Optimizing isotonic solutions for serological test accuracy
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions Background and Objectives
Isotonic solutions have played a crucial role in serological testing for decades, serving as a cornerstone in maintaining the integrity and accuracy of diagnostic procedures. These solutions, characterized by their osmotic pressure equivalence to bodily fluids, have evolved significantly since their inception in the early 20th century. The primary objective of isotonic solutions in serological tests is to provide a stable environment for blood components, particularly antibodies and antigens, ensuring their structural and functional preservation during the testing process.
The development of isotonic solutions has been closely tied to advancements in medical science and biochemistry. Initially, simple saline solutions were used, but as understanding of cellular physiology deepened, more sophisticated formulations emerged. These include balanced salt solutions and complex media that mimic the physiological environment more accurately. The evolution of isotonic solutions has been driven by the need for increased test sensitivity, specificity, and reproducibility in serological diagnostics.
In recent years, the focus has shifted towards optimizing isotonic solutions specifically for enhancing serological test accuracy. This objective is particularly pertinent in the context of emerging infectious diseases, autoimmune disorders, and the growing demand for rapid, point-of-care diagnostics. The optimization process involves fine-tuning the composition of isotonic solutions to minimize interference with test reagents while maximizing the stability and reactivity of target molecules.
Key objectives in the current landscape of isotonic solution development include reducing non-specific binding, enhancing signal-to-noise ratios, and improving the overall robustness of serological assays. Researchers are exploring novel additives, such as surfactants and stabilizers, to achieve these goals. Additionally, there is a growing interest in developing isotonic solutions that can extend the shelf life of serological test kits, particularly for use in resource-limited settings or during global health emergencies.
The optimization of isotonic solutions also aims to address challenges in multiplex serological testing, where multiple analytes are detected simultaneously. This requires solutions that can maintain the stability and specificity of various biomarkers without cross-reactivity. Furthermore, as personalized medicine gains traction, there is an emerging trend towards tailoring isotonic solutions for specific patient populations or disease profiles, potentially improving diagnostic accuracy in diverse clinical scenarios.
Looking ahead, the field of isotonic solution optimization is poised to integrate cutting-edge technologies such as nanotechnology and microfluidics. These advancements promise to revolutionize the formulation and application of isotonic solutions, potentially leading to more sensitive, rapid, and cost-effective serological tests. The ultimate goal remains to enhance the accuracy and reliability of serological diagnostics, contributing to improved patient care and public health outcomes worldwide.
The development of isotonic solutions has been closely tied to advancements in medical science and biochemistry. Initially, simple saline solutions were used, but as understanding of cellular physiology deepened, more sophisticated formulations emerged. These include balanced salt solutions and complex media that mimic the physiological environment more accurately. The evolution of isotonic solutions has been driven by the need for increased test sensitivity, specificity, and reproducibility in serological diagnostics.
In recent years, the focus has shifted towards optimizing isotonic solutions specifically for enhancing serological test accuracy. This objective is particularly pertinent in the context of emerging infectious diseases, autoimmune disorders, and the growing demand for rapid, point-of-care diagnostics. The optimization process involves fine-tuning the composition of isotonic solutions to minimize interference with test reagents while maximizing the stability and reactivity of target molecules.
Key objectives in the current landscape of isotonic solution development include reducing non-specific binding, enhancing signal-to-noise ratios, and improving the overall robustness of serological assays. Researchers are exploring novel additives, such as surfactants and stabilizers, to achieve these goals. Additionally, there is a growing interest in developing isotonic solutions that can extend the shelf life of serological test kits, particularly for use in resource-limited settings or during global health emergencies.
The optimization of isotonic solutions also aims to address challenges in multiplex serological testing, where multiple analytes are detected simultaneously. This requires solutions that can maintain the stability and specificity of various biomarkers without cross-reactivity. Furthermore, as personalized medicine gains traction, there is an emerging trend towards tailoring isotonic solutions for specific patient populations or disease profiles, potentially improving diagnostic accuracy in diverse clinical scenarios.
Looking ahead, the field of isotonic solution optimization is poised to integrate cutting-edge technologies such as nanotechnology and microfluidics. These advancements promise to revolutionize the formulation and application of isotonic solutions, potentially leading to more sensitive, rapid, and cost-effective serological tests. The ultimate goal remains to enhance the accuracy and reliability of serological diagnostics, contributing to improved patient care and public health outcomes worldwide.
Serological Test Market Analysis
The serological test market has experienced significant growth in recent years, driven by the increasing prevalence of infectious diseases and the growing demand for accurate diagnostic tools. This market segment is characterized by its diverse applications, ranging from clinical diagnostics to epidemiological studies and vaccine development.
The global serological test market size was valued at approximately $2.3 billion in 2020 and is projected to reach $3.8 billion by 2026, growing at a CAGR of 8.5% during the forecast period. This growth is primarily attributed to the rising incidence of chronic and infectious diseases, technological advancements in serological testing methods, and the increasing adoption of point-of-care testing.
Geographically, North America dominates the serological test market, accounting for the largest market share due to well-established healthcare infrastructure, high healthcare expenditure, and the presence of key market players. Europe follows closely, driven by increasing research and development activities and government initiatives to improve diagnostic capabilities. The Asia-Pacific region is expected to witness the highest growth rate, fueled by improving healthcare systems, rising awareness about early disease detection, and the growing geriatric population.
The market is segmented based on technology, application, and end-user. Enzyme-linked immunosorbent assay (ELISA) remains the most widely used technology, owing to its high sensitivity and specificity. However, rapid diagnostic tests are gaining traction due to their ease of use and quick turnaround time. In terms of applications, infectious disease testing holds the largest market share, followed by oncology and endocrinology.
Key players in the serological test market include Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers, and Bio-Rad Laboratories. These companies are focusing on strategic collaborations, product innovations, and geographical expansions to maintain their market positions and gain a competitive edge.
The COVID-19 pandemic has significantly impacted the serological test market, leading to a surge in demand for antibody tests. This has accelerated the development and adoption of novel serological testing technologies, including multiplex assays and automated platforms. The pandemic has also highlighted the importance of accurate and reliable serological tests in managing public health crises and guiding policy decisions.
Looking ahead, the serological test market is poised for continued growth, driven by factors such as the increasing focus on personalized medicine, advancements in biomarker discovery, and the rising demand for rapid and accurate diagnostic tools. However, challenges such as stringent regulatory requirements and the need for skilled professionals may hinder market growth to some extent.
The global serological test market size was valued at approximately $2.3 billion in 2020 and is projected to reach $3.8 billion by 2026, growing at a CAGR of 8.5% during the forecast period. This growth is primarily attributed to the rising incidence of chronic and infectious diseases, technological advancements in serological testing methods, and the increasing adoption of point-of-care testing.
Geographically, North America dominates the serological test market, accounting for the largest market share due to well-established healthcare infrastructure, high healthcare expenditure, and the presence of key market players. Europe follows closely, driven by increasing research and development activities and government initiatives to improve diagnostic capabilities. The Asia-Pacific region is expected to witness the highest growth rate, fueled by improving healthcare systems, rising awareness about early disease detection, and the growing geriatric population.
The market is segmented based on technology, application, and end-user. Enzyme-linked immunosorbent assay (ELISA) remains the most widely used technology, owing to its high sensitivity and specificity. However, rapid diagnostic tests are gaining traction due to their ease of use and quick turnaround time. In terms of applications, infectious disease testing holds the largest market share, followed by oncology and endocrinology.
Key players in the serological test market include Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers, and Bio-Rad Laboratories. These companies are focusing on strategic collaborations, product innovations, and geographical expansions to maintain their market positions and gain a competitive edge.
The COVID-19 pandemic has significantly impacted the serological test market, leading to a surge in demand for antibody tests. This has accelerated the development and adoption of novel serological testing technologies, including multiplex assays and automated platforms. The pandemic has also highlighted the importance of accurate and reliable serological tests in managing public health crises and guiding policy decisions.
Looking ahead, the serological test market is poised for continued growth, driven by factors such as the increasing focus on personalized medicine, advancements in biomarker discovery, and the rising demand for rapid and accurate diagnostic tools. However, challenges such as stringent regulatory requirements and the need for skilled professionals may hinder market growth to some extent.
Current Challenges in Isotonic Solution Formulation
The formulation of isotonic solutions for serological tests faces several significant challenges that impact test accuracy and reliability. One of the primary issues is maintaining the proper osmotic balance within the solution. Isotonic solutions must closely match the osmolarity of human blood to prevent cell lysis or crenation, which can lead to false results. Achieving this delicate balance is complicated by the variability in sample composition and the need for solutions to remain stable over time and across different storage conditions.
Another challenge lies in the selection and combination of buffer components. The choice of buffer can significantly affect the pH stability of the solution, which is crucial for maintaining the integrity of antibodies and antigens used in serological tests. Fluctuations in pH can alter the binding affinity between these biomolecules, potentially leading to inaccurate test results. Additionally, some buffer components may interact with test reagents or interfere with the detection methods, necessitating careful consideration and extensive testing of buffer formulations.
The presence of preservatives in isotonic solutions presents another hurdle. While preservatives are essential for preventing microbial growth and extending shelf life, they can also interfere with serological reactions or cause non-specific binding. Finding the right balance between effective preservation and minimal interference with test performance is a complex task that requires extensive optimization and validation studies.
Furthermore, the compatibility of isotonic solutions with various detection platforms and assay formats poses a significant challenge. Different serological tests may employ diverse detection methods, such as enzyme-linked immunosorbent assays (ELISA), chemiluminescence, or lateral flow assays. Each of these methods may have specific requirements for the isotonic solution, necessitating the development of versatile formulations that can perform consistently across multiple platforms.
Lastly, the scalability and cost-effectiveness of isotonic solution production present ongoing challenges. As serological testing becomes more widespread, particularly in resource-limited settings, there is a growing need for solutions that can be manufactured efficiently and economically without compromising quality or performance. This requires careful consideration of raw material sourcing, production processes, and quality control measures to ensure consistent and reliable formulations at scale.
Another challenge lies in the selection and combination of buffer components. The choice of buffer can significantly affect the pH stability of the solution, which is crucial for maintaining the integrity of antibodies and antigens used in serological tests. Fluctuations in pH can alter the binding affinity between these biomolecules, potentially leading to inaccurate test results. Additionally, some buffer components may interact with test reagents or interfere with the detection methods, necessitating careful consideration and extensive testing of buffer formulations.
The presence of preservatives in isotonic solutions presents another hurdle. While preservatives are essential for preventing microbial growth and extending shelf life, they can also interfere with serological reactions or cause non-specific binding. Finding the right balance between effective preservation and minimal interference with test performance is a complex task that requires extensive optimization and validation studies.
Furthermore, the compatibility of isotonic solutions with various detection platforms and assay formats poses a significant challenge. Different serological tests may employ diverse detection methods, such as enzyme-linked immunosorbent assays (ELISA), chemiluminescence, or lateral flow assays. Each of these methods may have specific requirements for the isotonic solution, necessitating the development of versatile formulations that can perform consistently across multiple platforms.
Lastly, the scalability and cost-effectiveness of isotonic solution production present ongoing challenges. As serological testing becomes more widespread, particularly in resource-limited settings, there is a growing need for solutions that can be manufactured efficiently and economically without compromising quality or performance. This requires careful consideration of raw material sourcing, production processes, and quality control measures to ensure consistent and reliable formulations at scale.
Existing Isotonic Solution Optimization Techniques
01 Formulation of isotonic solutions
Isotonic solutions are formulated to have the same osmotic pressure as body fluids, ensuring accuracy in medical applications. These solutions typically contain a balance of electrolytes and other solutes to match physiological conditions. Proper formulation is crucial for maintaining cell integrity and preventing osmotic shock in various medical and pharmaceutical applications.- Measurement techniques for isotonic solutions: Various measurement techniques are employed to ensure the accuracy of isotonic solutions. These may include osmometry, conductivity measurements, and refractive index analysis. Advanced sensors and analytical instruments are used to precisely determine the concentration and osmolality of the solutions, ensuring they match physiological conditions.
- Formulation and preparation methods: Accurate formulation and preparation methods are crucial for isotonic solutions. This involves precise weighing and mixing of solutes, careful pH adjustment, and sterile filtration processes. Advanced manufacturing techniques and quality control measures are implemented to ensure consistency and accuracy in large-scale production.
- Calibration and standardization procedures: Regular calibration and standardization of equipment and processes are essential for maintaining accuracy in isotonic solution preparation. This includes the use of reference standards, validation protocols, and inter-laboratory comparisons to ensure consistency and traceability of measurements across different batches and facilities.
- Automated systems for isotonic solution preparation: Automated systems and robotics are increasingly used in the preparation of isotonic solutions to improve accuracy and reduce human error. These systems incorporate precise liquid handling, real-time monitoring, and feedback control mechanisms to ensure consistent and accurate formulation of isotonic solutions.
- Quality control and stability testing: Rigorous quality control measures and stability testing protocols are implemented to ensure the accuracy and consistency of isotonic solutions over time. This includes periodic testing of osmolality, pH, and chemical composition, as well as accelerated stability studies to predict long-term performance under various storage conditions.
02 Measurement and control of osmolality
Accurate measurement and control of osmolality are essential for ensuring the isotonicity of solutions. Advanced techniques and instruments are employed to precisely determine and adjust the osmotic pressure of solutions. This includes the use of osmometers, freezing point depression methods, and vapor pressure techniques to achieve high accuracy in isotonic solution preparation.Expand Specific Solutions03 Quality control and stability testing
Rigorous quality control measures and stability testing are implemented to maintain the accuracy of isotonic solutions over time. This involves monitoring pH, conductivity, and osmolality during production and storage. Stability studies are conducted to ensure that the isotonic properties of the solutions remain consistent throughout their shelf life, guaranteeing their efficacy in medical applications.Expand Specific Solutions04 Automated systems for preparation and dispensing
Automated systems are developed for the preparation and dispensing of isotonic solutions to enhance accuracy and reduce human error. These systems utilize precise volumetric measurements, real-time monitoring of solution parameters, and advanced control algorithms to ensure consistent and accurate production of isotonic solutions. Automated dispensing systems also help maintain sterility and reduce contamination risks.Expand Specific Solutions05 Specialized packaging and delivery systems
Innovative packaging and delivery systems are designed to maintain the accuracy of isotonic solutions during storage and administration. These include specialized containers with barrier properties to prevent moisture loss or gain, as well as advanced delivery devices that ensure precise dosing. Such systems help preserve the isotonic properties of the solutions and enhance their overall accuracy in clinical applications.Expand Specific Solutions
Key Players in Serological Test Industry
The optimization of isotonic solutions for serological test accuracy is in a mature stage of development, with a substantial market size driven by the growing demand for precise diagnostic tools. The technology has reached a high level of maturity, with key players like Thermo Fisher Scientific, Novartis AG, and Roche leading the field. These companies leverage their extensive R&D capabilities and global presence to continually refine isotonic solution formulations. The competitive landscape is characterized by a mix of established pharmaceutical giants and specialized diagnostic firms, each contributing to advancements in serological testing accuracy through innovative approaches and proprietary technologies.
Thermo Fisher Scientific (Bremen) GmbH
Technical Solution: Thermo Fisher Scientific has developed advanced isotonic solution optimization techniques for serological test accuracy. Their approach involves using high-precision mass spectrometry to analyze and fine-tune the composition of isotonic solutions[1]. By employing their proprietary Orbitrap technology, they can detect minute changes in solution osmolality and ionic balance, allowing for real-time adjustments to maintain optimal conditions for serological tests[2]. The company has also implemented machine learning algorithms to predict the most effective isotonic formulations based on specific test requirements and environmental factors[3]. This predictive capability significantly reduces the time and resources needed for solution optimization, enhancing overall test efficiency and reliability.
Strengths: Cutting-edge mass spectrometry technology, predictive algorithms for rapid optimization, and extensive experience in life sciences. Weaknesses: Potentially higher costs associated with advanced technology implementation and the need for specialized training for operators.
Novartis AG
Technical Solution: Novartis AG has developed a multifaceted approach to optimizing isotonic solutions for serological test accuracy, leveraging their extensive experience in pharmaceutical research and development. Their method incorporates advanced biophysical characterization techniques, including dynamic light scattering and circular dichroism, to analyze the behavior of proteins and antibodies in various isotonic environments[1]. Novartis has also implemented high-throughput screening platforms to rapidly assess the performance of different isotonic formulations across a wide range of serological tests[2]. The company's approach includes the use of artificial intelligence algorithms to predict optimal solution compositions based on molecular interactions and test-specific requirements[3]. Additionally, Novartis has developed novel stabilizing agents and buffer systems that enhance the long-term stability of isotonic solutions, ensuring consistent test performance over extended periods.
Strengths: Comprehensive biophysical characterization capabilities, high-throughput screening platforms, and expertise in developing stable formulations. Weaknesses: Potential focus on pharmaceutical applications may limit adaptability to other fields of serological testing.
Innovative Approaches in Solution Formulation
Anti-tigit antibodies and uses thereof
PatentWO2021092196A1
Innovation
- Development of anti-TIGIT antibodies with specific epitope binding capabilities, including residues Q53, T55, Y113, and P114, that enhance antibody-dependent cell-mediated cytotoxicity and mixed lymphocyte reactions, and block interactions with CD155 and CD112, demonstrating potent ADCC and MLR potentiation across species.
Reagent for diluting blood sample and method for measuring mean corpuscular volume by using the same
PatentInactiveEP2169402A1
Innovation
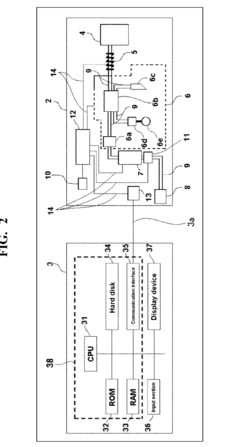
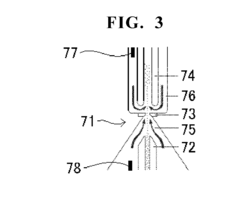
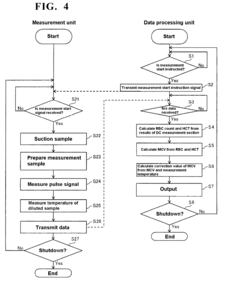
- A blood sample-diluting reagent comprising water, polyoxyethylene alkyl ether with a hydroxyl value of 52 to 60, and an osmo-regulator to maintain osmotic pressure between 150 to 400 mOsm/kg, which includes sodium chloride, and optional additives like buffers, oxidant inhibitors, and antiseptic agents, to stabilize MCV measurements at temperatures up to 20°C or less.
Regulatory Framework for Diagnostic Reagents
The regulatory framework for diagnostic reagents plays a crucial role in ensuring the safety, efficacy, and quality of serological tests, including those utilizing isotonic solutions. In the context of optimizing isotonic solutions for serological test accuracy, understanding and adhering to these regulations is paramount.
Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe have established comprehensive guidelines for the development, manufacturing, and marketing of diagnostic reagents. These regulations cover various aspects, including product classification, pre-market approval processes, quality management systems, and post-market surveillance.
For isotonic solutions used in serological tests, specific regulations focus on the composition, purity, and stability of the reagents. Manufacturers must demonstrate that their isotonic solutions meet stringent quality standards and do not interfere with the accuracy of test results. This often involves extensive validation studies and documentation of manufacturing processes.
The regulatory framework also addresses the labeling and packaging requirements for diagnostic reagents. Clear and accurate labeling is essential for proper use and interpretation of serological tests, particularly when optimized isotonic solutions are employed to enhance test accuracy.
In recent years, regulatory bodies have placed increased emphasis on the analytical and clinical performance of diagnostic tests. This has led to more rigorous requirements for demonstrating the sensitivity, specificity, and overall accuracy of serological tests, including the impact of isotonic solution optimization on these parameters.
Manufacturers seeking to introduce optimized isotonic solutions for serological tests must navigate a complex regulatory landscape. This often involves submitting detailed technical documentation, conducting clinical trials, and obtaining necessary certifications or approvals before bringing their products to market.
The regulatory framework also extends to quality control and assurance measures throughout the product lifecycle. Manufacturers are required to implement robust quality management systems, conduct regular audits, and maintain traceability of their products. This ensures that the optimized isotonic solutions consistently meet the required standards for serological test accuracy.
As the field of diagnostics continues to evolve, regulatory bodies are adapting their frameworks to address emerging technologies and methodologies. This includes considerations for novel approaches to optimizing isotonic solutions and their potential impact on serological test performance.
Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe have established comprehensive guidelines for the development, manufacturing, and marketing of diagnostic reagents. These regulations cover various aspects, including product classification, pre-market approval processes, quality management systems, and post-market surveillance.
For isotonic solutions used in serological tests, specific regulations focus on the composition, purity, and stability of the reagents. Manufacturers must demonstrate that their isotonic solutions meet stringent quality standards and do not interfere with the accuracy of test results. This often involves extensive validation studies and documentation of manufacturing processes.
The regulatory framework also addresses the labeling and packaging requirements for diagnostic reagents. Clear and accurate labeling is essential for proper use and interpretation of serological tests, particularly when optimized isotonic solutions are employed to enhance test accuracy.
In recent years, regulatory bodies have placed increased emphasis on the analytical and clinical performance of diagnostic tests. This has led to more rigorous requirements for demonstrating the sensitivity, specificity, and overall accuracy of serological tests, including the impact of isotonic solution optimization on these parameters.
Manufacturers seeking to introduce optimized isotonic solutions for serological tests must navigate a complex regulatory landscape. This often involves submitting detailed technical documentation, conducting clinical trials, and obtaining necessary certifications or approvals before bringing their products to market.
The regulatory framework also extends to quality control and assurance measures throughout the product lifecycle. Manufacturers are required to implement robust quality management systems, conduct regular audits, and maintain traceability of their products. This ensures that the optimized isotonic solutions consistently meet the required standards for serological test accuracy.
As the field of diagnostics continues to evolve, regulatory bodies are adapting their frameworks to address emerging technologies and methodologies. This includes considerations for novel approaches to optimizing isotonic solutions and their potential impact on serological test performance.
Quality Control Measures for Isotonic Solutions
Quality control measures for isotonic solutions are critical in ensuring the accuracy and reliability of serological tests. These measures encompass a range of procedures and protocols designed to maintain the integrity of the solutions throughout their preparation, storage, and use.
One of the primary quality control measures is the precise formulation of isotonic solutions. This involves carefully calculating and measuring the concentrations of solutes to achieve the desired osmolality. Typically, a solution with an osmolality of 290-310 mOsm/kg is considered isotonic. Regular calibration of equipment used for measuring osmolality, such as osmometers, is essential to maintain accuracy.
pH monitoring is another crucial aspect of quality control. Isotonic solutions used in serological tests should generally have a pH between 7.2 and 7.4 to mimic physiological conditions. Routine pH checks using calibrated pH meters help ensure that the solutions remain within the acceptable range throughout their shelf life.
Sterility testing is a vital quality control measure to prevent microbial contamination. This involves aseptic techniques during preparation and regular microbiological testing of samples. Membrane filtration or direct inoculation methods can be employed to detect any bacterial or fungal growth in the solutions.
Stability testing is conducted to assess the long-term integrity of isotonic solutions. This includes evaluating the solutions under various storage conditions and time periods to determine their shelf life. Accelerated stability studies can provide valuable information on potential degradation products and help establish appropriate storage recommendations.
Visual inspection is a simple yet effective quality control measure. Solutions should be examined for clarity, color, and the absence of visible particles. Any signs of turbidity, discoloration, or particulate matter warrant further investigation and potential rejection of the batch.
Chemical analysis forms an integral part of quality control for isotonic solutions. This may include techniques such as high-performance liquid chromatography (HPLC) or mass spectrometry to verify the concentration and purity of key components. Regular analysis helps detect any degradation or contamination that may occur over time.
Documentation and traceability are essential components of quality control. Detailed records of raw materials, preparation methods, batch numbers, and test results should be maintained. This allows for easy tracking and investigation in case of any issues or discrepancies.
Implementing these quality control measures ensures that isotonic solutions used in serological tests meet the required specifications, thereby contributing to the overall accuracy and reliability of the test results.
One of the primary quality control measures is the precise formulation of isotonic solutions. This involves carefully calculating and measuring the concentrations of solutes to achieve the desired osmolality. Typically, a solution with an osmolality of 290-310 mOsm/kg is considered isotonic. Regular calibration of equipment used for measuring osmolality, such as osmometers, is essential to maintain accuracy.
pH monitoring is another crucial aspect of quality control. Isotonic solutions used in serological tests should generally have a pH between 7.2 and 7.4 to mimic physiological conditions. Routine pH checks using calibrated pH meters help ensure that the solutions remain within the acceptable range throughout their shelf life.
Sterility testing is a vital quality control measure to prevent microbial contamination. This involves aseptic techniques during preparation and regular microbiological testing of samples. Membrane filtration or direct inoculation methods can be employed to detect any bacterial or fungal growth in the solutions.
Stability testing is conducted to assess the long-term integrity of isotonic solutions. This includes evaluating the solutions under various storage conditions and time periods to determine their shelf life. Accelerated stability studies can provide valuable information on potential degradation products and help establish appropriate storage recommendations.
Visual inspection is a simple yet effective quality control measure. Solutions should be examined for clarity, color, and the absence of visible particles. Any signs of turbidity, discoloration, or particulate matter warrant further investigation and potential rejection of the batch.
Chemical analysis forms an integral part of quality control for isotonic solutions. This may include techniques such as high-performance liquid chromatography (HPLC) or mass spectrometry to verify the concentration and purity of key components. Regular analysis helps detect any degradation or contamination that may occur over time.
Documentation and traceability are essential components of quality control. Detailed records of raw materials, preparation methods, batch numbers, and test results should be maintained. This allows for easy tracking and investigation in case of any issues or discrepancies.
Implementing these quality control measures ensures that isotonic solutions used in serological tests meet the required specifications, thereby contributing to the overall accuracy and reliability of the test results.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!