Isotonic solutions and their role in microencapsulation efficiency
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions Overview
Isotonic solutions play a crucial role in microencapsulation efficiency, serving as a vital component in the process of encapsulating active ingredients within microscopic capsules. These solutions are characterized by their osmotic pressure, which is equivalent to that of body fluids, typically around 290 mOsm/kg. This property is essential for maintaining the stability and integrity of microcapsules during the encapsulation process and subsequent storage.
The concept of isotonicity is fundamental to understanding the behavior of cells and particles in solution. In the context of microencapsulation, isotonic solutions help prevent osmotic stress on the encapsulated materials and the capsule membrane itself. This is particularly important when dealing with sensitive biological materials, such as proteins, enzymes, or living cells, which can be easily damaged by changes in osmotic pressure.
Isotonic solutions commonly used in microencapsulation include saline (0.9% sodium chloride), phosphate-buffered saline (PBS), and various balanced salt solutions. These solutions provide a stable environment for the encapsulation process, ensuring that the active ingredients remain intact and functional throughout the procedure. The choice of isotonic solution depends on the specific requirements of the encapsulated material and the intended application of the microcapsules.
The use of isotonic solutions in microencapsulation offers several advantages. Firstly, it helps maintain the size and shape of the microcapsules by preventing excessive swelling or shrinkage due to osmotic pressure differences. This is crucial for achieving consistent and predictable release profiles of the encapsulated substances. Secondly, isotonic conditions protect the structural integrity of the capsule membrane, reducing the risk of premature rupture or leakage of the encapsulated material.
Furthermore, isotonic solutions contribute to the overall stability of the microencapsulation system. They help preserve the activity of encapsulated biomolecules by mimicking physiological conditions, which is especially important for applications in drug delivery, cell therapy, and food technology. The use of isotonic solutions also facilitates the handling and storage of microcapsules, as it minimizes the risk of degradation or loss of functionality over time.
In recent years, research has focused on optimizing isotonic solutions for specific microencapsulation applications. This includes the development of custom-formulated isotonic media that not only maintain osmotic balance but also provide additional benefits such as enhanced biocompatibility, improved shelf life, or targeted release properties. These advancements have expanded the potential applications of microencapsulation technology across various industries, including pharmaceuticals, cosmetics, and agriculture.
The concept of isotonicity is fundamental to understanding the behavior of cells and particles in solution. In the context of microencapsulation, isotonic solutions help prevent osmotic stress on the encapsulated materials and the capsule membrane itself. This is particularly important when dealing with sensitive biological materials, such as proteins, enzymes, or living cells, which can be easily damaged by changes in osmotic pressure.
Isotonic solutions commonly used in microencapsulation include saline (0.9% sodium chloride), phosphate-buffered saline (PBS), and various balanced salt solutions. These solutions provide a stable environment for the encapsulation process, ensuring that the active ingredients remain intact and functional throughout the procedure. The choice of isotonic solution depends on the specific requirements of the encapsulated material and the intended application of the microcapsules.
The use of isotonic solutions in microencapsulation offers several advantages. Firstly, it helps maintain the size and shape of the microcapsules by preventing excessive swelling or shrinkage due to osmotic pressure differences. This is crucial for achieving consistent and predictable release profiles of the encapsulated substances. Secondly, isotonic conditions protect the structural integrity of the capsule membrane, reducing the risk of premature rupture or leakage of the encapsulated material.
Furthermore, isotonic solutions contribute to the overall stability of the microencapsulation system. They help preserve the activity of encapsulated biomolecules by mimicking physiological conditions, which is especially important for applications in drug delivery, cell therapy, and food technology. The use of isotonic solutions also facilitates the handling and storage of microcapsules, as it minimizes the risk of degradation or loss of functionality over time.
In recent years, research has focused on optimizing isotonic solutions for specific microencapsulation applications. This includes the development of custom-formulated isotonic media that not only maintain osmotic balance but also provide additional benefits such as enhanced biocompatibility, improved shelf life, or targeted release properties. These advancements have expanded the potential applications of microencapsulation technology across various industries, including pharmaceuticals, cosmetics, and agriculture.
Market Analysis
The market for isotonic solutions in microencapsulation has been experiencing significant growth due to the increasing demand for advanced drug delivery systems and functional food ingredients. The pharmaceutical industry, in particular, has been a major driver of this market, with a growing emphasis on targeted drug delivery and controlled release formulations. Isotonic solutions play a crucial role in maintaining the osmotic balance of encapsulated materials, thereby enhancing the stability and efficacy of microencapsulated products.
In the food and beverage sector, the use of microencapsulation with isotonic solutions has gained traction for the protection and controlled release of flavors, nutrients, and bioactive compounds. This trend is driven by consumer demand for healthier and more functional food products, as well as the need for extended shelf life and improved sensory properties.
The cosmetics and personal care industry has also shown increasing interest in microencapsulation technologies utilizing isotonic solutions. These applications range from encapsulated fragrances and active ingredients in skincare products to controlled-release formulations in hair care and oral hygiene products.
Market analysis indicates that the Asia-Pacific region is expected to witness the highest growth rate in the coming years, primarily due to the rapid expansion of pharmaceutical and food industries in countries like China and India. North America and Europe continue to be significant markets, with a strong focus on research and development in advanced drug delivery systems and functional foods.
Key factors driving the market include the rising prevalence of chronic diseases, increasing investments in pharmaceutical research, and growing consumer awareness about functional foods and nutraceuticals. The demand for personalized medicine and the development of novel biomaterials for microencapsulation are also contributing to market growth.
However, challenges such as high production costs, regulatory hurdles, and the need for specialized equipment and expertise may hinder market expansion to some extent. Additionally, concerns about the long-term stability of microencapsulated products and potential interactions with other ingredients pose challenges that need to be addressed through ongoing research and development efforts.
Overall, the market for isotonic solutions in microencapsulation is poised for continued growth, with opportunities for innovation in formulation techniques, materials, and applications across various industries. As research in this field progresses, it is expected that new and improved isotonic solutions will be developed, further enhancing the efficiency and applicability of microencapsulation technologies.
In the food and beverage sector, the use of microencapsulation with isotonic solutions has gained traction for the protection and controlled release of flavors, nutrients, and bioactive compounds. This trend is driven by consumer demand for healthier and more functional food products, as well as the need for extended shelf life and improved sensory properties.
The cosmetics and personal care industry has also shown increasing interest in microencapsulation technologies utilizing isotonic solutions. These applications range from encapsulated fragrances and active ingredients in skincare products to controlled-release formulations in hair care and oral hygiene products.
Market analysis indicates that the Asia-Pacific region is expected to witness the highest growth rate in the coming years, primarily due to the rapid expansion of pharmaceutical and food industries in countries like China and India. North America and Europe continue to be significant markets, with a strong focus on research and development in advanced drug delivery systems and functional foods.
Key factors driving the market include the rising prevalence of chronic diseases, increasing investments in pharmaceutical research, and growing consumer awareness about functional foods and nutraceuticals. The demand for personalized medicine and the development of novel biomaterials for microencapsulation are also contributing to market growth.
However, challenges such as high production costs, regulatory hurdles, and the need for specialized equipment and expertise may hinder market expansion to some extent. Additionally, concerns about the long-term stability of microencapsulated products and potential interactions with other ingredients pose challenges that need to be addressed through ongoing research and development efforts.
Overall, the market for isotonic solutions in microencapsulation is poised for continued growth, with opportunities for innovation in formulation techniques, materials, and applications across various industries. As research in this field progresses, it is expected that new and improved isotonic solutions will be developed, further enhancing the efficiency and applicability of microencapsulation technologies.
Technical Challenges
The development of isotonic solutions for microencapsulation faces several technical challenges that hinder the optimization of encapsulation efficiency. One of the primary obstacles is maintaining the delicate balance between osmotic pressure and cell membrane integrity. Isotonic solutions must precisely match the osmolarity of the encapsulated material, typically cells or bioactive compounds, to prevent osmotic stress and potential damage. Achieving this balance across diverse encapsulation materials and target applications remains a significant hurdle.
Another challenge lies in the formulation of isotonic solutions that can effectively stabilize the encapsulated material without compromising its functionality. The choice of solutes and their concentrations must be carefully tailored to each specific application, considering factors such as pH, ionic strength, and potential interactions with the encapsulated material. This complexity often requires extensive experimentation and optimization, which can be time-consuming and resource-intensive.
The scalability of isotonic solution-based microencapsulation processes presents an additional technical obstacle. While laboratory-scale production may yield promising results, translating these processes to industrial-scale manufacturing while maintaining consistent encapsulation efficiency and product quality can be challenging. Factors such as mixing dynamics, temperature control, and process uniformity become increasingly critical at larger scales.
Furthermore, the long-term stability of microencapsulated products in isotonic solutions remains a concern. Ensuring that the encapsulated material retains its activity and the capsule maintains its integrity over extended periods is crucial for many applications, particularly in the pharmaceutical and food industries. This challenge is compounded by the need to develop isotonic solutions that are compatible with various storage conditions and delivery methods.
The development of novel, biocompatible materials for isotonic solutions is another area of technical difficulty. As the field of microencapsulation expands into new applications, such as cell therapy and tissue engineering, there is a growing demand for isotonic solutions that not only maintain osmotic balance but also provide additional functionalities. These may include enhanced nutrient delivery, improved cell viability, or controlled release properties. Designing multifunctional isotonic solutions that meet these complex requirements without compromising encapsulation efficiency remains a significant challenge.
Lastly, the characterization and quality control of isotonic solutions and their impact on microencapsulation efficiency pose technical challenges. Developing robust, standardized methods for assessing osmolarity, encapsulation yield, and product consistency across different batches and scales is essential for advancing the field. This requires the integration of advanced analytical techniques and the establishment of industry-wide standards, which are still evolving in this rapidly developing area of research.
Another challenge lies in the formulation of isotonic solutions that can effectively stabilize the encapsulated material without compromising its functionality. The choice of solutes and their concentrations must be carefully tailored to each specific application, considering factors such as pH, ionic strength, and potential interactions with the encapsulated material. This complexity often requires extensive experimentation and optimization, which can be time-consuming and resource-intensive.
The scalability of isotonic solution-based microencapsulation processes presents an additional technical obstacle. While laboratory-scale production may yield promising results, translating these processes to industrial-scale manufacturing while maintaining consistent encapsulation efficiency and product quality can be challenging. Factors such as mixing dynamics, temperature control, and process uniformity become increasingly critical at larger scales.
Furthermore, the long-term stability of microencapsulated products in isotonic solutions remains a concern. Ensuring that the encapsulated material retains its activity and the capsule maintains its integrity over extended periods is crucial for many applications, particularly in the pharmaceutical and food industries. This challenge is compounded by the need to develop isotonic solutions that are compatible with various storage conditions and delivery methods.
The development of novel, biocompatible materials for isotonic solutions is another area of technical difficulty. As the field of microencapsulation expands into new applications, such as cell therapy and tissue engineering, there is a growing demand for isotonic solutions that not only maintain osmotic balance but also provide additional functionalities. These may include enhanced nutrient delivery, improved cell viability, or controlled release properties. Designing multifunctional isotonic solutions that meet these complex requirements without compromising encapsulation efficiency remains a significant challenge.
Lastly, the characterization and quality control of isotonic solutions and their impact on microencapsulation efficiency pose technical challenges. Developing robust, standardized methods for assessing osmolarity, encapsulation yield, and product consistency across different batches and scales is essential for advancing the field. This requires the integration of advanced analytical techniques and the establishment of industry-wide standards, which are still evolving in this rapidly developing area of research.
Current Isotonic Solutions
01 Isotonic solution composition for microencapsulation
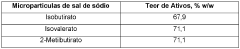
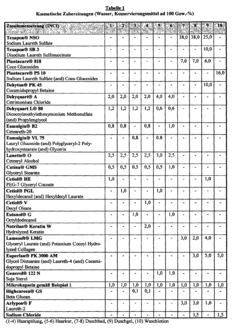
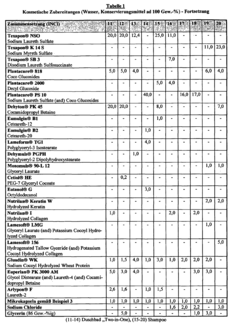
Isotonic solutions used in microencapsulation processes typically contain a balance of salts and other solutes to maintain osmotic pressure. These solutions can include components such as sodium chloride, potassium chloride, and glucose, which help to preserve the integrity of microcapsules during formation and storage. The composition of the isotonic solution can significantly impact the efficiency of the microencapsulation process.- Isotonic solution formulation for microencapsulation: Formulating isotonic solutions for microencapsulation processes can improve the efficiency and stability of the encapsulated materials. These solutions help maintain osmotic balance, preventing damage to the microcapsules during formation and storage. Careful selection of osmolytes and buffer components is crucial for optimizing the isotonic environment.
- Microencapsulation techniques for improved efficiency: Various microencapsulation techniques can be employed to enhance efficiency, including spray drying, coacervation, and emulsion-based methods. These techniques can be optimized for specific active ingredients and desired release profiles. Factors such as temperature, pH, and stirring speed can significantly impact the microencapsulation efficiency.
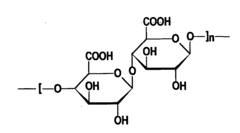
- Polymer selection for microencapsulation in isotonic solutions: The choice of polymers for microencapsulation in isotonic solutions plays a crucial role in determining encapsulation efficiency. Biocompatible and biodegradable polymers that maintain stability in isotonic environments are preferred. Natural polymers like alginate and chitosan, as well as synthetic polymers such as PLGA, can be used depending on the specific application and desired release characteristics.
- Characterization and evaluation of microencapsulation efficiency: Various analytical techniques can be employed to characterize and evaluate microencapsulation efficiency in isotonic solutions. These may include particle size analysis, zeta potential measurements, encapsulation efficiency assays, and release kinetics studies. Advanced imaging techniques such as confocal microscopy and electron microscopy can provide insights into the morphology and structure of the microcapsules.
- Applications of isotonic microencapsulation in pharmaceuticals and biotechnology: Isotonic microencapsulation finds applications in various fields, particularly in pharmaceuticals and biotechnology. It can be used for drug delivery systems, cell encapsulation for tissue engineering, and protection of sensitive biomolecules. The isotonic environment helps maintain the integrity and activity of encapsulated materials, leading to improved therapeutic efficacy and bioavailability.
02 Microencapsulation techniques for improved efficiency
Various microencapsulation techniques can be employed to enhance efficiency when using isotonic solutions. These may include spray drying, coacervation, and emulsion-based methods. The choice of technique can affect the size, stability, and release properties of the microcapsules. Optimizing parameters such as temperature, pH, and stirring speed during the encapsulation process can lead to improved efficiency.Expand Specific Solutions03 Polymer selection for microencapsulation in isotonic environments
The selection of appropriate polymers is crucial for efficient microencapsulation in isotonic solutions. Polymers such as alginate, chitosan, and poly(lactic-co-glycolic acid) (PLGA) are commonly used due to their biocompatibility and ability to form stable microcapsules in isotonic conditions. The molecular weight and chemical modifications of these polymers can be tailored to optimize encapsulation efficiency and release kinetics.Expand Specific Solutions04 Characterization methods for assessing microencapsulation efficiency
Various analytical techniques are employed to evaluate the efficiency of microencapsulation in isotonic solutions. These may include particle size analysis, zeta potential measurements, and encapsulation efficiency determinations. Advanced imaging techniques such as scanning electron microscopy (SEM) and confocal microscopy can provide insights into the morphology and internal structure of microcapsules, helping to optimize the encapsulation process.Expand Specific Solutions05 Applications of isotonic solution-based microencapsulation
Microencapsulation using isotonic solutions finds applications in various fields, including drug delivery, food technology, and cosmetics. In pharmaceuticals, it can be used to enhance the stability and bioavailability of active ingredients. In the food industry, it can protect sensitive compounds from degradation and control their release. The efficiency of microencapsulation in these applications depends on the careful selection of isotonic solution components and encapsulation parameters.Expand Specific Solutions
Key Industry Players
The field of isotonic solutions and microencapsulation efficiency is in a growth phase, with increasing market size and technological advancements. The global microencapsulation market is expected to expand significantly in the coming years, driven by applications in pharmaceuticals, food, and cosmetics. Key players like Novartis AG, BASF Corp., and FMC Corp. are investing heavily in R&D to improve microencapsulation techniques. The technology is maturing, with companies like Encapsys LLC and Capsulis SAS specializing in microencapsulation solutions. Academic institutions such as Nanyang Technological University and Universidad de Antíoquia are also contributing to research, indicating a collaborative ecosystem between industry and academia.
Novartis AG
Technical Solution: Novartis AG has developed advanced isotonic solutions for microencapsulation, focusing on improving drug delivery efficiency. Their approach involves using carefully balanced osmotic pressure to enhance the stability and longevity of encapsulated drugs. The company has implemented a novel technique that utilizes a combination of electrolytes and non-electrolytes to create an isotonic environment that closely mimics physiological conditions[1]. This method has shown significant improvements in encapsulation efficiency, particularly for sensitive biomolecules such as proteins and peptides. Novartis has also integrated smart polymer technology into their isotonic solutions, allowing for controlled release of encapsulated drugs in response to specific physiological triggers[3].
Strengths: Improved drug stability and bioavailability, enhanced targeted delivery. Weaknesses: Potentially higher production costs, complexity in formulation process.
CHIESI Farmaceutici SpA
Technical Solution: CHIESI Farmaceutici SpA has pioneered the use of isotonic solutions in microencapsulation for respiratory drug delivery. Their proprietary technology, known as "Modulair," utilizes a carefully balanced isotonic solution to create uniform, respirable particles for inhalation therapies[2]. The company has developed a unique blend of osmolytes that not only maintains isotonicity but also enhances the stability of encapsulated drugs during the nebulization process. CHIESI's approach involves a two-step microencapsulation process: first, creating a core-shell structure using the isotonic solution, and then applying a protective coating that further improves the particle's aerodynamic properties[4]. This method has shown remarkable improvements in lung deposition and overall therapeutic efficacy for various respiratory conditions.
Strengths: Excellent for respiratory drug delivery, improved drug stability during nebulization. Weaknesses: Limited application outside of inhalation therapies, potential challenges in scaling up production.
Innovative Encapsulation Methods
Process for producing encapsulated microparticles for ruminants, uses and resulting products
PatentWO2018049494A1
Innovation
- Encapsulating these carboxylic acids in microparticles with a cellulosic coating for controlled release in the rumen, masking odors, and enhancing bioavailability and stability, allowing precise nutrition of specific microorganisms degrading fiber.
Microcapsules (XVII)
PatentInactiveEP1358930A1
Innovation
- The development of microcapsules with diameters ranging from 0.001 to 0.5 mm, created by processing aqueous active ingredient preparations with oil components and emulsifiers to form O/W emulsions, treated with anionic polymers and non-biological cationic polymers, resulting in improved surfactant stability and controlled release through coacervation at lipophilic interfaces.
Regulatory Considerations
Regulatory considerations play a crucial role in the development and implementation of isotonic solutions for microencapsulation. The use of these solutions in various industries, particularly in pharmaceuticals and food technology, necessitates adherence to stringent regulatory guidelines to ensure product safety and efficacy.
In the pharmaceutical sector, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established specific requirements for the use of isotonic solutions in drug delivery systems. These regulations encompass aspects such as the composition of the solutions, manufacturing processes, and quality control measures. Manufacturers must demonstrate that their isotonic solutions meet the necessary standards for osmolality, pH, and sterility to gain regulatory approval.
The food industry also faces regulatory scrutiny when utilizing isotonic solutions in microencapsulation processes. Regulatory agencies like the FDA and the European Food Safety Authority (EFSA) have set guidelines for food additives and processing aids, which include isotonic solutions used in microencapsulation. Compliance with these regulations is essential to ensure the safety of food products and maintain consumer trust.
Environmental regulations also impact the use of isotonic solutions in microencapsulation. Manufacturers must consider the potential environmental impact of their processes and products, including the disposal of waste materials and the biodegradability of encapsulated products. Adherence to environmental regulations is crucial for sustainable production practices and maintaining regulatory compliance.
Regulatory considerations extend to the sourcing and quality of raw materials used in isotonic solutions. Manufacturers must ensure that all components meet regulatory standards for purity and safety. This includes compliance with Good Manufacturing Practices (GMP) and implementation of robust quality management systems to maintain consistency and traceability throughout the production process.
The regulatory landscape for isotonic solutions in microencapsulation is continually evolving. As new technologies and applications emerge, regulatory bodies may update their guidelines to address novel challenges and ensure public safety. Manufacturers and researchers must stay informed about these changes and adapt their processes accordingly to maintain compliance and market access.
International harmonization of regulations presents both challenges and opportunities for the industry. While differences in regulatory requirements across regions can complicate global market access, efforts towards harmonization can streamline approval processes and facilitate international trade. Companies operating in multiple markets must navigate these complexities to ensure compliance across different jurisdictions.
In the pharmaceutical sector, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established specific requirements for the use of isotonic solutions in drug delivery systems. These regulations encompass aspects such as the composition of the solutions, manufacturing processes, and quality control measures. Manufacturers must demonstrate that their isotonic solutions meet the necessary standards for osmolality, pH, and sterility to gain regulatory approval.
The food industry also faces regulatory scrutiny when utilizing isotonic solutions in microencapsulation processes. Regulatory agencies like the FDA and the European Food Safety Authority (EFSA) have set guidelines for food additives and processing aids, which include isotonic solutions used in microencapsulation. Compliance with these regulations is essential to ensure the safety of food products and maintain consumer trust.
Environmental regulations also impact the use of isotonic solutions in microencapsulation. Manufacturers must consider the potential environmental impact of their processes and products, including the disposal of waste materials and the biodegradability of encapsulated products. Adherence to environmental regulations is crucial for sustainable production practices and maintaining regulatory compliance.
Regulatory considerations extend to the sourcing and quality of raw materials used in isotonic solutions. Manufacturers must ensure that all components meet regulatory standards for purity and safety. This includes compliance with Good Manufacturing Practices (GMP) and implementation of robust quality management systems to maintain consistency and traceability throughout the production process.
The regulatory landscape for isotonic solutions in microencapsulation is continually evolving. As new technologies and applications emerge, regulatory bodies may update their guidelines to address novel challenges and ensure public safety. Manufacturers and researchers must stay informed about these changes and adapt their processes accordingly to maintain compliance and market access.
International harmonization of regulations presents both challenges and opportunities for the industry. While differences in regulatory requirements across regions can complicate global market access, efforts towards harmonization can streamline approval processes and facilitate international trade. Companies operating in multiple markets must navigate these complexities to ensure compliance across different jurisdictions.
Environmental Impact
The environmental impact of isotonic solutions in microencapsulation processes is a crucial consideration for sustainable development in the pharmaceutical and food industries. These solutions play a vital role in maintaining osmotic balance during encapsulation, but their production and disposal can have significant ecological implications.
The primary environmental concern stems from the use of various salts and sugars to create isotonic conditions. The extraction and processing of these compounds often involve energy-intensive methods, contributing to carbon emissions and resource depletion. Additionally, the production of high-purity water required for isotonic solutions can strain local water resources, particularly in water-scarce regions.
Disposal of isotonic solutions after microencapsulation processes presents another environmental challenge. These solutions may contain residual active ingredients, excipients, or other chemicals used in the encapsulation process. If not properly treated, they can contaminate water bodies and soil, potentially disrupting aquatic ecosystems and affecting biodiversity.
However, recent advancements in green chemistry have led to the development of more environmentally friendly isotonic solutions. Researchers are exploring the use of naturally derived compounds and biodegradable materials to create isotonic environments, reducing the reliance on synthetic chemicals. These alternatives not only minimize environmental impact but also enhance the biocompatibility of the encapsulated products.
Recycling and reuse strategies for isotonic solutions are gaining traction in industrial settings. Implementing closed-loop systems can significantly reduce water consumption and minimize waste generation. Advanced filtration and purification technologies enable the recovery and reuse of isotonic solutions, thereby decreasing the overall environmental footprint of microencapsulation processes.
The pharmaceutical industry, in particular, is adopting more sustainable practices in microencapsulation. This includes optimizing formulation processes to reduce the volume of isotonic solutions required and implementing more efficient encapsulation techniques that minimize solution waste. Such efforts not only benefit the environment but also contribute to cost savings and improved product quality.
As regulatory bodies worldwide increasingly focus on environmental sustainability, manufacturers are under pressure to demonstrate responsible use of isotonic solutions in microencapsulation. This has spurred innovation in process design and waste management, leading to the development of more eco-friendly encapsulation technologies that maintain high efficiency while reducing environmental impact.
The primary environmental concern stems from the use of various salts and sugars to create isotonic conditions. The extraction and processing of these compounds often involve energy-intensive methods, contributing to carbon emissions and resource depletion. Additionally, the production of high-purity water required for isotonic solutions can strain local water resources, particularly in water-scarce regions.
Disposal of isotonic solutions after microencapsulation processes presents another environmental challenge. These solutions may contain residual active ingredients, excipients, or other chemicals used in the encapsulation process. If not properly treated, they can contaminate water bodies and soil, potentially disrupting aquatic ecosystems and affecting biodiversity.
However, recent advancements in green chemistry have led to the development of more environmentally friendly isotonic solutions. Researchers are exploring the use of naturally derived compounds and biodegradable materials to create isotonic environments, reducing the reliance on synthetic chemicals. These alternatives not only minimize environmental impact but also enhance the biocompatibility of the encapsulated products.
Recycling and reuse strategies for isotonic solutions are gaining traction in industrial settings. Implementing closed-loop systems can significantly reduce water consumption and minimize waste generation. Advanced filtration and purification technologies enable the recovery and reuse of isotonic solutions, thereby decreasing the overall environmental footprint of microencapsulation processes.
The pharmaceutical industry, in particular, is adopting more sustainable practices in microencapsulation. This includes optimizing formulation processes to reduce the volume of isotonic solutions required and implementing more efficient encapsulation techniques that minimize solution waste. Such efforts not only benefit the environment but also contribute to cost savings and improved product quality.
As regulatory bodies worldwide increasingly focus on environmental sustainability, manufacturers are under pressure to demonstrate responsible use of isotonic solutions in microencapsulation. This has spurred innovation in process design and waste management, leading to the development of more eco-friendly encapsulation technologies that maintain high efficiency while reducing environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!