Isotonic solutions influence on chemiluminescence assays
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chemiluminescence Assay Background and Objectives
Chemiluminescence assays have emerged as powerful analytical tools in various fields, including clinical diagnostics, environmental monitoring, and biomedical research. These assays leverage the principle of light emission resulting from chemical reactions, offering high sensitivity and wide dynamic range. The evolution of chemiluminescence technology has been marked by continuous improvements in reagent formulations, detection systems, and application methodologies.
The primary objective of researching the influence of isotonic solutions on chemiluminescence assays is to enhance the accuracy, reliability, and applicability of these assays in diverse biological and clinical settings. Isotonic solutions, which maintain osmotic balance with cellular environments, play a crucial role in preserving sample integrity and ensuring consistent assay performance. Understanding their impact is essential for optimizing assay conditions and expanding the utility of chemiluminescence-based techniques.
Recent advancements in chemiluminescence technology have focused on increasing signal intensity, reducing background noise, and improving the stability of light-emitting reactions. These developments have led to the creation of more sensitive and robust assay platforms capable of detecting analytes at extremely low concentrations. However, the interaction between assay components and sample matrices, particularly isotonic solutions, remains a critical area of investigation.
The exploration of isotonic solution effects on chemiluminescence assays aims to address several key challenges. These include minimizing matrix interference, enhancing signal-to-noise ratios, and ensuring consistent assay performance across different sample types. By elucidating the mechanisms through which isotonic solutions influence chemiluminescent reactions, researchers seek to develop standardized protocols that can be applied across a broad spectrum of analytical applications.
Furthermore, this research endeavors to expand the applicability of chemiluminescence assays to complex biological samples and challenging environmental matrices. The ultimate goal is to create versatile assay systems that maintain high sensitivity and specificity while accommodating the diverse physiological conditions encountered in real-world samples. This includes optimizing assay performance in the presence of various electrolytes, proteins, and other biomolecules commonly found in isotonic biological fluids.
As the field of chemiluminescence continues to evolve, the integration of novel technologies such as microfluidics, nanotechnology, and advanced imaging systems presents new opportunities for innovation. These developments, combined with a deeper understanding of isotonic solution effects, are expected to drive the next generation of chemiluminescence assays, enabling more precise, rapid, and cost-effective analytical solutions across multiple industries and research domains.
The primary objective of researching the influence of isotonic solutions on chemiluminescence assays is to enhance the accuracy, reliability, and applicability of these assays in diverse biological and clinical settings. Isotonic solutions, which maintain osmotic balance with cellular environments, play a crucial role in preserving sample integrity and ensuring consistent assay performance. Understanding their impact is essential for optimizing assay conditions and expanding the utility of chemiluminescence-based techniques.
Recent advancements in chemiluminescence technology have focused on increasing signal intensity, reducing background noise, and improving the stability of light-emitting reactions. These developments have led to the creation of more sensitive and robust assay platforms capable of detecting analytes at extremely low concentrations. However, the interaction between assay components and sample matrices, particularly isotonic solutions, remains a critical area of investigation.
The exploration of isotonic solution effects on chemiluminescence assays aims to address several key challenges. These include minimizing matrix interference, enhancing signal-to-noise ratios, and ensuring consistent assay performance across different sample types. By elucidating the mechanisms through which isotonic solutions influence chemiluminescent reactions, researchers seek to develop standardized protocols that can be applied across a broad spectrum of analytical applications.
Furthermore, this research endeavors to expand the applicability of chemiluminescence assays to complex biological samples and challenging environmental matrices. The ultimate goal is to create versatile assay systems that maintain high sensitivity and specificity while accommodating the diverse physiological conditions encountered in real-world samples. This includes optimizing assay performance in the presence of various electrolytes, proteins, and other biomolecules commonly found in isotonic biological fluids.
As the field of chemiluminescence continues to evolve, the integration of novel technologies such as microfluidics, nanotechnology, and advanced imaging systems presents new opportunities for innovation. These developments, combined with a deeper understanding of isotonic solution effects, are expected to drive the next generation of chemiluminescence assays, enabling more precise, rapid, and cost-effective analytical solutions across multiple industries and research domains.
Market Analysis for Chemiluminescence Diagnostics
The chemiluminescence diagnostics market has experienced significant growth in recent years, driven by the increasing prevalence of chronic and infectious diseases, growing demand for rapid and accurate diagnostic tests, and technological advancements in the field. This market segment is a crucial component of the broader in vitro diagnostics (IVD) industry, which is projected to reach substantial market value in the coming years.
The demand for chemiluminescence-based diagnostic tests is particularly strong in developed regions such as North America and Europe, where healthcare infrastructure is well-established and there is a high adoption rate of advanced diagnostic technologies. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential due to improving healthcare systems, rising disposable incomes, and increasing awareness about early disease detection.
Key factors driving the market include the high sensitivity and specificity of chemiluminescence assays, their ability to detect a wide range of analytes, and the growing trend towards automation in clinical laboratories. Chemiluminescence technology offers advantages over other diagnostic methods, such as faster turnaround times, reduced sample volumes, and improved workflow efficiency, which are highly valued in modern healthcare settings.
The COVID-19 pandemic has further accelerated the demand for rapid and accurate diagnostic tests, including those based on chemiluminescence technology. This has led to increased investment in research and development of new chemiluminescence-based assays for infectious diseases, potentially expanding the market's scope beyond its traditional applications in hormone testing, cancer markers, and cardiac biomarkers.
Market segmentation reveals that clinical diagnostics hold the largest share in the chemiluminescence market, followed by research applications and other sectors such as food safety testing. Within clinical diagnostics, immunoassays dominate the market due to their wide applicability in detecting various biomarkers and pathogens.
Geographically, North America currently leads the global chemiluminescence diagnostics market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about preventive healthcare.
The market is characterized by intense competition among major players, who are focusing on product innovations, strategic collaborations, and mergers and acquisitions to maintain their market positions. Key trends shaping the market include the development of multiplex assays, integration of artificial intelligence and machine learning in data analysis, and the shift towards point-of-care testing solutions.
The demand for chemiluminescence-based diagnostic tests is particularly strong in developed regions such as North America and Europe, where healthcare infrastructure is well-established and there is a high adoption rate of advanced diagnostic technologies. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential due to improving healthcare systems, rising disposable incomes, and increasing awareness about early disease detection.
Key factors driving the market include the high sensitivity and specificity of chemiluminescence assays, their ability to detect a wide range of analytes, and the growing trend towards automation in clinical laboratories. Chemiluminescence technology offers advantages over other diagnostic methods, such as faster turnaround times, reduced sample volumes, and improved workflow efficiency, which are highly valued in modern healthcare settings.
The COVID-19 pandemic has further accelerated the demand for rapid and accurate diagnostic tests, including those based on chemiluminescence technology. This has led to increased investment in research and development of new chemiluminescence-based assays for infectious diseases, potentially expanding the market's scope beyond its traditional applications in hormone testing, cancer markers, and cardiac biomarkers.
Market segmentation reveals that clinical diagnostics hold the largest share in the chemiluminescence market, followed by research applications and other sectors such as food safety testing. Within clinical diagnostics, immunoassays dominate the market due to their wide applicability in detecting various biomarkers and pathogens.
Geographically, North America currently leads the global chemiluminescence diagnostics market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and rising awareness about preventive healthcare.
The market is characterized by intense competition among major players, who are focusing on product innovations, strategic collaborations, and mergers and acquisitions to maintain their market positions. Key trends shaping the market include the development of multiplex assays, integration of artificial intelligence and machine learning in data analysis, and the shift towards point-of-care testing solutions.
Current Challenges in Chemiluminescence Assays
Chemiluminescence assays have become increasingly popular in clinical diagnostics and research due to their high sensitivity and wide dynamic range. However, several challenges persist in optimizing these assays, particularly when considering the influence of isotonic solutions. One of the primary challenges is maintaining assay sensitivity in the presence of isotonic buffers, which are essential for preserving cell integrity in biological samples.
The interference of isotonic solutions with chemiluminescent reactions is a significant concern. These solutions, typically containing salts and other osmolytes, can affect the kinetics and efficiency of the light-producing reactions. This interference may lead to reduced signal intensity or altered reaction rates, potentially compromising the accuracy and reliability of assay results. Researchers are grappling with the need to balance the physiological requirements of samples with the optimal conditions for chemiluminescence.
Another challenge lies in the stability of chemiluminescent reagents in isotonic environments. Many chemiluminescent substrates and enzymes exhibit altered stability or activity when exposed to high salt concentrations or specific osmolytes present in isotonic solutions. This instability can result in decreased assay performance over time, limiting the shelf life of reagents and potentially introducing variability in long-term studies or clinical applications.
The quenching effect of certain components in isotonic solutions poses an additional hurdle. Some ions or molecules commonly found in these solutions can act as quenchers, reducing the intensity of the emitted light. This phenomenon necessitates careful consideration of buffer composition and may require the development of specialized formulations to minimize quenching while maintaining isotonicity.
Standardization across different isotonic solutions and their effects on chemiluminescence assays remains a challenge. The variety of isotonic formulations used in different laboratories and clinical settings can lead to inconsistencies in assay performance and inter-laboratory variability. Establishing standardized protocols that account for the specific effects of different isotonic solutions on chemiluminescence is crucial for improving assay reproducibility and comparability.
The development of robust calibration methods that compensate for the influence of isotonic solutions is an ongoing challenge. Current calibration approaches may not fully account for the complex interactions between sample matrices, isotonic buffers, and chemiluminescent reactions. This gap highlights the need for advanced calibration strategies that can effectively normalize results across different isotonic conditions.
Lastly, the challenge of miniaturization and integration of chemiluminescence assays with microfluidic or point-of-care devices is compounded by the presence of isotonic solutions. Designing compact systems that maintain assay performance while accommodating the necessary isotonic environment requires innovative engineering solutions and careful optimization of reaction conditions.
The interference of isotonic solutions with chemiluminescent reactions is a significant concern. These solutions, typically containing salts and other osmolytes, can affect the kinetics and efficiency of the light-producing reactions. This interference may lead to reduced signal intensity or altered reaction rates, potentially compromising the accuracy and reliability of assay results. Researchers are grappling with the need to balance the physiological requirements of samples with the optimal conditions for chemiluminescence.
Another challenge lies in the stability of chemiluminescent reagents in isotonic environments. Many chemiluminescent substrates and enzymes exhibit altered stability or activity when exposed to high salt concentrations or specific osmolytes present in isotonic solutions. This instability can result in decreased assay performance over time, limiting the shelf life of reagents and potentially introducing variability in long-term studies or clinical applications.
The quenching effect of certain components in isotonic solutions poses an additional hurdle. Some ions or molecules commonly found in these solutions can act as quenchers, reducing the intensity of the emitted light. This phenomenon necessitates careful consideration of buffer composition and may require the development of specialized formulations to minimize quenching while maintaining isotonicity.
Standardization across different isotonic solutions and their effects on chemiluminescence assays remains a challenge. The variety of isotonic formulations used in different laboratories and clinical settings can lead to inconsistencies in assay performance and inter-laboratory variability. Establishing standardized protocols that account for the specific effects of different isotonic solutions on chemiluminescence is crucial for improving assay reproducibility and comparability.
The development of robust calibration methods that compensate for the influence of isotonic solutions is an ongoing challenge. Current calibration approaches may not fully account for the complex interactions between sample matrices, isotonic buffers, and chemiluminescent reactions. This gap highlights the need for advanced calibration strategies that can effectively normalize results across different isotonic conditions.
Lastly, the challenge of miniaturization and integration of chemiluminescence assays with microfluidic or point-of-care devices is compounded by the presence of isotonic solutions. Designing compact systems that maintain assay performance while accommodating the necessary isotonic environment requires innovative engineering solutions and careful optimization of reaction conditions.
Existing Solutions for Isotonic Interference
01 Chemiluminescence assay methods and reagents
Various methods and reagents are developed for chemiluminescence assays, including novel compounds, reaction systems, and detection techniques. These advancements aim to improve sensitivity, specificity, and reliability of chemiluminescence-based analytical methods.- Chemiluminescence assay methods and reagents: Various methods and reagents are developed for chemiluminescence assays, including novel compounds, reaction systems, and detection techniques. These advancements aim to improve sensitivity, specificity, and reliability of chemiluminescence-based analytical methods.
- Instrumentation for chemiluminescence detection: Specialized instruments and devices are designed for chemiluminescence detection, incorporating features such as automated sample handling, precise light measurement, and data analysis capabilities. These instruments enhance the efficiency and accuracy of chemiluminescence assays.
- Applications in biomedical and clinical diagnostics: Chemiluminescence assays are widely applied in biomedical research and clinical diagnostics. They are used for detecting various analytes, including hormones, proteins, nucleic acids, and small molecules, offering high sensitivity and wide dynamic range for quantitative analysis.
- Influence of environmental factors on chemiluminescence: Environmental factors such as temperature, pH, and interfering substances can significantly impact chemiluminescence reactions. Understanding and controlling these influences is crucial for optimizing assay performance and ensuring reliable results.
- Chemiluminescence signal enhancement strategies: Various strategies are employed to enhance chemiluminescence signals, including the use of catalysts, energy transfer systems, and nanoparticles. These approaches aim to improve detection limits and expand the applicability of chemiluminescence assays in different fields.
02 Instrumentation for chemiluminescence detection
Specialized instruments and devices are designed for chemiluminescence detection, incorporating features such as automated sample handling, signal amplification, and data processing. These instruments enhance the efficiency and accuracy of chemiluminescence assays in various applications.Expand Specific Solutions03 Applications in biomedical and clinical diagnostics
Chemiluminescence assays are widely applied in biomedical research and clinical diagnostics, including detection of specific biomarkers, pathogens, and disease indicators. These applications leverage the high sensitivity and wide dynamic range of chemiluminescence techniques.Expand Specific Solutions04 Influence of environmental factors on chemiluminescence
Various environmental factors, such as temperature, pH, and interfering substances, can affect the performance of chemiluminescence assays. Understanding and controlling these influences is crucial for optimizing assay conditions and ensuring reliable results.Expand Specific Solutions05 Chemiluminescence signal enhancement strategies
Techniques for enhancing chemiluminescence signals are developed to improve assay sensitivity and detection limits. These strategies may include chemical modifications, nanoparticle-based amplification, or enzymatic cascades to boost light emission intensity and duration.Expand Specific Solutions
Key Players in Chemiluminescence Industry
The research on the influence of isotonic solutions on chemiluminescence assays is in a mature stage of development, with a significant market presence and established technological foundations. The competitive landscape is characterized by a mix of large multinational corporations and specialized diagnostic companies. Key players like Siemens Healthcare Diagnostics, Roche Diagnostics, and Abbott Laboratories dominate the market with their extensive product portfolios and global reach. Smaller, specialized firms such as Chemclin Diagnostics and Lumigen contribute to innovation in niche areas. The technology's maturity is evident from its widespread adoption in clinical diagnostics, but ongoing research by academic institutions and industry collaborations continues to refine and expand its applications.
Siemens Healthcare Diagnostics, Inc.
Technical Solution: Siemens Healthcare Diagnostics has developed advanced chemiluminescence immunoassay systems that utilize optimized isotonic solutions to enhance signal stability and reduce background noise. Their ADVIA Centaur XP Immunoassay System employs a proprietary AE (acridinium ester) chemiluminescent label technology, which is less susceptible to interference from isotonic solutions[1]. The system incorporates a specially formulated wash buffer that maintains optimal ionic strength, minimizing the impact of sample matrix effects on assay performance[2]. Additionally, Siemens has implemented a unique magnetic particle separation technique that allows for efficient washing steps, further reducing the influence of isotonic solutions on the chemiluminescence signal[3].
Strengths: Highly sensitive and specific assays with reduced interference from isotonic solutions. Weaknesses: Complex system design may increase costs and maintenance requirements.
Roche Diagnostics Operations, Inc.
Technical Solution: Roche Diagnostics has pioneered the development of electrochemiluminescence (ECL) technology, which offers superior performance in the presence of isotonic solutions compared to traditional chemiluminescence methods. Their cobas e analyzers utilize ruthenium-based ECL labels that are less affected by changes in ionic strength[4]. Roche has implemented a unique approach to sample handling, where the assay buffer is precisely controlled to maintain optimal ionic conditions throughout the reaction process[5]. This strategy minimizes the impact of varying isotonic environments on assay performance. Furthermore, Roche has developed specialized surfactants and stabilizers that protect the ECL labels from potential quenching effects caused by isotonic solutions, ensuring consistent and reliable results across a wide range of sample types[6].
Strengths: Highly robust assays with excellent performance in various sample matrices. Weaknesses: Proprietary technology may limit compatibility with other systems.
Core Innovations in Chemiluminescence Assays
Stabilizing compositions, methods and kits for chemiluminescent assays
PatentWO2009076330A1
Innovation
- A stabilizing composition comprising an acridinium compound, such as acridinium-9-carboxamide or acridinium-9-carboxylate aryl ester, combined with a hydrogen peroxide consuming agent like myeloperoxidase or a transition metal complex, is used to stabilize the assay against oxidative degradation, ensuring precise detection or quantification of analytes.
Equipment for measuring luminescence reaction
PatentInactiveEP1387163A1
Innovation
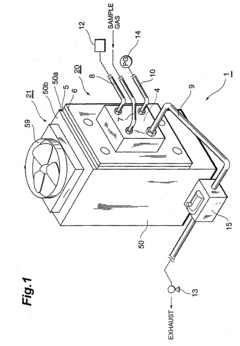
- A luminescent reaction measurement device with a light reflecting reaction chamber and a side-on type photomultiplier tube, where emitted chemiluminescence light is transmitted through a window to the detector, and reflected back through a gold-coated dynode surface to increase light incidence on the photoelectric surface, allowing for higher sensitivity detection without cooling the reaction chamber.
Regulatory Considerations for Diagnostic Assays
Regulatory considerations play a crucial role in the development and implementation of diagnostic assays, including chemiluminescence assays influenced by isotonic solutions. These considerations are essential to ensure the safety, efficacy, and reliability of diagnostic tests used in clinical settings.
The regulatory landscape for diagnostic assays varies across different regions and countries. In the United States, the Food and Drug Administration (FDA) oversees the regulation of diagnostic tests through the Center for Devices and Radiological Health (CDRH). The FDA classifies diagnostic assays into different risk categories, with chemiluminescence assays typically falling under Class II or Class III devices, depending on their intended use and potential risks.
In the European Union, the In Vitro Diagnostic Regulation (IVDR) governs the development and marketing of diagnostic assays. The IVDR introduces stricter requirements for clinical evidence, risk classification, and post-market surveillance compared to its predecessor, the In Vitro Diagnostic Directive (IVDD).
When developing chemiluminescence assays influenced by isotonic solutions, manufacturers must consider several regulatory aspects. These include analytical performance characteristics, such as sensitivity, specificity, precision, and accuracy. The impact of isotonic solutions on these parameters must be thoroughly evaluated and documented to meet regulatory requirements.
Clinical validation is another critical regulatory consideration. Manufacturers must demonstrate that the assay performs as intended in the target population and provides clinically relevant results. This involves conducting clinical studies to assess the diagnostic accuracy and clinical utility of the assay under various conditions, including the presence of isotonic solutions.
Quality control and assurance measures are essential components of regulatory compliance. Manufacturers must implement robust quality management systems to ensure consistent production and performance of their assays. This includes establishing procedures for batch testing, stability studies, and ongoing monitoring of assay performance in the presence of isotonic solutions.
Labeling and instructions for use are subject to regulatory scrutiny. Manufacturers must provide clear and comprehensive information on the proper use of the assay, including any limitations or precautions related to the influence of isotonic solutions. This information should be based on scientific evidence and clinical data obtained during the development and validation process.
Post-market surveillance is a critical regulatory requirement for diagnostic assays. Manufacturers must have systems in place to monitor the performance of their assays in real-world settings, collect and analyze adverse event reports, and implement corrective actions when necessary. This is particularly important for assays influenced by isotonic solutions, as unforeseen interactions or effects may emerge over time.
The regulatory landscape for diagnostic assays varies across different regions and countries. In the United States, the Food and Drug Administration (FDA) oversees the regulation of diagnostic tests through the Center for Devices and Radiological Health (CDRH). The FDA classifies diagnostic assays into different risk categories, with chemiluminescence assays typically falling under Class II or Class III devices, depending on their intended use and potential risks.
In the European Union, the In Vitro Diagnostic Regulation (IVDR) governs the development and marketing of diagnostic assays. The IVDR introduces stricter requirements for clinical evidence, risk classification, and post-market surveillance compared to its predecessor, the In Vitro Diagnostic Directive (IVDD).
When developing chemiluminescence assays influenced by isotonic solutions, manufacturers must consider several regulatory aspects. These include analytical performance characteristics, such as sensitivity, specificity, precision, and accuracy. The impact of isotonic solutions on these parameters must be thoroughly evaluated and documented to meet regulatory requirements.
Clinical validation is another critical regulatory consideration. Manufacturers must demonstrate that the assay performs as intended in the target population and provides clinically relevant results. This involves conducting clinical studies to assess the diagnostic accuracy and clinical utility of the assay under various conditions, including the presence of isotonic solutions.
Quality control and assurance measures are essential components of regulatory compliance. Manufacturers must implement robust quality management systems to ensure consistent production and performance of their assays. This includes establishing procedures for batch testing, stability studies, and ongoing monitoring of assay performance in the presence of isotonic solutions.
Labeling and instructions for use are subject to regulatory scrutiny. Manufacturers must provide clear and comprehensive information on the proper use of the assay, including any limitations or precautions related to the influence of isotonic solutions. This information should be based on scientific evidence and clinical data obtained during the development and validation process.
Post-market surveillance is a critical regulatory requirement for diagnostic assays. Manufacturers must have systems in place to monitor the performance of their assays in real-world settings, collect and analyze adverse event reports, and implement corrective actions when necessary. This is particularly important for assays influenced by isotonic solutions, as unforeseen interactions or effects may emerge over time.
Environmental Impact of Chemiluminescence Reagents
Chemiluminescence assays have become increasingly popular in analytical and diagnostic applications due to their high sensitivity and wide dynamic range. However, the environmental impact of chemiluminescence reagents is a growing concern that warrants careful consideration. The chemicals used in these assays, while effective for their intended purpose, can have significant ecological consequences if not properly managed.
One of the primary environmental concerns associated with chemiluminescence reagents is their potential for water pollution. Many of these compounds are water-soluble and can easily enter aquatic ecosystems through laboratory waste disposal or accidental spills. Once in the environment, they may persist and accumulate, potentially affecting aquatic life and disrupting ecological balance. For instance, luminol and its derivatives, commonly used in forensic applications and biomedical research, have been shown to have toxic effects on certain aquatic organisms at high concentrations.
The production and disposal of chemiluminescence reagents also contribute to the overall environmental footprint of laboratories and industries utilizing these techniques. The synthesis of these compounds often involves complex chemical processes that may generate hazardous by-products and consume significant energy resources. Furthermore, the disposal of unused or expired reagents requires specialized handling to prevent environmental contamination, adding to the waste management challenges faced by research institutions and clinical laboratories.
Another aspect of environmental concern is the potential for atmospheric pollution. Some chemiluminescence reactions produce volatile organic compounds (VOCs) as by-products, which can contribute to air quality issues if released in significant quantities. While the scale of this problem is generally small compared to industrial emissions, it is still an important consideration for large-scale applications or in areas with already compromised air quality.
The use of chemiluminescence reagents in field-based environmental monitoring presents a paradoxical situation. While these assays can be valuable tools for detecting pollutants and assessing environmental health, the reagents themselves may introduce new contaminants into the ecosystems being studied. This necessitates careful protocol design and waste management practices to minimize the environmental impact of the monitoring activities themselves.
To address these environmental concerns, there is a growing focus on developing more eco-friendly chemiluminescence reagents and assay protocols. This includes research into biodegradable luminescent compounds, the use of less toxic catalysts, and the optimization of reaction conditions to minimize reagent consumption. Additionally, efforts are being made to improve waste management practices in laboratories, such as implementing recycling programs for certain reagents and developing more efficient disposal methods for chemiluminescent waste.
One of the primary environmental concerns associated with chemiluminescence reagents is their potential for water pollution. Many of these compounds are water-soluble and can easily enter aquatic ecosystems through laboratory waste disposal or accidental spills. Once in the environment, they may persist and accumulate, potentially affecting aquatic life and disrupting ecological balance. For instance, luminol and its derivatives, commonly used in forensic applications and biomedical research, have been shown to have toxic effects on certain aquatic organisms at high concentrations.
The production and disposal of chemiluminescence reagents also contribute to the overall environmental footprint of laboratories and industries utilizing these techniques. The synthesis of these compounds often involves complex chemical processes that may generate hazardous by-products and consume significant energy resources. Furthermore, the disposal of unused or expired reagents requires specialized handling to prevent environmental contamination, adding to the waste management challenges faced by research institutions and clinical laboratories.
Another aspect of environmental concern is the potential for atmospheric pollution. Some chemiluminescence reactions produce volatile organic compounds (VOCs) as by-products, which can contribute to air quality issues if released in significant quantities. While the scale of this problem is generally small compared to industrial emissions, it is still an important consideration for large-scale applications or in areas with already compromised air quality.
The use of chemiluminescence reagents in field-based environmental monitoring presents a paradoxical situation. While these assays can be valuable tools for detecting pollutants and assessing environmental health, the reagents themselves may introduce new contaminants into the ecosystems being studied. This necessitates careful protocol design and waste management practices to minimize the environmental impact of the monitoring activities themselves.
To address these environmental concerns, there is a growing focus on developing more eco-friendly chemiluminescence reagents and assay protocols. This includes research into biodegradable luminescent compounds, the use of less toxic catalysts, and the optimization of reaction conditions to minimize reagent consumption. Additionally, efforts are being made to improve waste management practices in laboratories, such as implementing recycling programs for certain reagents and developing more efficient disposal methods for chemiluminescent waste.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!