Role of isotonic solutions in cell sheet engineering techniques
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cell Sheet Engineering Background and Objectives
Cell sheet engineering has emerged as a groundbreaking approach in tissue engineering and regenerative medicine over the past two decades. This innovative technique allows for the creation of three-dimensional tissue constructs without the need for scaffolds, overcoming limitations associated with traditional tissue engineering methods. The primary objective of cell sheet engineering is to develop functional tissue equivalents that can be used for transplantation, drug screening, and disease modeling.
The evolution of cell sheet engineering can be traced back to the early 2000s when researchers first demonstrated the ability to harvest intact cell sheets using temperature-responsive culture surfaces. Since then, the field has witnessed significant advancements in both methodology and applications. The technology has been successfully applied to various tissue types, including cardiac, corneal, and periodontal tissues, showcasing its versatility and potential impact on multiple medical domains.
One of the key challenges in cell sheet engineering has been maintaining cell viability and functionality during the detachment and transfer processes. This is where isotonic solutions play a crucial role. Isotonic solutions, which have the same osmotic pressure as the cell's internal environment, are essential for preserving cell integrity and function during these critical stages of cell sheet manipulation.
The use of isotonic solutions in cell sheet engineering aims to achieve several objectives. Firstly, they help maintain cellular homeostasis by preventing osmotic stress during the detachment process. Secondly, isotonic solutions facilitate the preservation of cell-cell junctions and extracellular matrix components, which are vital for the structural and functional integrity of the cell sheet. Lastly, these solutions contribute to the overall viability and metabolic activity of the cells, ensuring that the engineered tissue remains functional post-transplantation.
As the field of cell sheet engineering continues to advance, researchers are exploring novel isotonic solution formulations to further enhance cell sheet quality and functionality. The ongoing research focuses on optimizing the composition of these solutions to better mimic the physiological environment of specific tissue types. This tailored approach aims to improve the survival and integration of transplanted cell sheets, ultimately leading to more successful clinical outcomes.
The technological trajectory of cell sheet engineering, particularly in relation to isotonic solutions, is expected to have far-reaching implications for personalized medicine and organ transplantation. As we look towards the future, the continued refinement of isotonic solution protocols in cell sheet engineering techniques promises to unlock new possibilities in tissue regeneration and organ repair, potentially revolutionizing the treatment of various degenerative diseases and injuries.
The evolution of cell sheet engineering can be traced back to the early 2000s when researchers first demonstrated the ability to harvest intact cell sheets using temperature-responsive culture surfaces. Since then, the field has witnessed significant advancements in both methodology and applications. The technology has been successfully applied to various tissue types, including cardiac, corneal, and periodontal tissues, showcasing its versatility and potential impact on multiple medical domains.
One of the key challenges in cell sheet engineering has been maintaining cell viability and functionality during the detachment and transfer processes. This is where isotonic solutions play a crucial role. Isotonic solutions, which have the same osmotic pressure as the cell's internal environment, are essential for preserving cell integrity and function during these critical stages of cell sheet manipulation.
The use of isotonic solutions in cell sheet engineering aims to achieve several objectives. Firstly, they help maintain cellular homeostasis by preventing osmotic stress during the detachment process. Secondly, isotonic solutions facilitate the preservation of cell-cell junctions and extracellular matrix components, which are vital for the structural and functional integrity of the cell sheet. Lastly, these solutions contribute to the overall viability and metabolic activity of the cells, ensuring that the engineered tissue remains functional post-transplantation.
As the field of cell sheet engineering continues to advance, researchers are exploring novel isotonic solution formulations to further enhance cell sheet quality and functionality. The ongoing research focuses on optimizing the composition of these solutions to better mimic the physiological environment of specific tissue types. This tailored approach aims to improve the survival and integration of transplanted cell sheets, ultimately leading to more successful clinical outcomes.
The technological trajectory of cell sheet engineering, particularly in relation to isotonic solutions, is expected to have far-reaching implications for personalized medicine and organ transplantation. As we look towards the future, the continued refinement of isotonic solution protocols in cell sheet engineering techniques promises to unlock new possibilities in tissue regeneration and organ repair, potentially revolutionizing the treatment of various degenerative diseases and injuries.
Market Analysis for Cell Sheet Applications
The cell sheet engineering market has shown significant growth potential in recent years, driven by increasing demand for regenerative medicine and tissue engineering solutions. This technology offers advantages over traditional cell-based therapies by preserving cell-cell junctions and extracellular matrix, leading to improved tissue functionality and integration.
The global market for cell sheet engineering applications is expected to expand rapidly, with a compound annual growth rate projected to exceed 15% over the next five years. This growth is primarily fueled by rising investments in regenerative medicine research and development, as well as the increasing prevalence of chronic diseases requiring innovative treatment approaches.
Key application areas for cell sheet technology include cardiovascular diseases, ophthalmology, orthopedics, and wound healing. The cardiovascular segment currently holds the largest market share, driven by the high incidence of heart diseases and the need for advanced cardiac repair solutions. Ophthalmology applications, particularly for corneal regeneration, are also gaining traction due to the growing aging population and increasing cases of eye disorders.
Geographically, North America and Europe dominate the cell sheet engineering market, owing to well-established healthcare infrastructure, substantial research funding, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth, propelled by increasing healthcare expenditure, rising awareness of regenerative medicine, and government initiatives to promote advanced medical technologies.
The market landscape is characterized by a mix of established biotechnology companies, academic institutions, and emerging startups. Collaborations between research organizations and industry players are becoming increasingly common, accelerating the translation of cell sheet technologies from laboratory to clinical applications.
Despite the promising outlook, several factors may impact market growth. These include high development costs, complex regulatory pathways, and the need for long-term clinical data to demonstrate efficacy and safety. Additionally, scalability and manufacturing challenges associated with cell sheet production need to be addressed to facilitate widespread adoption.
As the field advances, there is growing interest in combining cell sheet engineering with other cutting-edge technologies, such as 3D bioprinting and gene editing, to enhance therapeutic outcomes. This convergence is expected to open up new market opportunities and drive innovation in tissue engineering and regenerative medicine.
The global market for cell sheet engineering applications is expected to expand rapidly, with a compound annual growth rate projected to exceed 15% over the next five years. This growth is primarily fueled by rising investments in regenerative medicine research and development, as well as the increasing prevalence of chronic diseases requiring innovative treatment approaches.
Key application areas for cell sheet technology include cardiovascular diseases, ophthalmology, orthopedics, and wound healing. The cardiovascular segment currently holds the largest market share, driven by the high incidence of heart diseases and the need for advanced cardiac repair solutions. Ophthalmology applications, particularly for corneal regeneration, are also gaining traction due to the growing aging population and increasing cases of eye disorders.
Geographically, North America and Europe dominate the cell sheet engineering market, owing to well-established healthcare infrastructure, substantial research funding, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth, propelled by increasing healthcare expenditure, rising awareness of regenerative medicine, and government initiatives to promote advanced medical technologies.
The market landscape is characterized by a mix of established biotechnology companies, academic institutions, and emerging startups. Collaborations between research organizations and industry players are becoming increasingly common, accelerating the translation of cell sheet technologies from laboratory to clinical applications.
Despite the promising outlook, several factors may impact market growth. These include high development costs, complex regulatory pathways, and the need for long-term clinical data to demonstrate efficacy and safety. Additionally, scalability and manufacturing challenges associated with cell sheet production need to be addressed to facilitate widespread adoption.
As the field advances, there is growing interest in combining cell sheet engineering with other cutting-edge technologies, such as 3D bioprinting and gene editing, to enhance therapeutic outcomes. This convergence is expected to open up new market opportunities and drive innovation in tissue engineering and regenerative medicine.
Current Challenges in Cell Sheet Engineering
Cell sheet engineering has emerged as a promising approach in tissue engineering and regenerative medicine. However, several challenges persist in this field, hindering its widespread application and clinical translation. One of the primary obstacles is maintaining cell viability and functionality during the cell sheet detachment process. The use of isotonic solutions plays a crucial role in addressing this challenge, but optimizing their composition and application remains complex.
The detachment of cell sheets from culture surfaces is a critical step that can significantly impact the quality and integrity of the engineered tissue. Traditional enzymatic methods often compromise cell-cell junctions and extracellular matrix (ECM) proteins, leading to reduced functionality of the resulting tissue. While temperature-responsive culture surfaces have shown promise, they still face limitations in terms of cell sheet thickness and detachment efficiency.
Another significant challenge is the preservation of cell sheet structure and function during transportation and implantation. Isotonic solutions are essential in maintaining osmotic balance and preventing cell damage, but finding the ideal formulation that supports both structural integrity and cellular metabolism is an ongoing area of research. The balance between providing necessary nutrients and avoiding excessive swelling or shrinkage of the cell sheet is delicate and requires further optimization.
The scalability of cell sheet engineering techniques also presents a considerable hurdle. As researchers aim to create larger and more complex tissue constructs, the role of isotonic solutions becomes increasingly important in maintaining uniform cell viability across the entire sheet. Ensuring adequate nutrient and oxygen diffusion throughout thicker cell sheets remains a significant challenge, particularly in the absence of vascularization.
Furthermore, the long-term stability of engineered cell sheets in vivo is a concern that requires attention. While isotonic solutions can help in the initial preservation and implantation of cell sheets, their role in promoting long-term integration and functionality is still not fully understood. Developing strategies to enhance the survival and integration of implanted cell sheets, possibly through the incorporation of growth factors or other bioactive molecules in isotonic solutions, is an area of active investigation.
Lastly, the standardization of protocols for cell sheet engineering, including the composition and application of isotonic solutions, remains a challenge. Variability in cell sources, culture conditions, and detachment methods can lead to inconsistent results across different laboratories and studies. Establishing robust, reproducible protocols that incorporate optimized isotonic solutions is crucial for the advancement of cell sheet engineering towards clinical applications.
The detachment of cell sheets from culture surfaces is a critical step that can significantly impact the quality and integrity of the engineered tissue. Traditional enzymatic methods often compromise cell-cell junctions and extracellular matrix (ECM) proteins, leading to reduced functionality of the resulting tissue. While temperature-responsive culture surfaces have shown promise, they still face limitations in terms of cell sheet thickness and detachment efficiency.
Another significant challenge is the preservation of cell sheet structure and function during transportation and implantation. Isotonic solutions are essential in maintaining osmotic balance and preventing cell damage, but finding the ideal formulation that supports both structural integrity and cellular metabolism is an ongoing area of research. The balance between providing necessary nutrients and avoiding excessive swelling or shrinkage of the cell sheet is delicate and requires further optimization.
The scalability of cell sheet engineering techniques also presents a considerable hurdle. As researchers aim to create larger and more complex tissue constructs, the role of isotonic solutions becomes increasingly important in maintaining uniform cell viability across the entire sheet. Ensuring adequate nutrient and oxygen diffusion throughout thicker cell sheets remains a significant challenge, particularly in the absence of vascularization.
Furthermore, the long-term stability of engineered cell sheets in vivo is a concern that requires attention. While isotonic solutions can help in the initial preservation and implantation of cell sheets, their role in promoting long-term integration and functionality is still not fully understood. Developing strategies to enhance the survival and integration of implanted cell sheets, possibly through the incorporation of growth factors or other bioactive molecules in isotonic solutions, is an area of active investigation.
Lastly, the standardization of protocols for cell sheet engineering, including the composition and application of isotonic solutions, remains a challenge. Variability in cell sources, culture conditions, and detachment methods can lead to inconsistent results across different laboratories and studies. Establishing robust, reproducible protocols that incorporate optimized isotonic solutions is crucial for the advancement of cell sheet engineering towards clinical applications.
Isotonic Solution Formulations for Cell Sheets
01 Composition of isotonic solutions
Isotonic solutions are formulated to have the same osmotic pressure as body fluids, typically containing a balance of electrolytes and other solutes. These solutions are crucial in medical applications, including intravenous therapy and cell culture media. The composition often includes sodium chloride, potassium chloride, and other essential ions to maintain cellular balance.- Composition of isotonic solutions: Isotonic solutions are formulated to have the same osmotic pressure as body fluids, typically containing a balance of electrolytes and other solutes. These solutions are crucial in medical applications, including intravenous therapy and cell culture media, as they maintain cellular integrity and prevent osmotic shock.
- Medical applications of isotonic solutions: Isotonic solutions are widely used in various medical procedures, such as wound irrigation, eye care, and nasal sprays. They are also employed in dialysis treatments and as a base for drug delivery systems, ensuring optimal absorption and minimizing adverse effects on body tissues.
- Isotonic solutions in sports and fitness: Isotonic drinks and solutions play a significant role in sports nutrition and hydration. These formulations help athletes maintain proper fluid balance, replace electrolytes lost through sweat, and improve performance during intense physical activities or prolonged exercise.
- Manufacturing and quality control of isotonic solutions: The production of isotonic solutions involves precise formulation, mixing, and sterilization processes. Quality control measures are implemented to ensure consistency, purity, and safety of the final product. Advanced technologies and equipment are used to monitor and maintain the isotonicity of solutions during manufacturing.
- Innovative applications and delivery systems for isotonic solutions: Recent advancements in isotonic solution technology include the development of novel delivery systems, such as smart infusion pumps and wearable devices for continuous administration. Additionally, research is ongoing to explore the use of isotonic solutions in emerging fields like regenerative medicine and 3D bioprinting.
02 Medical applications of isotonic solutions
Isotonic solutions have various medical applications, including use in intravenous fluids, dialysis, wound irrigation, and ophthalmic preparations. They are designed to be compatible with body tissues and fluids, minimizing cellular disturbances. These solutions play a critical role in maintaining hydration, electrolyte balance, and proper cellular function in medical treatments.Expand Specific Solutions03 Isotonic solutions in sports and exercise
Isotonic solutions are widely used in sports and exercise for hydration and electrolyte replacement. These beverages are formulated to closely match the concentration of solutes in the body's fluids, allowing for rapid absorption and rehydration. They typically contain carbohydrates and electrolytes to replenish energy and maintain fluid balance during physical activity.Expand Specific Solutions04 Production and quality control of isotonic solutions
The production of isotonic solutions involves precise formulation and quality control measures to ensure consistency and safety. This includes careful selection and measurement of ingredients, sterile manufacturing processes, and rigorous testing for osmolality, pH, and contaminants. Advanced technologies and automated systems are often employed to maintain accuracy and sterility throughout the production process.Expand Specific Solutions05 Innovative applications of isotonic solutions
Recent innovations have expanded the use of isotonic solutions beyond traditional applications. These include their use in advanced drug delivery systems, tissue engineering, and regenerative medicine. Isotonic solutions are being tailored for specific cellular environments, incorporating growth factors or other bioactive molecules to enhance their therapeutic potential in various medical and biotechnological applications.Expand Specific Solutions
Key Players in Cell Sheet Technology
The cell sheet engineering techniques using isotonic solutions are in a nascent stage of development, with the market still emerging and relatively small. The technology is progressing but remains in early phases of maturity, with significant potential for growth. Key players like Novartis AG and Factor Bioscience are driving innovation in this field, leveraging their expertise in biotechnology and regenerative medicine. Universities such as Sichuan University and the Technical University of Denmark are contributing to fundamental research, while companies like Eastman Chemical Co. and Solvay Specialty Polymers Italy SpA are exploring materials applications. The competitive landscape is diverse, with pharmaceutical, chemical, and biotech firms collaborating with academic institutions to advance the technology.
Novartis AG
Technical Solution: Novartis has made significant advancements in cell sheet engineering techniques using isotonic solutions, particularly in the context of regenerative medicine and tissue engineering. Their approach involves the use of specialized isotonic culture media supplemented with specific growth factors and small molecules to promote the formation and maintenance of cell sheets[5]. One of their key innovations is the development of a gradient isotonic solution system that allows for the controlled detachment and manipulation of cell sheets. This system creates a subtle osmotic gradient that facilitates the gentle lifting of cell sheets from culture surfaces without disrupting cell-cell interactions[6]. Additionally, Novartis has explored the use of isotonic hydrogel-based substrates that can be dissolved under physiological conditions, allowing for the easy release of intact cell sheets[7].
Strengths: Advanced control over cell sheet formation and detachment, potential for large-scale production of cell sheets for clinical applications. Weaknesses: May require complex and costly culture media formulations, potential regulatory challenges for clinical translation.
Sichuan University
Technical Solution: Researchers at Sichuan University have developed innovative approaches to cell sheet engineering using isotonic solutions. Their work focuses on creating biomimetic microenvironments that promote the formation of functional cell sheets. One of their key contributions is the development of a novel isotonic hydrogel system that mimics the extracellular matrix composition of specific tissues[8]. This hydrogel, when used as a culture substrate, allows for the growth of cells in sheet-like formations while providing essential biochemical and mechanical cues. The isotonic nature of the hydrogel ensures optimal cell viability and function. Additionally, the team has explored the use of magnetically responsive nanoparticles incorporated into isotonic solutions to facilitate the non-invasive manipulation and stacking of cell sheets[9]. This approach enables the creation of complex, multi-layered tissue constructs while maintaining physiological osmolarity throughout the process.
Strengths: Biomimetic approach enhances cell sheet functionality, innovative methods for multi-layer tissue construction. Weaknesses: Complexity of hydrogel formulations may pose challenges for large-scale production, potential long-term effects of nanoparticles need further investigation.
Innovations in Isotonic Solution Research
Method for producing sheet-like cell culture
PatentWO2019177148A1
Innovation
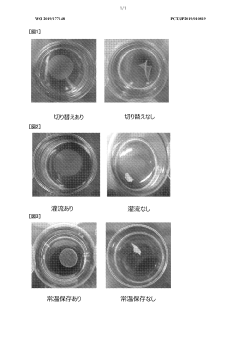
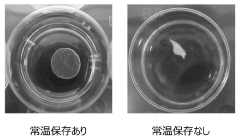
- A method involving immersion in a low-nutrient isotonic solution to suppress shrinkage and twisting, including soaking the cell culture before detachment, perfusing with a low-nutrient solution after detachment, and storing in a low-nutrient isotonic solution to maintain the integrity and quality of the cell culture.
Ionomeric membrane
PatentActiveEP1702670B1
Innovation
- Development of ionomeric membranes with sulphonic (per)fluorinated ionomers having side chains of the formula -O-CF2-CF2-SO3-, with controlled size variations and equivalent weight between 700 and 1600 g/eq, prepared through a process involving hydrolysis of -SO2F groups to -SO3H, and a specific membrane preparation process including dispersion deposition and thermal treatment, ensuring stability and integrity in hydroalcoholic solutions.
Regulatory Considerations for Cell-Based Products
The regulatory landscape for cell-based products is complex and evolving, particularly in the context of cell sheet engineering techniques utilizing isotonic solutions. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established guidelines for the development and commercialization of cell-based therapies. These guidelines address various aspects, including product characterization, quality control, safety, and efficacy.
For cell sheet engineering techniques involving isotonic solutions, regulatory considerations focus on several key areas. First, the composition and quality of the isotonic solutions used in the process must be carefully controlled and documented. This includes ensuring the purity, sterility, and consistency of the solutions, as well as demonstrating their compatibility with the cell types being used.
Safety considerations are paramount in regulatory assessments. Manufacturers must provide comprehensive data on the potential risks associated with the use of isotonic solutions in cell sheet engineering, including any potential for contamination, immunogenicity, or unintended cellular modifications. Long-term stability studies and shelf-life determinations are also crucial components of the regulatory submission.
Efficacy data is another critical aspect of regulatory review. Developers must demonstrate that the use of isotonic solutions in cell sheet engineering techniques results in consistent and reproducible outcomes. This includes providing evidence of cell viability, functionality, and structural integrity of the engineered cell sheets.
Manufacturing processes and quality control measures are subject to stringent regulatory scrutiny. Good Manufacturing Practice (GMP) compliance is essential, with particular attention paid to the production and handling of both the isotonic solutions and the resulting cell sheets. Validation of analytical methods used to characterize the products is also a key regulatory requirement.
Clinical trial design and execution for cell-based products using these techniques must adhere to regulatory guidelines. This includes appropriate patient selection, endpoint definition, and long-term follow-up protocols. Regulatory agencies may require specific post-market surveillance plans to monitor the safety and efficacy of these products in real-world settings.
As the field of cell sheet engineering advances, regulatory frameworks are likely to evolve. Developers and manufacturers must stay abreast of changing regulations and engage in ongoing dialogue with regulatory authorities to ensure compliance and facilitate the path to market approval for these innovative therapies.
For cell sheet engineering techniques involving isotonic solutions, regulatory considerations focus on several key areas. First, the composition and quality of the isotonic solutions used in the process must be carefully controlled and documented. This includes ensuring the purity, sterility, and consistency of the solutions, as well as demonstrating their compatibility with the cell types being used.
Safety considerations are paramount in regulatory assessments. Manufacturers must provide comprehensive data on the potential risks associated with the use of isotonic solutions in cell sheet engineering, including any potential for contamination, immunogenicity, or unintended cellular modifications. Long-term stability studies and shelf-life determinations are also crucial components of the regulatory submission.
Efficacy data is another critical aspect of regulatory review. Developers must demonstrate that the use of isotonic solutions in cell sheet engineering techniques results in consistent and reproducible outcomes. This includes providing evidence of cell viability, functionality, and structural integrity of the engineered cell sheets.
Manufacturing processes and quality control measures are subject to stringent regulatory scrutiny. Good Manufacturing Practice (GMP) compliance is essential, with particular attention paid to the production and handling of both the isotonic solutions and the resulting cell sheets. Validation of analytical methods used to characterize the products is also a key regulatory requirement.
Clinical trial design and execution for cell-based products using these techniques must adhere to regulatory guidelines. This includes appropriate patient selection, endpoint definition, and long-term follow-up protocols. Regulatory agencies may require specific post-market surveillance plans to monitor the safety and efficacy of these products in real-world settings.
As the field of cell sheet engineering advances, regulatory frameworks are likely to evolve. Developers and manufacturers must stay abreast of changing regulations and engage in ongoing dialogue with regulatory authorities to ensure compliance and facilitate the path to market approval for these innovative therapies.
Scalability of Cell Sheet Production
The scalability of cell sheet production is a critical factor in the widespread adoption of cell sheet engineering techniques for regenerative medicine and tissue engineering applications. As the demand for cell-based therapies continues to grow, the ability to produce large quantities of high-quality cell sheets becomes increasingly important.
One of the key challenges in scaling up cell sheet production is maintaining consistent quality across larger batches. This includes ensuring uniform cell distribution, thickness, and extracellular matrix composition throughout the sheet. The use of isotonic solutions plays a crucial role in addressing these challenges by providing a stable environment for cell growth and sheet formation.
Automated systems have been developed to enhance the scalability of cell sheet production. These systems often incorporate temperature-responsive culture surfaces and precise control of isotonic solution composition and flow. By automating the process, it becomes possible to produce multiple cell sheets simultaneously while maintaining consistent quality.
The choice of culture vessel is another important consideration for scalable production. Large-scale bioreactors designed specifically for cell sheet engineering have been developed, allowing for the cultivation of cell sheets with larger surface areas. These bioreactors often incorporate advanced monitoring and control systems to maintain optimal conditions throughout the culture period.
Optimizing the composition of isotonic solutions is crucial for successful scale-up. Factors such as pH, osmolarity, and nutrient concentration must be carefully balanced to support cell growth and sheet formation across larger volumes. Additionally, the use of serum-free or chemically defined media formulations can improve reproducibility and reduce batch-to-batch variability.
The development of cryopreservation techniques for cell sheets has also contributed to improved scalability. By allowing for the storage and transport of pre-formed cell sheets, it becomes possible to decouple production from clinical application, enabling centralized manufacturing and distribution.
As production scales increase, quality control measures become increasingly important. Non-invasive imaging techniques and automated analysis tools have been developed to assess cell sheet quality without compromising their integrity. These tools allow for real-time monitoring of sheet formation and can help identify and address issues early in the production process.
While significant progress has been made in scaling up cell sheet production, challenges remain. Further research is needed to optimize isotonic solution formulations for different cell types and to develop more efficient harvesting methods for large-scale production. Additionally, regulatory considerations and standardization of production processes will be crucial for the widespread clinical adoption of cell sheet therapies.
One of the key challenges in scaling up cell sheet production is maintaining consistent quality across larger batches. This includes ensuring uniform cell distribution, thickness, and extracellular matrix composition throughout the sheet. The use of isotonic solutions plays a crucial role in addressing these challenges by providing a stable environment for cell growth and sheet formation.
Automated systems have been developed to enhance the scalability of cell sheet production. These systems often incorporate temperature-responsive culture surfaces and precise control of isotonic solution composition and flow. By automating the process, it becomes possible to produce multiple cell sheets simultaneously while maintaining consistent quality.
The choice of culture vessel is another important consideration for scalable production. Large-scale bioreactors designed specifically for cell sheet engineering have been developed, allowing for the cultivation of cell sheets with larger surface areas. These bioreactors often incorporate advanced monitoring and control systems to maintain optimal conditions throughout the culture period.
Optimizing the composition of isotonic solutions is crucial for successful scale-up. Factors such as pH, osmolarity, and nutrient concentration must be carefully balanced to support cell growth and sheet formation across larger volumes. Additionally, the use of serum-free or chemically defined media formulations can improve reproducibility and reduce batch-to-batch variability.
The development of cryopreservation techniques for cell sheets has also contributed to improved scalability. By allowing for the storage and transport of pre-formed cell sheets, it becomes possible to decouple production from clinical application, enabling centralized manufacturing and distribution.
As production scales increase, quality control measures become increasingly important. Non-invasive imaging techniques and automated analysis tools have been developed to assess cell sheet quality without compromising their integrity. These tools allow for real-time monitoring of sheet formation and can help identify and address issues early in the production process.
While significant progress has been made in scaling up cell sheet production, challenges remain. Further research is needed to optimize isotonic solution formulations for different cell types and to develop more efficient harvesting methods for large-scale production. Additionally, regulatory considerations and standardization of production processes will be crucial for the widespread clinical adoption of cell sheet therapies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!