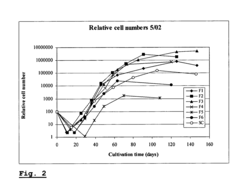
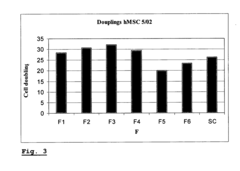
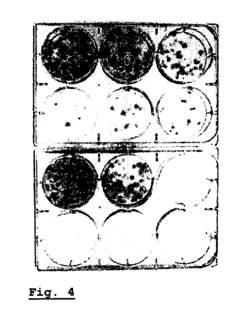
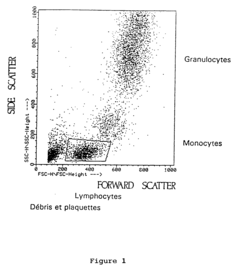
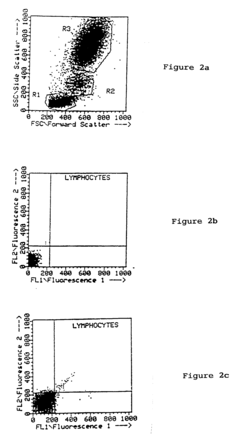
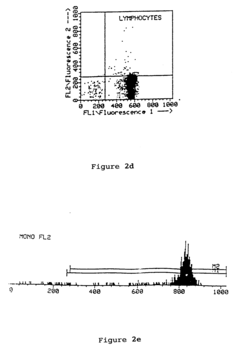
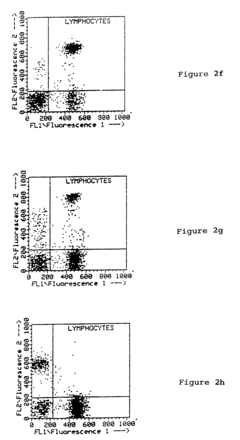
Enhancing leucocyte isolation with isotonic solution formulations
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Leucocyte Isolation Background and Objectives
Leucocyte isolation has been a critical technique in immunology and hematology research for decades. The process involves separating white blood cells from other blood components, allowing for detailed study and analysis of these crucial immune system cells. Historically, this isolation was performed using density gradient centrifugation methods, which, while effective, often resulted in cell damage and loss.
The development of isotonic solution formulations marked a significant advancement in leucocyte isolation techniques. These solutions maintain osmotic balance, closely mimicking the natural environment of blood cells. This approach has greatly improved cell viability and functionality post-isolation, leading to more accurate and reliable research outcomes.
The primary objective of enhancing leucocyte isolation with isotonic solution formulations is to optimize the separation process while preserving cell integrity and function. Researchers aim to develop formulations that not only effectively separate leucocytes but also maintain their physiological state, ensuring that isolated cells closely represent their in vivo counterparts.
Current research focuses on fine-tuning the composition of isotonic solutions to address specific challenges in leucocyte isolation. These include minimizing cell activation during the isolation process, reducing contamination from other cell types, and improving the overall yield of viable leucocytes. Additionally, there is a growing interest in developing specialized formulations for isolating specific leucocyte subpopulations, such as neutrophils, lymphocytes, or monocytes.
The evolution of leucocyte isolation techniques is closely tied to advancements in biomedical research and clinical diagnostics. As our understanding of immune system function and dysfunction deepens, the demand for more sophisticated and precise isolation methods increases. This drives the continuous improvement of isotonic solution formulations, pushing the boundaries of what is possible in cellular isolation and analysis.
Looking ahead, the field of leucocyte isolation is poised for further innovation. Emerging technologies, such as microfluidics and immunomagnetic separation, are being integrated with advanced isotonic solutions to create more efficient and targeted isolation methods. These developments promise to enhance not only basic research but also clinical applications, including personalized medicine and immunotherapy.
The development of isotonic solution formulations marked a significant advancement in leucocyte isolation techniques. These solutions maintain osmotic balance, closely mimicking the natural environment of blood cells. This approach has greatly improved cell viability and functionality post-isolation, leading to more accurate and reliable research outcomes.
The primary objective of enhancing leucocyte isolation with isotonic solution formulations is to optimize the separation process while preserving cell integrity and function. Researchers aim to develop formulations that not only effectively separate leucocytes but also maintain their physiological state, ensuring that isolated cells closely represent their in vivo counterparts.
Current research focuses on fine-tuning the composition of isotonic solutions to address specific challenges in leucocyte isolation. These include minimizing cell activation during the isolation process, reducing contamination from other cell types, and improving the overall yield of viable leucocytes. Additionally, there is a growing interest in developing specialized formulations for isolating specific leucocyte subpopulations, such as neutrophils, lymphocytes, or monocytes.
The evolution of leucocyte isolation techniques is closely tied to advancements in biomedical research and clinical diagnostics. As our understanding of immune system function and dysfunction deepens, the demand for more sophisticated and precise isolation methods increases. This drives the continuous improvement of isotonic solution formulations, pushing the boundaries of what is possible in cellular isolation and analysis.
Looking ahead, the field of leucocyte isolation is poised for further innovation. Emerging technologies, such as microfluidics and immunomagnetic separation, are being integrated with advanced isotonic solutions to create more efficient and targeted isolation methods. These developments promise to enhance not only basic research but also clinical applications, including personalized medicine and immunotherapy.
Market Analysis for Improved Leucocyte Isolation Methods
The market for improved leucocyte isolation methods is experiencing significant growth, driven by the increasing demand for precision medicine and advanced cellular therapies. The global cell separation technology market, which includes leucocyte isolation, is projected to reach $10.5 billion by 2025, with a compound annual growth rate of 18.8% from 2020 to 2025. This growth is primarily fueled by the rising incidence of chronic diseases, technological advancements in cell separation techniques, and the expanding applications of cell-based therapies.
In the field of leucocyte isolation, there is a growing need for more efficient and reliable methods, particularly those utilizing isotonic solution formulations. The current market is dominated by traditional density gradient centrifugation techniques, which, while effective, can be time-consuming and may result in cell loss or activation. This has created a substantial opportunity for innovative solutions that can enhance the speed, purity, and viability of isolated leucocytes.
The healthcare sector represents the largest end-user segment for leucocyte isolation technologies, accounting for approximately 65% of the market share. Within this sector, research institutions, pharmaceutical companies, and biotechnology firms are the primary consumers, driven by their focus on developing novel cell-based therapies and conducting immunological research.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure and significant investments in biomedical research. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the highest growth rates in the coming years, propelled by increasing healthcare expenditure and growing research activities.
The market for leucocyte isolation methods is highly competitive, with several key players dominating the landscape. These include Thermo Fisher Scientific, Becton Dickinson and Company, Merck KGaA, and STEMCELL Technologies. These companies are continuously investing in research and development to improve their product offerings and gain a competitive edge.
A notable trend in the market is the shift towards automated and closed-system isolation methods, which offer improved standardization and reduced risk of contamination. This trend is particularly relevant for clinical applications, where regulatory compliance and reproducibility are crucial.
The COVID-19 pandemic has further highlighted the importance of efficient leucocyte isolation techniques, particularly in the context of studying immune responses to viral infections. This has led to an increased focus on developing rapid and reliable isolation methods, potentially accelerating market growth in the coming years.
In the field of leucocyte isolation, there is a growing need for more efficient and reliable methods, particularly those utilizing isotonic solution formulations. The current market is dominated by traditional density gradient centrifugation techniques, which, while effective, can be time-consuming and may result in cell loss or activation. This has created a substantial opportunity for innovative solutions that can enhance the speed, purity, and viability of isolated leucocytes.
The healthcare sector represents the largest end-user segment for leucocyte isolation technologies, accounting for approximately 65% of the market share. Within this sector, research institutions, pharmaceutical companies, and biotechnology firms are the primary consumers, driven by their focus on developing novel cell-based therapies and conducting immunological research.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure and significant investments in biomedical research. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the highest growth rates in the coming years, propelled by increasing healthcare expenditure and growing research activities.
The market for leucocyte isolation methods is highly competitive, with several key players dominating the landscape. These include Thermo Fisher Scientific, Becton Dickinson and Company, Merck KGaA, and STEMCELL Technologies. These companies are continuously investing in research and development to improve their product offerings and gain a competitive edge.
A notable trend in the market is the shift towards automated and closed-system isolation methods, which offer improved standardization and reduced risk of contamination. This trend is particularly relevant for clinical applications, where regulatory compliance and reproducibility are crucial.
The COVID-19 pandemic has further highlighted the importance of efficient leucocyte isolation techniques, particularly in the context of studying immune responses to viral infections. This has led to an increased focus on developing rapid and reliable isolation methods, potentially accelerating market growth in the coming years.
Current Challenges in Leucocyte Isolation Techniques
Leucocyte isolation techniques face several significant challenges that hinder their effectiveness and reliability. One of the primary issues is the maintenance of cell viability and functionality during the isolation process. Current methods often subject cells to mechanical stress, osmotic shock, or chemical exposure, which can lead to cell damage or activation, potentially altering their biological properties and compromising downstream analyses.
Another major challenge is the purity of isolated leucocyte populations. Conventional isolation techniques frequently result in contamination from other cell types, particularly erythrocytes and platelets. This contamination can interfere with subsequent experimental procedures and skew research outcomes, especially in sensitive applications such as flow cytometry or gene expression studies.
The efficiency of leucocyte isolation is also a persistent concern. Current methods often yield low recovery rates, particularly for rare cell subpopulations. This inefficiency necessitates larger initial sample volumes, which may not always be feasible, especially in clinical settings with limited patient material available.
Reproducibility across different laboratories and operators remains a significant hurdle. The variability in isolation outcomes can be attributed to differences in sample handling, reagent quality, and protocol execution. This lack of standardization makes it challenging to compare results between studies and impedes the translation of research findings into clinical applications.
Time and cost considerations pose additional challenges. Many existing isolation techniques are labor-intensive and time-consuming, limiting their applicability in high-throughput settings or urgent clinical scenarios. Moreover, the reliance on expensive specialized equipment or proprietary reagents can make these methods cost-prohibitive for many research laboratories or healthcare facilities.
The preservation of leucocyte subpopulations presents another obstacle. Current isolation methods may inadvertently alter the relative proportions of different leucocyte subtypes or selectively enrich certain populations while depleting others. This can lead to a misrepresentation of the original cellular composition and potentially skew biological interpretations.
Lastly, the adaptability of isolation techniques to diverse sample types remains a challenge. Methods optimized for peripheral blood may not be directly applicable to other biological fluids or tissue samples, necessitating time-consuming protocol modifications and validation for each new sample type.
Another major challenge is the purity of isolated leucocyte populations. Conventional isolation techniques frequently result in contamination from other cell types, particularly erythrocytes and platelets. This contamination can interfere with subsequent experimental procedures and skew research outcomes, especially in sensitive applications such as flow cytometry or gene expression studies.
The efficiency of leucocyte isolation is also a persistent concern. Current methods often yield low recovery rates, particularly for rare cell subpopulations. This inefficiency necessitates larger initial sample volumes, which may not always be feasible, especially in clinical settings with limited patient material available.
Reproducibility across different laboratories and operators remains a significant hurdle. The variability in isolation outcomes can be attributed to differences in sample handling, reagent quality, and protocol execution. This lack of standardization makes it challenging to compare results between studies and impedes the translation of research findings into clinical applications.
Time and cost considerations pose additional challenges. Many existing isolation techniques are labor-intensive and time-consuming, limiting their applicability in high-throughput settings or urgent clinical scenarios. Moreover, the reliance on expensive specialized equipment or proprietary reagents can make these methods cost-prohibitive for many research laboratories or healthcare facilities.
The preservation of leucocyte subpopulations presents another obstacle. Current isolation methods may inadvertently alter the relative proportions of different leucocyte subtypes or selectively enrich certain populations while depleting others. This can lead to a misrepresentation of the original cellular composition and potentially skew biological interpretations.
Lastly, the adaptability of isolation techniques to diverse sample types remains a challenge. Methods optimized for peripheral blood may not be directly applicable to other biological fluids or tissue samples, necessitating time-consuming protocol modifications and validation for each new sample type.
Existing Isotonic Solutions for Leucocyte Isolation
01 Isotonic solution compositions for leukocyte isolation
Specific compositions of isotonic solutions are designed to maintain cell viability and integrity during leukocyte isolation processes. These solutions typically contain balanced electrolytes, buffers, and other components to mimic physiological conditions and prevent cell damage or lysis during isolation procedures.- Isotonic solution compositions for leukocyte isolation: Specific compositions of isotonic solutions are designed for the effective isolation of leukocytes. These solutions typically contain balanced electrolytes and other components to maintain cell viability and facilitate separation. The formulations may include buffers, salts, and osmolarity adjusters to create an environment suitable for leukocyte isolation without damaging the cells.
- Methods for leukocyte isolation using isotonic solutions: Various techniques and procedures are employed for isolating leukocytes using isotonic solutions. These methods may involve centrifugation, density gradient separation, or filtration processes. The use of specific isotonic formulations in conjunction with these techniques enhances the efficiency and purity of leukocyte isolation while maintaining cell integrity.
- Additives to enhance leukocyte isolation in isotonic solutions: Certain additives are incorporated into isotonic solutions to improve the efficiency of leukocyte isolation. These may include specific proteins, polymers, or other compounds that aid in cell separation or preservation. The additives can help prevent cell clumping, reduce contamination from other cell types, or improve the overall yield of isolated leukocytes.
- Devices and systems for leukocyte isolation using isotonic solutions: Specialized devices and systems are developed for leukocyte isolation that utilize isotonic solutions. These may include automated separation systems, microfluidic devices, or specialized containers designed to optimize the isolation process. The integration of these devices with specific isotonic formulations can enhance the efficiency and reproducibility of leukocyte isolation procedures.
- Preservation of isolated leukocytes in isotonic solutions: Isotonic solutions are formulated to maintain the viability and functionality of isolated leukocytes for extended periods. These preservation solutions may contain specific nutrients, antioxidants, or stabilizers to protect the cells from degradation. The composition of these solutions is crucial for downstream applications such as cell culture, analysis, or therapeutic use of the isolated leukocytes.
02 Methods for isolating leukocytes using isotonic solutions
Various techniques and procedures are employed for isolating leukocytes using isotonic solutions. These methods may include density gradient centrifugation, immunomagnetic separation, or filtration processes, all of which utilize isotonic solutions to maintain cell viability and function throughout the isolation process.Expand Specific Solutions03 Additives to enhance leukocyte isolation in isotonic solutions
Specific additives are incorporated into isotonic solutions to improve the efficiency and yield of leukocyte isolation. These may include antioxidants, enzyme inhibitors, or specific proteins that help preserve cell integrity and function during the isolation process.Expand Specific Solutions04 Devices and systems for leukocyte isolation using isotonic solutions
Specialized devices and systems are developed for efficient leukocyte isolation using isotonic solutions. These may include automated cell separation systems, microfluidic devices, or specialized centrifugation equipment designed to work with specific isotonic solution formulations.Expand Specific Solutions05 Quality control and storage of isolated leukocytes in isotonic solutions
Procedures and formulations are developed for maintaining the quality and viability of isolated leukocytes in isotonic solutions. This includes optimizing storage conditions, developing preservation media, and establishing quality control measures to ensure the isolated cells remain functional for downstream applications.Expand Specific Solutions
Key Players in Cell Isolation Technology
The field of enhancing leucocyte isolation with isotonic solution formulations is in a growth phase, with increasing market demand driven by advancements in cell therapy and immunology research. The global market for cell isolation technologies is expanding, estimated to reach several billion dollars by 2025. Technologically, the field is progressing rapidly, with companies like Gammadelta Therapeutics and Chongqing Precision Biotechnology leading innovations in cell therapy applications. Established players such as QIAGEN GmbH and Bio-Rad Laboratories are contributing to the maturation of isolation techniques, while emerging companies like Generation Biotech are introducing novel approaches to DNA analysis and cell separation, indicating a dynamic and competitive landscape.
QIAGEN GmbH
Technical Solution: QIAGEN GmbH has developed advanced leucocyte isolation techniques using optimized isotonic solution formulations. Their RBC Lysis Buffer system efficiently removes red blood cells while preserving leucocytes for downstream applications[1]. The company's proprietary buffer composition maintains osmotic balance, minimizing cell stress during isolation. QIAGEN's method incorporates a two-step process: first, a brief incubation with the lysis buffer to selectively disrupt erythrocytes, followed by a washing step with a specialized stabilization solution to neutralize the lysis and preserve leucocyte integrity[2]. This approach yields high-purity leucocyte populations with over 95% viability, suitable for various analytical and research applications[3].
Strengths: High leucocyte purity and viability, versatile for multiple downstream applications. Weaknesses: May require specialized equipment for optimal results, potentially higher cost compared to basic methods.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad Laboratories has innovated leucocyte isolation techniques using advanced isotonic solution formulations. Their Lymphocyte Separation Medium (LSM) is a carefully formulated solution designed to create density gradients for efficient separation of mononuclear cells from whole blood[1]. The company's approach involves layering diluted blood over the LSM, followed by centrifugation, which results in distinct layers with leucocytes concentrated at the interface[2]. Bio-Rad's formulation maintains physiological osmolality, crucial for preserving cell viability and functionality. Additionally, they have developed supplementary buffers that enhance the separation process and stabilize isolated cells, allowing for extended storage and analysis periods[3].
Strengths: Efficient separation of specific leucocyte populations, maintains cell functionality for downstream applications. Weaknesses: Requires centrifugation equipment, may have limitations in processing large sample volumes quickly.
Innovative Approaches in Isotonic Formulation Design
Method for purifying mesenchymal stem cells
PatentInactiveUS20110195497A1
Innovation
- Isolating MSCs using density gradient centrifugation with a density of less than 1.073 g/ml, preferably around 1.068 g/ml, using solutions like Ficoll® or Percoll®, which enriches cells with enhanced proliferation capacity while retaining multipotency and typical antigen characteristics, thereby minimizing the number of flattened cells and maintaining differentiation potential.
Method to protect leucocytes, protective composition, protected blood composition and method to analyse blood
PatentInactiveEP0625706A1
Innovation
- A method using a substantially isotonic composition containing an aliphatic aldehyde, a salt of an alkali or alkaline earth metal, and a weak acid to protect leukocytes before hypotonic lysis, preventing aggregate formation and maintaining cellular morphology, thereby allowing accurate leukocyte analysis without washing.
Regulatory Considerations for Cell Isolation Products
Regulatory considerations play a crucial role in the development and commercialization of cell isolation products, particularly those aimed at enhancing leucocyte isolation with isotonic solution formulations. These products are subject to stringent oversight by regulatory bodies such as the FDA in the United States and the EMA in Europe.
The primary regulatory framework for cell isolation products falls under medical device regulations. In the US, these products are typically classified as Class II medical devices, requiring a 510(k) premarket notification. The FDA evaluates the safety and effectiveness of these products, focusing on their intended use, performance characteristics, and potential risks.
Manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and implement robust quality management systems. This includes maintaining detailed documentation of product development, manufacturing processes, and quality control measures. Validation of the isotonic solution formulations is critical, ensuring consistency in osmolality, pH, and other key parameters that affect leucocyte viability and function.
Clinical evidence is often necessary to support regulatory submissions. This may involve conducting clinical studies to demonstrate the efficacy of the enhanced leucocyte isolation technique using the isotonic solution formulations. The studies should address aspects such as cell yield, purity, and functionality of isolated leucocytes.
Labeling and packaging requirements are another important regulatory consideration. Clear instructions for use, storage conditions, and any limitations or precautions must be accurately communicated to end-users. Additionally, manufacturers need to implement post-market surveillance systems to monitor product performance and address any adverse events or complaints.
As cell isolation products may be used in various clinical applications, including diagnostics and cell therapies, manufacturers must consider the regulatory implications of these downstream uses. This may involve additional regulatory pathways or requirements, depending on the specific applications and jurisdictions involved.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), can help streamline regulatory processes for global markets. However, manufacturers must still navigate country-specific requirements and obtain necessary approvals or certifications in each target market.
Staying abreast of evolving regulatory landscapes is crucial for manufacturers of cell isolation products. Emerging technologies and novel applications may lead to updates in regulatory guidance or the introduction of new standards. Proactive engagement with regulatory authorities through pre-submission consultations can help address potential challenges early in the product development process.
The primary regulatory framework for cell isolation products falls under medical device regulations. In the US, these products are typically classified as Class II medical devices, requiring a 510(k) premarket notification. The FDA evaluates the safety and effectiveness of these products, focusing on their intended use, performance characteristics, and potential risks.
Manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and implement robust quality management systems. This includes maintaining detailed documentation of product development, manufacturing processes, and quality control measures. Validation of the isotonic solution formulations is critical, ensuring consistency in osmolality, pH, and other key parameters that affect leucocyte viability and function.
Clinical evidence is often necessary to support regulatory submissions. This may involve conducting clinical studies to demonstrate the efficacy of the enhanced leucocyte isolation technique using the isotonic solution formulations. The studies should address aspects such as cell yield, purity, and functionality of isolated leucocytes.
Labeling and packaging requirements are another important regulatory consideration. Clear instructions for use, storage conditions, and any limitations or precautions must be accurately communicated to end-users. Additionally, manufacturers need to implement post-market surveillance systems to monitor product performance and address any adverse events or complaints.
As cell isolation products may be used in various clinical applications, including diagnostics and cell therapies, manufacturers must consider the regulatory implications of these downstream uses. This may involve additional regulatory pathways or requirements, depending on the specific applications and jurisdictions involved.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), can help streamline regulatory processes for global markets. However, manufacturers must still navigate country-specific requirements and obtain necessary approvals or certifications in each target market.
Staying abreast of evolving regulatory landscapes is crucial for manufacturers of cell isolation products. Emerging technologies and novel applications may lead to updates in regulatory guidance or the introduction of new standards. Proactive engagement with regulatory authorities through pre-submission consultations can help address potential challenges early in the product development process.
Quality Control in Leucocyte Isolation Processes
Quality control is a critical aspect of leucocyte isolation processes, ensuring the reliability and reproducibility of results in both research and clinical applications. The use of isotonic solution formulations plays a crucial role in maintaining cell viability and functionality during isolation procedures.
Standardization of quality control measures is essential for consistent leucocyte isolation outcomes. This includes the implementation of rigorous protocols for sample handling, processing, and analysis. Regular calibration and maintenance of equipment used in isolation processes, such as centrifuges and cell counters, are fundamental to maintaining accuracy and precision.
The composition of isotonic solutions used in leucocyte isolation must be carefully controlled to optimize cell yield and purity. Factors such as pH, osmolality, and ion concentrations are critical parameters that require continuous monitoring. Automated systems for solution preparation and quality checks can significantly reduce variability and human error in this process.
Viability assessment of isolated leucocytes is a key quality control measure. Flow cytometry techniques, coupled with specific fluorescent dyes, allow for rapid and accurate determination of cell viability and subset composition. This information is crucial for evaluating the effectiveness of the isolation process and the suitability of the isolated cells for downstream applications.
Contamination control is another vital aspect of quality assurance in leucocyte isolation. Sterile techniques and the use of appropriate antimicrobial agents in isolation solutions help minimize the risk of microbial contamination. Regular environmental monitoring and aseptic processing validation are essential components of a comprehensive quality control program.
Batch-to-batch consistency in isotonic solution formulations is critical for reproducible leucocyte isolation. Implementation of robust quality management systems, including detailed documentation of raw materials, preparation processes, and quality control results, ensures traceability and facilitates troubleshooting when issues arise.
The development and validation of rapid, on-site quality control tests for isotonic solutions can significantly enhance the efficiency of leucocyte isolation processes. These may include real-time monitoring of solution parameters using specialized sensors or the integration of microfluidic devices for rapid assessment of solution properties and their effects on cell populations.
Continuous improvement in quality control measures is driven by ongoing research and technological advancements. The incorporation of machine learning algorithms for predictive quality control and the development of novel biomarkers for assessing leucocyte functionality post-isolation are promising areas for enhancing the reliability and effectiveness of isolation processes.
Standardization of quality control measures is essential for consistent leucocyte isolation outcomes. This includes the implementation of rigorous protocols for sample handling, processing, and analysis. Regular calibration and maintenance of equipment used in isolation processes, such as centrifuges and cell counters, are fundamental to maintaining accuracy and precision.
The composition of isotonic solutions used in leucocyte isolation must be carefully controlled to optimize cell yield and purity. Factors such as pH, osmolality, and ion concentrations are critical parameters that require continuous monitoring. Automated systems for solution preparation and quality checks can significantly reduce variability and human error in this process.
Viability assessment of isolated leucocytes is a key quality control measure. Flow cytometry techniques, coupled with specific fluorescent dyes, allow for rapid and accurate determination of cell viability and subset composition. This information is crucial for evaluating the effectiveness of the isolation process and the suitability of the isolated cells for downstream applications.
Contamination control is another vital aspect of quality assurance in leucocyte isolation. Sterile techniques and the use of appropriate antimicrobial agents in isolation solutions help minimize the risk of microbial contamination. Regular environmental monitoring and aseptic processing validation are essential components of a comprehensive quality control program.
Batch-to-batch consistency in isotonic solution formulations is critical for reproducible leucocyte isolation. Implementation of robust quality management systems, including detailed documentation of raw materials, preparation processes, and quality control results, ensures traceability and facilitates troubleshooting when issues arise.
The development and validation of rapid, on-site quality control tests for isotonic solutions can significantly enhance the efficiency of leucocyte isolation processes. These may include real-time monitoring of solution parameters using specialized sensors or the integration of microfluidic devices for rapid assessment of solution properties and their effects on cell populations.
Continuous improvement in quality control measures is driven by ongoing research and technological advancements. The incorporation of machine learning algorithms for predictive quality control and the development of novel biomarkers for assessing leucocyte functionality post-isolation are promising areas for enhancing the reliability and effectiveness of isolation processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!