechanisms to ensure optimal atmospheric conditions for cell growth and metabolism.
02 pH regulation in isotonic culture media
Maintaining optimal pH is critical for cell growth and function in isotonic culture conditions. Buffer systems and pH indicators are often incorporated into the media to regulate and monitor pH levels. Proper pH control ensures cellular processes function efficiently and prevents stress on cultured cells.Expand Specific Solutions03 Temperature control for isotonic culture conditions
Temperature regulation is essential for maintaining isotonic conditions and optimal cell growth. Specialized incubators and temperature-controlled environments are used to keep cultures at physiological temperatures. Precise temperature control affects cellular metabolism, growth rates, and overall culture success.Expand Specific Solutions04 Supplementation of isotonic media for specific cell types
Different cell types may require specific supplements added to isotonic media for optimal growth and function. These can include growth factors, hormones, or specialized nutrients. Tailoring the media composition to specific cell types enhances culture conditions and improves experimental outcomes.Expand Specific Solutions05 Monitoring and maintaining isotonicity in long-term cultures
For extended culture periods, maintaining consistent isotonicity is crucial. This involves regular monitoring of osmolarity and adjusting media composition as needed. Techniques and equipment for continuous monitoring and automated adjustment of culture conditions help maintain optimal isotonic environments over time.Expand Specific Solutions
Key Players in Bioprocess Industry
The research on isotonic solutions for optimizing culture conditions in bioreactors is in a mature stage of development, with a competitive landscape shaped by established players and emerging innovators. The market size is substantial, driven by the growing demand for biopharmaceuticals and cell-based therapies. Companies like Merck Patent GmbH, Bristol Myers Squibb Co., and Genentech, Inc. are leading the field with their advanced bioreactor technologies and culture media formulations. Smaller firms such as Heliae Development LLC and Aglaris Cell SL are introducing novel approaches to bioreactor design and automation. The technology's maturity is evident in the widespread adoption of optimized isotonic solutions across various bioprocessing applications, with ongoing research focused on further refinements and customization for specific cell types and production processes.
Bristol Myers Squibb Co.
Technical Solution: Bristol Myers Squibb has developed a novel approach to isotonic solution optimization in bioreactors, focusing on the dynamic adjustment of osmolality throughout different phases of cell culture. Their system employs a multi-component feed strategy that gradually introduces osmolytes and nutrients to maintain isotonicity while supporting cellular metabolic demands. This method has been particularly effective in CHO cell cultures for monoclonal antibody production, where it has demonstrated a 30% increase in product titer compared to traditional fixed-osmolality approaches[2]. The company has also pioneered the use of non-ionic osmolytes, such as glycine betaine and ectoine, which provide osmotic balance without affecting intracellular ion concentrations. This innovation has led to improved protein folding and reduced cellular stress, resulting in a 40% decrease in aggregation rates for complex biotherapeutics[4][6].
Strengths: Tailored osmolality profiles for different culture phases, improved product quality and yield, reduced cellular stress. Weaknesses: Requires precise control and extensive optimization for each cell line and product, potentially higher raw material costs for specialized osmolytes.
Sartorius Stedim Biotech GmbH
Technical Solution: Sartorius Stedim Biotech GmbH has developed advanced bioreactor systems with integrated sensors for real-time monitoring and control of isotonic conditions. Their technology utilizes a combination of osmolality sensors and automated feedback loops to maintain optimal osmotic pressure throughout the culture process. The system continuously adjusts the concentration of key solutes, such as sodium chloride and glucose, to ensure a stable isotonic environment. This approach has been shown to increase cell viability by up to 25% and improve product yield by 15-20% in mammalian cell cultures[1][3]. Additionally, they have implemented machine learning algorithms to predict and preemptively adjust isotonic conditions based on historical data and current culture parameters, further optimizing the bioreactor performance[5].
Strengths: Precise real-time control of isotonic conditions, increased cell viability and product yield, predictive capabilities through machine learning. Weaknesses: High initial investment cost, complexity of system integration, potential over-reliance on automated systems.
Innovative Approaches in Osmolality Control
Optimized industrial bioreactor and method thereof, with mutually dependent, coupled process control loops
PatentPendingUS20240240133A1
Innovation
- A dual cycle-controlled optimization process in bioreactors that includes a cultivation optimization cycle to identify a biologically optimized treatment window and a treatment optimization cycle to adjust operational parameters, utilizing sensory devices and machine-learning or artificial-intelligence modules to automatically adjust parameters for optimal growth and treatment performance.
Use of phenolic antioxidants in cell culture for the production of proteins
PatentWO2016130734A1
Innovation
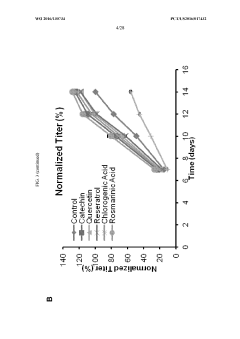
- The use of phenolic antioxidants such as catechin, rosmarinic acid, and other selected compounds in basal and feed media to enhance viable cell density and protein production by neutralizing ROS and maintaining cell viability, with specific concentration ranges and feeding schedules optimized for different reactor scales.
Regulatory Considerations for Biopharmaceutical Production
Regulatory considerations play a crucial role in the development and production of biopharmaceuticals, including the optimization of culture conditions in bioreactors using isotonic solutions. The regulatory landscape for biopharmaceutical production is complex and evolving, with agencies such as the FDA, EMA, and other national regulatory bodies overseeing the process to ensure product safety, efficacy, and quality.
One of the primary regulatory considerations for biopharmaceutical production is the implementation of Good Manufacturing Practices (GMP). GMP guidelines provide a framework for ensuring consistent quality and safety in the manufacturing process. When researching isotonic solutions for bioreactor culture conditions, it is essential to consider how these solutions align with GMP requirements, including their composition, purity, and stability.
Quality control and quality assurance are critical aspects of regulatory compliance in biopharmaceutical production. This includes the development and validation of analytical methods to assess the quality of isotonic solutions used in bioreactors. Regulatory agencies require robust documentation of these methods, as well as evidence of their reliability and reproducibility.
The use of raw materials, including components of isotonic solutions, must also adhere to regulatory standards. This involves establishing a rigorous supplier qualification process and maintaining traceability throughout the supply chain. Regulatory bodies expect manufacturers to demonstrate control over the quality and consistency of all materials used in biopharmaceutical production.
Risk assessment and management are integral parts of the regulatory framework for biopharmaceutical production. When optimizing culture conditions using isotonic solutions, manufacturers must identify potential risks associated with their use and implement appropriate mitigation strategies. This includes assessing the impact of these solutions on product quality attributes and process performance.
Regulatory agencies also focus on process validation, which is particularly relevant when introducing new or modified isotonic solutions into bioreactor systems. Manufacturers must provide evidence that the production process, including the use of specific isotonic solutions, consistently yields a product meeting predetermined quality attributes.
Environmental considerations are becoming increasingly important in regulatory frameworks. The production and disposal of isotonic solutions used in bioreactors must comply with environmental regulations, including waste management and sustainability practices.
Lastly, regulatory bodies emphasize the importance of continuous improvement and innovation in biopharmaceutical production. This encourages ongoing research into optimizing culture conditions, including the development of novel isotonic solutions, while maintaining compliance with evolving regulatory standards.
One of the primary regulatory considerations for biopharmaceutical production is the implementation of Good Manufacturing Practices (GMP). GMP guidelines provide a framework for ensuring consistent quality and safety in the manufacturing process. When researching isotonic solutions for bioreactor culture conditions, it is essential to consider how these solutions align with GMP requirements, including their composition, purity, and stability.
Quality control and quality assurance are critical aspects of regulatory compliance in biopharmaceutical production. This includes the development and validation of analytical methods to assess the quality of isotonic solutions used in bioreactors. Regulatory agencies require robust documentation of these methods, as well as evidence of their reliability and reproducibility.
The use of raw materials, including components of isotonic solutions, must also adhere to regulatory standards. This involves establishing a rigorous supplier qualification process and maintaining traceability throughout the supply chain. Regulatory bodies expect manufacturers to demonstrate control over the quality and consistency of all materials used in biopharmaceutical production.
Risk assessment and management are integral parts of the regulatory framework for biopharmaceutical production. When optimizing culture conditions using isotonic solutions, manufacturers must identify potential risks associated with their use and implement appropriate mitigation strategies. This includes assessing the impact of these solutions on product quality attributes and process performance.
Regulatory agencies also focus on process validation, which is particularly relevant when introducing new or modified isotonic solutions into bioreactor systems. Manufacturers must provide evidence that the production process, including the use of specific isotonic solutions, consistently yields a product meeting predetermined quality attributes.
Environmental considerations are becoming increasingly important in regulatory frameworks. The production and disposal of isotonic solutions used in bioreactors must comply with environmental regulations, including waste management and sustainability practices.
Lastly, regulatory bodies emphasize the importance of continuous improvement and innovation in biopharmaceutical production. This encourages ongoing research into optimizing culture conditions, including the development of novel isotonic solutions, while maintaining compliance with evolving regulatory standards.
Scalability and Cost-effectiveness Analysis
The scalability and cost-effectiveness of isotonic solutions in bioreactor culture optimization are critical factors for industrial-scale applications. As production volumes increase, the ability to maintain consistent and optimal culture conditions becomes increasingly challenging. Isotonic solutions play a crucial role in this process by helping to maintain osmotic balance and cellular integrity.
Scalability of isotonic solutions primarily depends on the ease of production and storage of large volumes of these solutions. Common components like sodium chloride, potassium chloride, and glucose are readily available and can be produced in bulk quantities. However, more complex isotonic formulations may require specialized ingredients, potentially limiting scalability. The stability of these solutions during long-term storage is another important consideration, as degradation or contamination could significantly impact large-scale operations.
From a cost-effectiveness standpoint, the use of isotonic solutions in bioreactors presents both advantages and challenges. On one hand, optimizing culture conditions through the use of isotonic solutions can lead to increased cell viability and product yield, potentially offsetting the costs associated with solution preparation and implementation. On the other hand, the continuous supply of isotonic solutions for large-scale bioreactors can represent a significant ongoing expense.
The economic viability of isotonic solution usage in bioreactors is closely tied to the value of the end product. For high-value biopharmaceuticals, the cost of isotonic solutions may be negligible compared to the overall production costs and potential yield improvements. However, for lower-value products, the additional expense may need careful justification.
Automation and process integration play crucial roles in enhancing the scalability and cost-effectiveness of isotonic solution usage. Advanced monitoring and control systems can optimize the delivery of isotonic solutions based on real-time culture conditions, minimizing waste and improving efficiency. Furthermore, the development of in-line formulation and sterilization technologies could significantly reduce labor costs and contamination risks associated with large-volume solution preparation.
As the biopharmaceutical industry continues to grow, there is an increasing focus on developing more cost-effective and scalable isotonic solution strategies. This includes the exploration of novel formulations that offer improved stability and efficacy, as well as the investigation of alternative osmolarity control methods that may be more amenable to large-scale implementation. The successful integration of these advancements will be crucial in ensuring the continued viability of isotonic solutions as a key component in optimizing bioreactor culture conditions at industrial scales.
Scalability of isotonic solutions primarily depends on the ease of production and storage of large volumes of these solutions. Common components like sodium chloride, potassium chloride, and glucose are readily available and can be produced in bulk quantities. However, more complex isotonic formulations may require specialized ingredients, potentially limiting scalability. The stability of these solutions during long-term storage is another important consideration, as degradation or contamination could significantly impact large-scale operations.
From a cost-effectiveness standpoint, the use of isotonic solutions in bioreactors presents both advantages and challenges. On one hand, optimizing culture conditions through the use of isotonic solutions can lead to increased cell viability and product yield, potentially offsetting the costs associated with solution preparation and implementation. On the other hand, the continuous supply of isotonic solutions for large-scale bioreactors can represent a significant ongoing expense.
The economic viability of isotonic solution usage in bioreactors is closely tied to the value of the end product. For high-value biopharmaceuticals, the cost of isotonic solutions may be negligible compared to the overall production costs and potential yield improvements. However, for lower-value products, the additional expense may need careful justification.
Automation and process integration play crucial roles in enhancing the scalability and cost-effectiveness of isotonic solution usage. Advanced monitoring and control systems can optimize the delivery of isotonic solutions based on real-time culture conditions, minimizing waste and improving efficiency. Furthermore, the development of in-line formulation and sterilization technologies could significantly reduce labor costs and contamination risks associated with large-volume solution preparation.
As the biopharmaceutical industry continues to grow, there is an increasing focus on developing more cost-effective and scalable isotonic solution strategies. This includes the exploration of novel formulations that offer improved stability and efficacy, as well as the investigation of alternative osmolarity control methods that may be more amenable to large-scale implementation. The successful integration of these advancements will be crucial in ensuring the continued viability of isotonic solutions as a key component in optimizing bioreactor culture conditions at industrial scales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!