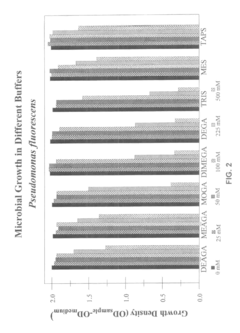
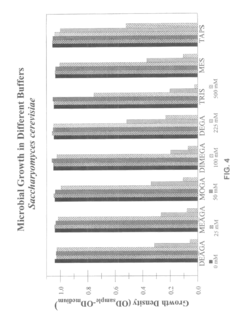
Isotonic solutions as pH buffers in biological systems
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Buffer Evolution
The evolution of isotonic buffers in biological systems has been a critical area of research, spanning several decades of scientific advancement. Initially, simple saline solutions were used to maintain osmotic balance in cells and tissues. However, as our understanding of cellular physiology deepened, the need for more sophisticated buffer systems became apparent.
In the 1950s and 1960s, researchers began to recognize the importance of maintaining not only osmotic balance but also pH stability in biological samples. This led to the development of more complex buffer solutions, such as phosphate-buffered saline (PBS), which combined the osmotic properties of saline with the pH buffering capacity of phosphate ions.
The 1970s saw a significant leap forward with the introduction of HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer by Norman Good and colleagues. HEPES and other Good's buffers were designed to address the limitations of previous buffer systems, offering improved pH stability and reduced interference with biological processes.
As molecular biology techniques advanced in the 1980s and 1990s, the demand for specialized isotonic buffers grew. Researchers developed buffers tailored to specific applications, such as cell culture media, electrophoresis buffers, and solutions for protein purification. These buffers often incorporated additional components like amino acids, vitamins, and trace elements to better mimic physiological conditions.
The turn of the millennium brought about a renewed focus on mimicking the intracellular environment. This led to the development of more sophisticated buffer systems that not only maintained osmolarity and pH but also replicated the ionic composition of cytoplasm. These advanced buffers have been crucial in improving the accuracy of in vitro experiments and the preservation of biological samples.
Recent years have seen the emergence of "smart" buffer systems that can respond dynamically to changes in their environment. These include pH-responsive polymers and nanoparticle-based buffers that can maintain stable pH over a broader range of conditions. Additionally, there has been growing interest in developing biocompatible and biodegradable buffer systems for use in medical applications, such as drug delivery and tissue engineering.
The evolution of isotonic buffers continues to be driven by advances in our understanding of cellular biology and the increasing demands of biotechnology and medical research. As we look to the future, the development of even more sophisticated and application-specific buffer systems is likely to play a crucial role in furthering our ability to study and manipulate biological systems with ever-increasing precision and control.
In the 1950s and 1960s, researchers began to recognize the importance of maintaining not only osmotic balance but also pH stability in biological samples. This led to the development of more complex buffer solutions, such as phosphate-buffered saline (PBS), which combined the osmotic properties of saline with the pH buffering capacity of phosphate ions.
The 1970s saw a significant leap forward with the introduction of HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer by Norman Good and colleagues. HEPES and other Good's buffers were designed to address the limitations of previous buffer systems, offering improved pH stability and reduced interference with biological processes.
As molecular biology techniques advanced in the 1980s and 1990s, the demand for specialized isotonic buffers grew. Researchers developed buffers tailored to specific applications, such as cell culture media, electrophoresis buffers, and solutions for protein purification. These buffers often incorporated additional components like amino acids, vitamins, and trace elements to better mimic physiological conditions.
The turn of the millennium brought about a renewed focus on mimicking the intracellular environment. This led to the development of more sophisticated buffer systems that not only maintained osmolarity and pH but also replicated the ionic composition of cytoplasm. These advanced buffers have been crucial in improving the accuracy of in vitro experiments and the preservation of biological samples.
Recent years have seen the emergence of "smart" buffer systems that can respond dynamically to changes in their environment. These include pH-responsive polymers and nanoparticle-based buffers that can maintain stable pH over a broader range of conditions. Additionally, there has been growing interest in developing biocompatible and biodegradable buffer systems for use in medical applications, such as drug delivery and tissue engineering.
The evolution of isotonic buffers continues to be driven by advances in our understanding of cellular biology and the increasing demands of biotechnology and medical research. As we look to the future, the development of even more sophisticated and application-specific buffer systems is likely to play a crucial role in furthering our ability to study and manipulate biological systems with ever-increasing precision and control.
Biological System Demands
The demand for isotonic solutions as pH buffers in biological systems stems from the critical need to maintain stable physiological conditions within living organisms. Biological systems are highly sensitive to pH fluctuations, and even minor changes can significantly impact cellular functions, enzyme activities, and overall system performance. Isotonic solutions, which have the same osmotic pressure as the surrounding cellular environment, are particularly valuable in this context as they prevent osmotic stress while simultaneously regulating pH.
In research and clinical settings, there is a growing requirement for more effective and versatile pH buffer systems that can closely mimic the complex physiological environments of various biological systems. This demand is driven by the increasing complexity of in vitro experiments, tissue engineering applications, and advanced cell culture techniques. Researchers and clinicians seek isotonic buffer solutions that can maintain stable pH levels across a wide range of temperatures, ion concentrations, and metabolic conditions.
The pharmaceutical and biotechnology industries also contribute significantly to the demand for isotonic pH buffers. These sectors require stable buffer systems for drug formulation, protein stabilization, and the development of biopharmaceuticals. The ability of isotonic solutions to preserve the structural integrity and functionality of biomolecules while maintaining optimal pH conditions is crucial for product efficacy and shelf-life.
In medical applications, there is a continuous need for improved isotonic buffer solutions for intravenous therapies, dialysis fluids, and organ preservation solutions. These applications demand pH buffers that can effectively maintain physiological pH while being compatible with the complex ionic and metabolic requirements of living tissues. The aging population and increasing prevalence of chronic diseases further amplify this demand, as more patients require interventions that rely on precisely controlled physiological environments.
The field of regenerative medicine and tissue engineering presents another significant area of demand for advanced isotonic pH buffer systems. As researchers work on developing complex tissue constructs and organoids, there is a critical need for buffer solutions that can support long-term cell viability and tissue function while maintaining stable pH conditions. This demand extends to the development of bioinks for 3D bioprinting applications, where pH stability is essential for maintaining cell viability during the printing process and subsequent tissue maturation.
Environmental and agricultural sectors also contribute to the market demand for isotonic pH buffers. In aquaculture and hydroponics, maintaining optimal pH conditions is crucial for organism health and crop productivity. There is a growing interest in developing sustainable and eco-friendly buffer solutions that can effectively regulate pH in these large-scale biological systems without adverse environmental impacts.
In research and clinical settings, there is a growing requirement for more effective and versatile pH buffer systems that can closely mimic the complex physiological environments of various biological systems. This demand is driven by the increasing complexity of in vitro experiments, tissue engineering applications, and advanced cell culture techniques. Researchers and clinicians seek isotonic buffer solutions that can maintain stable pH levels across a wide range of temperatures, ion concentrations, and metabolic conditions.
The pharmaceutical and biotechnology industries also contribute significantly to the demand for isotonic pH buffers. These sectors require stable buffer systems for drug formulation, protein stabilization, and the development of biopharmaceuticals. The ability of isotonic solutions to preserve the structural integrity and functionality of biomolecules while maintaining optimal pH conditions is crucial for product efficacy and shelf-life.
In medical applications, there is a continuous need for improved isotonic buffer solutions for intravenous therapies, dialysis fluids, and organ preservation solutions. These applications demand pH buffers that can effectively maintain physiological pH while being compatible with the complex ionic and metabolic requirements of living tissues. The aging population and increasing prevalence of chronic diseases further amplify this demand, as more patients require interventions that rely on precisely controlled physiological environments.
The field of regenerative medicine and tissue engineering presents another significant area of demand for advanced isotonic pH buffer systems. As researchers work on developing complex tissue constructs and organoids, there is a critical need for buffer solutions that can support long-term cell viability and tissue function while maintaining stable pH conditions. This demand extends to the development of bioinks for 3D bioprinting applications, where pH stability is essential for maintaining cell viability during the printing process and subsequent tissue maturation.
Environmental and agricultural sectors also contribute to the market demand for isotonic pH buffers. In aquaculture and hydroponics, maintaining optimal pH conditions is crucial for organism health and crop productivity. There is a growing interest in developing sustainable and eco-friendly buffer solutions that can effectively regulate pH in these large-scale biological systems without adverse environmental impacts.
pH Buffer Challenges
The development of effective pH buffers for biological systems faces several significant challenges. One of the primary issues is maintaining buffer capacity across a wide range of physiological pH values. Biological processes often require precise pH control, typically between 6.8 and 7.4, but can extend to more extreme ranges in certain cellular compartments. Traditional buffer systems may struggle to provide consistent performance across this entire spectrum.
Another critical challenge is the potential toxicity of buffer components to living cells and tissues. Many commonly used buffers contain chemicals that can interfere with cellular processes or cause damage at higher concentrations. This toxicity concern limits the selection of suitable buffer compounds and necessitates extensive biocompatibility testing for new formulations.
The ionic strength of buffer solutions presents an additional hurdle. Isotonic solutions are crucial for maintaining cellular osmotic balance, but achieving isotonicity while simultaneously providing adequate buffering capacity can be complex. Balancing these two requirements often involves intricate formulation adjustments and may compromise overall buffer effectiveness.
Temperature sensitivity is yet another factor complicating pH buffer design. Many biological experiments and applications involve temperature fluctuations, which can significantly affect buffer performance. Developing temperature-resistant buffers that maintain consistent pH levels across varying thermal conditions remains a persistent challenge in the field.
Furthermore, the interaction between buffer components and biological molecules poses a substantial obstacle. Proteins, nucleic acids, and other biomolecules can bind to buffer constituents, altering their structure or function. This interaction may lead to experimental artifacts or unintended physiological effects, necessitating careful consideration of buffer composition in relation to the specific biological system under study.
The long-term stability of pH buffers in biological environments is also a significant concern. Degradation of buffer components over time can result in pH drift, potentially compromising experimental results or the viability of biological samples. Developing buffers that remain stable for extended periods under physiological conditions is crucial for many applications, particularly in long-term cell culture or storage of biological materials.
Lastly, the scalability and cost-effectiveness of buffer production present practical challenges for widespread adoption. While some highly effective buffer systems may be developed at a laboratory scale, translating these formulations to industrial-scale production while maintaining quality and minimizing costs can be a substantial hurdle in commercialization efforts.
Another critical challenge is the potential toxicity of buffer components to living cells and tissues. Many commonly used buffers contain chemicals that can interfere with cellular processes or cause damage at higher concentrations. This toxicity concern limits the selection of suitable buffer compounds and necessitates extensive biocompatibility testing for new formulations.
The ionic strength of buffer solutions presents an additional hurdle. Isotonic solutions are crucial for maintaining cellular osmotic balance, but achieving isotonicity while simultaneously providing adequate buffering capacity can be complex. Balancing these two requirements often involves intricate formulation adjustments and may compromise overall buffer effectiveness.
Temperature sensitivity is yet another factor complicating pH buffer design. Many biological experiments and applications involve temperature fluctuations, which can significantly affect buffer performance. Developing temperature-resistant buffers that maintain consistent pH levels across varying thermal conditions remains a persistent challenge in the field.
Furthermore, the interaction between buffer components and biological molecules poses a substantial obstacle. Proteins, nucleic acids, and other biomolecules can bind to buffer constituents, altering their structure or function. This interaction may lead to experimental artifacts or unintended physiological effects, necessitating careful consideration of buffer composition in relation to the specific biological system under study.
The long-term stability of pH buffers in biological environments is also a significant concern. Degradation of buffer components over time can result in pH drift, potentially compromising experimental results or the viability of biological samples. Developing buffers that remain stable for extended periods under physiological conditions is crucial for many applications, particularly in long-term cell culture or storage of biological materials.
Lastly, the scalability and cost-effectiveness of buffer production present practical challenges for widespread adoption. While some highly effective buffer systems may be developed at a laboratory scale, translating these formulations to industrial-scale production while maintaining quality and minimizing costs can be a substantial hurdle in commercialization efforts.
Current Isotonic Buffers
01 pH range for isotonic solutions
Isotonic solutions typically have a pH range that is compatible with physiological conditions. This range is usually between 6.5 and 7.5, which is close to the pH of blood and other bodily fluids. Maintaining the proper pH is crucial for the stability and effectiveness of the solution, as well as for minimizing irritation when used in medical applications.- pH range for isotonic solutions: Isotonic solutions typically have a pH range that is compatible with physiological conditions. This range is usually between 6.5 and 7.5, which is close to the pH of blood and other bodily fluids. Maintaining the correct pH is crucial for the stability and effectiveness of the solution, as well as for patient comfort and safety when used in medical applications.
- Buffer systems for pH control: Buffer systems are often incorporated into isotonic solutions to maintain a stable pH. Common buffer systems include phosphate buffers, citrate buffers, and bicarbonate buffers. These buffers help to resist changes in pH that may occur due to the addition of other components or environmental factors, ensuring the solution remains isotonic and at the desired pH level.
- pH adjustment methods: Various methods can be employed to adjust the pH of isotonic solutions. These may include the addition of acids or bases, such as hydrochloric acid or sodium hydroxide, in small quantities to achieve the desired pH. The adjustment process often requires precise measurements and careful titration to maintain the isotonicity of the solution while reaching the target pH.
- pH considerations for specific applications: The optimal pH of isotonic solutions can vary depending on their specific application. For example, ophthalmic solutions may require a pH closer to that of tears, while solutions for intravenous use might need to match blood pH more closely. Consideration of the intended use and the physiological environment in which the solution will be applied is crucial when determining the appropriate pH range.
- pH monitoring and stability: Continuous monitoring and maintenance of pH in isotonic solutions is essential for ensuring their stability and efficacy over time. This may involve the use of pH indicators, regular testing, and appropriate storage conditions. Stability studies are often conducted to determine how the pH of the solution may change during storage and to establish appropriate shelf-life for the product.
02 Buffer systems for pH control
Buffer systems are often incorporated into isotonic solutions to maintain a stable pH. Common buffer systems include phosphate buffers, citrate buffers, and bicarbonate buffers. These buffers help to resist changes in pH that may occur due to the addition of other components or environmental factors, ensuring the solution remains isotonic and at the desired pH level.Expand Specific Solutions03 pH adjustment methods
Various methods can be employed to adjust the pH of isotonic solutions. These may include the addition of acids or bases, such as hydrochloric acid or sodium hydroxide, in small quantities to achieve the desired pH. The adjustment process often requires precise measurements and careful titration to maintain the isotonicity of the solution while reaching the target pH.Expand Specific Solutions04 pH considerations for specific applications
The optimal pH of isotonic solutions can vary depending on their specific application. For example, ophthalmic solutions may require a pH closer to that of tears, while solutions for intravenous use might need to match blood pH more closely. Consideration of the intended use and the physiological environment in which the solution will be applied is crucial when determining the appropriate pH range.Expand Specific Solutions05 pH monitoring and stability
Maintaining pH stability in isotonic solutions is essential for their efficacy and shelf life. This often involves careful selection of ingredients, appropriate packaging, and storage conditions. Regular pH monitoring during production and storage may be necessary to ensure the solution remains within the specified pH range throughout its intended use period.Expand Specific Solutions
Key Buffer Manufacturers
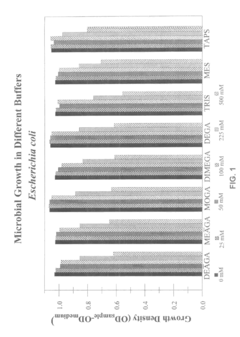
The research on isotonic solutions as pH buffers in biological systems is in a mature stage, with a well-established market and significant industry players. The global market for pH buffers is substantial, driven by applications in pharmaceuticals, biotechnology, and life sciences. Companies like Life Technologies Corp., Novo Nordisk A/S, and Novartis AG are major contributors, leveraging their extensive R&D capabilities and global presence. Emerging players such as Anb Sensors Ltd. are introducing innovative technologies, like calibration-free solid-state pH sensors, indicating ongoing technological advancements. The involvement of diverse companies, from pharmaceutical giants to specialized sensor manufacturers, suggests a competitive and evolving landscape with opportunities for both established and niche players.
Life Technologies Corp.
Technical Solution: Life Technologies Corp. has developed advanced isotonic buffer solutions for biological systems, focusing on pH stability and cell viability. Their proprietary formulations incorporate zwitterionic organic compounds, such as HEPES and MOPS, which provide excellent buffering capacity in the physiological pH range (6.8-7.4)[1]. These solutions are designed to maintain osmolarity and pH balance in cell culture media, ensuring optimal conditions for cellular functions. The company has also introduced novel phosphate-based buffer systems that offer improved long-term stability and reduced precipitation in high-protein environments[3]. Additionally, Life Technologies has developed specialized isotonic buffers for specific applications, such as nucleic acid preservation and protein purification, which incorporate antioxidants and chelating agents to prevent degradation[5].
Strengths: Broad range of specialized buffer solutions, excellent pH stability in physiological range, and compatibility with various biological applications. Weaknesses: Some formulations may be costly, and certain buffer components might interfere with specific assays or cellular processes.
Anb Sensors Ltd.
Technical Solution: Anb Sensors Ltd. has pioneered the development of innovative pH sensing technologies that can be integrated into isotonic buffer systems for real-time monitoring in biological applications. Their approach combines miniaturized solid-state pH sensors with biocompatible coatings, allowing for continuous pH measurement in complex biological environments[2]. The company's technology utilizes ion-sensitive field-effect transistors (ISFETs) modified with pH-responsive hydrogels, enabling rapid and accurate pH detection in isotonic solutions. Anb Sensors has also developed algorithms for compensating temperature and ionic strength effects, ensuring reliable pH measurements across various physiological conditions[4]. Their sensors can be incorporated into microfluidic devices and bioreactors, providing valuable data for optimizing buffer performance and maintaining pH homeostasis in biological systems[6].
Strengths: High-precision, real-time pH monitoring in isotonic solutions; miniaturized sensors suitable for integration into various biological platforms. Weaknesses: May require specialized equipment for sensor readout; potential long-term drift in sensor performance in complex biological matrices.
Innovative Buffer Patents
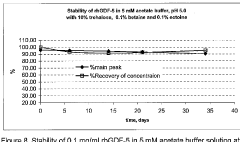
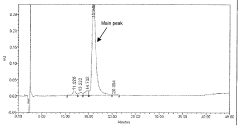
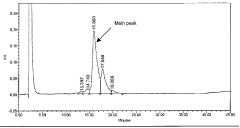
Liquid buffered GDF-5 formulations
PatentInactiveSG165647A1
Innovation
- Development of an acetate buffered solution for stabilizing GDF-5 protein in a specific pH range (4.2 to 5.3).
- Creation of a biologically isotonic solution that improves GDF-5 stability during storage, handling, and use.
- Optimization of the formulation to maintain GDF-5 stability while ensuring biological isotonicity.
Buffer compounds
PatentActiveUS20120129240A1
Innovation
- Development of amine-quaternary ammonium buffer compounds with a wide range of pKa values (2-13) that provide selectable, predetermined charge properties, low toxicity, and chemical compatibility, allowing for buffering across the pH range of 2 to 13, synthesized from symmetric and unsymmetric diamines and amine-guanidines with specific structural types.
Regulatory Considerations
The regulatory landscape for isotonic solutions used as pH buffers in biological systems is complex and multifaceted, requiring careful consideration of various guidelines and standards. Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe play crucial roles in overseeing the development, production, and use of these solutions in medical and research applications.
For pharmaceutical and medical device applications, isotonic buffer solutions must comply with Good Manufacturing Practice (GMP) regulations. These guidelines ensure consistent quality, safety, and efficacy of the products. Manufacturers are required to implement robust quality management systems, maintain detailed documentation, and undergo regular inspections to ensure compliance.
In the context of biological research, the use of isotonic pH buffers is subject to laboratory safety regulations and ethical guidelines. Institutional Review Boards (IRBs) and Animal Care and Use Committees (ACUCs) often review research protocols involving these solutions to ensure the safety of human subjects and the ethical treatment of animals in experimental settings.
Environmental regulations also come into play, particularly concerning the disposal of isotonic buffer solutions. Many jurisdictions have specific requirements for the handling and disposal of laboratory chemicals, including buffers, to prevent environmental contamination and protect public health.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide important standards for the composition and quality of isotonic buffer solutions used in pharmaceutical applications. These compendia outline specific requirements for pH, osmolality, and purity, which manufacturers must adhere to for regulatory compliance.
For isotonic buffer solutions used in medical devices or diagnostic kits, additional regulations such as the Medical Device Regulation (MDR) in Europe and the FDA's medical device regulations in the US apply. These frameworks ensure that the solutions meet specific performance criteria and are safe for their intended use.
Labeling and packaging regulations are another critical aspect to consider. Accurate labeling of isotonic buffer solutions, including pH values, composition, and expiration dates, is essential for regulatory compliance and user safety. In some cases, specific warning statements or storage instructions may be required on the packaging.
As the field of biological research and medical applications continues to evolve, regulatory frameworks are also adapting. Researchers and manufacturers must stay informed about emerging regulations and guidelines that may impact the development, production, and use of isotonic buffer solutions in biological systems.
For pharmaceutical and medical device applications, isotonic buffer solutions must comply with Good Manufacturing Practice (GMP) regulations. These guidelines ensure consistent quality, safety, and efficacy of the products. Manufacturers are required to implement robust quality management systems, maintain detailed documentation, and undergo regular inspections to ensure compliance.
In the context of biological research, the use of isotonic pH buffers is subject to laboratory safety regulations and ethical guidelines. Institutional Review Boards (IRBs) and Animal Care and Use Committees (ACUCs) often review research protocols involving these solutions to ensure the safety of human subjects and the ethical treatment of animals in experimental settings.
Environmental regulations also come into play, particularly concerning the disposal of isotonic buffer solutions. Many jurisdictions have specific requirements for the handling and disposal of laboratory chemicals, including buffers, to prevent environmental contamination and protect public health.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) provide important standards for the composition and quality of isotonic buffer solutions used in pharmaceutical applications. These compendia outline specific requirements for pH, osmolality, and purity, which manufacturers must adhere to for regulatory compliance.
For isotonic buffer solutions used in medical devices or diagnostic kits, additional regulations such as the Medical Device Regulation (MDR) in Europe and the FDA's medical device regulations in the US apply. These frameworks ensure that the solutions meet specific performance criteria and are safe for their intended use.
Labeling and packaging regulations are another critical aspect to consider. Accurate labeling of isotonic buffer solutions, including pH values, composition, and expiration dates, is essential for regulatory compliance and user safety. In some cases, specific warning statements or storage instructions may be required on the packaging.
As the field of biological research and medical applications continues to evolve, regulatory frameworks are also adapting. Researchers and manufacturers must stay informed about emerging regulations and guidelines that may impact the development, production, and use of isotonic buffer solutions in biological systems.
Biocompatibility Analysis
Biocompatibility is a critical factor in the development and application of isotonic solutions as pH buffers in biological systems. These solutions must maintain a delicate balance between effectively regulating pH and ensuring minimal adverse effects on living tissues and cells. The biocompatibility of isotonic pH buffers is primarily determined by their chemical composition, osmolarity, and potential interactions with biological molecules and cellular structures.
One of the key considerations in biocompatibility analysis is the impact of isotonic pH buffers on cell viability and function. Studies have shown that certain buffer compositions can influence cellular metabolism, membrane integrity, and protein function. For instance, phosphate-based buffers, while effective in maintaining pH, may interfere with calcium-dependent cellular processes. In contrast, HEPES-based buffers have demonstrated superior biocompatibility in many cell culture applications, with minimal effects on cellular physiology.
The osmolarity of isotonic pH buffers plays a crucial role in their biocompatibility. Solutions that are not properly isotonic can cause osmotic stress, leading to cell shrinkage or swelling, which can compromise cellular function and viability. Careful formulation and testing are required to ensure that the buffer maintains physiological osmolarity while still providing effective pH regulation.
Interactions between buffer components and biological molecules must also be considered. Some buffers may bind to proteins or other biomolecules, potentially altering their structure or function. This can have implications for both in vitro experiments and in vivo applications. For example, Tris buffers have been shown to interact with certain enzymes, potentially affecting experimental outcomes in biochemical assays.
Long-term exposure to isotonic pH buffers is another important aspect of biocompatibility analysis. While a buffer may show good short-term compatibility, prolonged contact with biological systems can reveal subtle effects on cellular health and tissue function. This is particularly relevant for applications such as organ preservation, where tissues may be exposed to buffer solutions for extended periods.
The biocompatibility of isotonic pH buffers also extends to their potential for systemic effects when used in vivo. Factors such as toxicity, immunogenicity, and metabolic fate must be carefully evaluated. Some buffer components may be metabolized or cleared differently by various organs, potentially leading to accumulation or unexpected physiological effects.
In conclusion, biocompatibility analysis of isotonic solutions as pH buffers in biological systems is a multifaceted process that requires consideration of various factors. It involves assessing the impact on cellular function, osmotic balance, molecular interactions, and long-term effects. This comprehensive evaluation is essential for developing safe and effective pH buffer solutions for diverse biological applications, from cell culture to medical treatments.
One of the key considerations in biocompatibility analysis is the impact of isotonic pH buffers on cell viability and function. Studies have shown that certain buffer compositions can influence cellular metabolism, membrane integrity, and protein function. For instance, phosphate-based buffers, while effective in maintaining pH, may interfere with calcium-dependent cellular processes. In contrast, HEPES-based buffers have demonstrated superior biocompatibility in many cell culture applications, with minimal effects on cellular physiology.
The osmolarity of isotonic pH buffers plays a crucial role in their biocompatibility. Solutions that are not properly isotonic can cause osmotic stress, leading to cell shrinkage or swelling, which can compromise cellular function and viability. Careful formulation and testing are required to ensure that the buffer maintains physiological osmolarity while still providing effective pH regulation.
Interactions between buffer components and biological molecules must also be considered. Some buffers may bind to proteins or other biomolecules, potentially altering their structure or function. This can have implications for both in vitro experiments and in vivo applications. For example, Tris buffers have been shown to interact with certain enzymes, potentially affecting experimental outcomes in biochemical assays.
Long-term exposure to isotonic pH buffers is another important aspect of biocompatibility analysis. While a buffer may show good short-term compatibility, prolonged contact with biological systems can reveal subtle effects on cellular health and tissue function. This is particularly relevant for applications such as organ preservation, where tissues may be exposed to buffer solutions for extended periods.
The biocompatibility of isotonic pH buffers also extends to their potential for systemic effects when used in vivo. Factors such as toxicity, immunogenicity, and metabolic fate must be carefully evaluated. Some buffer components may be metabolized or cleared differently by various organs, potentially leading to accumulation or unexpected physiological effects.
In conclusion, biocompatibility analysis of isotonic solutions as pH buffers in biological systems is a multifaceted process that requires consideration of various factors. It involves assessing the impact on cellular function, osmotic balance, molecular interactions, and long-term effects. This comprehensive evaluation is essential for developing safe and effective pH buffer solutions for diverse biological applications, from cell culture to medical treatments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!