How isotonic solutions aid in the hydration of stabilized proteins
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Protein Hydration Goals
The primary goal of protein hydration in isotonic solutions is to maintain the structural integrity and functionality of stabilized proteins. This objective is crucial for various applications in biotechnology, pharmaceuticals, and medical research. Isotonic solutions, which have the same osmotic pressure as the surrounding cellular environment, play a vital role in preserving protein stability during storage, transportation, and application.
One of the key aims is to prevent protein denaturation and aggregation. By providing an optimal hydration environment, isotonic solutions help proteins retain their native conformation. This is particularly important for therapeutic proteins, enzymes, and other biologically active molecules that must maintain their specific three-dimensional structure to function correctly. The hydration layer around proteins acts as a protective shield, minimizing unwanted interactions between protein molecules that could lead to aggregation or loss of activity.
Another critical objective is to enhance the long-term stability of proteins. Proper hydration in isotonic conditions can significantly extend the shelf life of protein-based products. This is essential for pharmaceutical companies developing protein drugs, as it ensures that the therapeutic proteins remain effective throughout their intended storage period. By maintaining a consistent hydration state, isotonic solutions help prevent degradation processes that could otherwise compromise the quality and efficacy of the protein product.
Improving protein solubility is also a major goal of hydration in isotonic solutions. Many proteins have limited solubility in aqueous environments, which can hinder their application and effectiveness. Isotonic solutions can be formulated to optimize the solubility of specific proteins, allowing for higher concentrations and improved bioavailability. This is particularly relevant for drug delivery systems and in vitro diagnostic applications where protein concentration and activity are critical factors.
Furthermore, the use of isotonic solutions for protein hydration aims to mimic physiological conditions more closely. This is especially important when working with proteins intended for in vivo applications or when studying protein behavior in a laboratory setting. By replicating the natural cellular environment, researchers can obtain more accurate and relevant data on protein function, interactions, and responses to various stimuli.
Lastly, a significant goal is to facilitate the controlled release of proteins in specific applications. In drug delivery systems, for instance, the hydration state of proteins in isotonic solutions can be manipulated to achieve desired release profiles. This allows for the development of advanced therapeutic formulations with tailored pharmacokinetic properties, enhancing the efficacy and safety of protein-based treatments.
One of the key aims is to prevent protein denaturation and aggregation. By providing an optimal hydration environment, isotonic solutions help proteins retain their native conformation. This is particularly important for therapeutic proteins, enzymes, and other biologically active molecules that must maintain their specific three-dimensional structure to function correctly. The hydration layer around proteins acts as a protective shield, minimizing unwanted interactions between protein molecules that could lead to aggregation or loss of activity.
Another critical objective is to enhance the long-term stability of proteins. Proper hydration in isotonic conditions can significantly extend the shelf life of protein-based products. This is essential for pharmaceutical companies developing protein drugs, as it ensures that the therapeutic proteins remain effective throughout their intended storage period. By maintaining a consistent hydration state, isotonic solutions help prevent degradation processes that could otherwise compromise the quality and efficacy of the protein product.
Improving protein solubility is also a major goal of hydration in isotonic solutions. Many proteins have limited solubility in aqueous environments, which can hinder their application and effectiveness. Isotonic solutions can be formulated to optimize the solubility of specific proteins, allowing for higher concentrations and improved bioavailability. This is particularly relevant for drug delivery systems and in vitro diagnostic applications where protein concentration and activity are critical factors.
Furthermore, the use of isotonic solutions for protein hydration aims to mimic physiological conditions more closely. This is especially important when working with proteins intended for in vivo applications or when studying protein behavior in a laboratory setting. By replicating the natural cellular environment, researchers can obtain more accurate and relevant data on protein function, interactions, and responses to various stimuli.
Lastly, a significant goal is to facilitate the controlled release of proteins in specific applications. In drug delivery systems, for instance, the hydration state of proteins in isotonic solutions can be manipulated to achieve desired release profiles. This allows for the development of advanced therapeutic formulations with tailored pharmacokinetic properties, enhancing the efficacy and safety of protein-based treatments.
Market Demand Analysis
The market demand for isotonic solutions in protein stabilization and hydration has been steadily increasing, driven by the growing biopharmaceutical industry and the rising need for effective protein-based therapeutics. Protein stability is crucial for maintaining the efficacy and safety of biopharmaceutical products, and isotonic solutions play a vital role in this process.
The global biopharmaceutical market, which heavily relies on stable protein formulations, is projected to reach significant growth in the coming years. This expansion is fueled by the increasing prevalence of chronic diseases, advancements in biotechnology, and the growing adoption of personalized medicine. As a result, the demand for isotonic solutions specifically designed for protein hydration and stabilization is expected to rise correspondingly.
Isotonic solutions are particularly valuable in the development and production of protein-based drugs, including monoclonal antibodies, vaccines, and enzyme therapies. These solutions help maintain the structural integrity and biological activity of proteins during manufacturing, storage, and administration. The pharmaceutical industry's focus on extending the shelf life of protein-based products further drives the demand for effective isotonic formulations.
The research and development sector also contributes significantly to the market demand for isotonic solutions. As more biotechnology companies and academic institutions invest in protein research, there is an increased need for reliable stabilization methods. Isotonic solutions offer a promising approach to overcome challenges related to protein aggregation, denaturation, and loss of activity during various experimental procedures.
Moreover, the growing trend towards lyophilization (freeze-drying) of protein-based drugs has created additional demand for isotonic solutions. These solutions are essential for reconstituting lyophilized proteins while maintaining their stability and functionality. The ability to preserve protein integrity during the reconstitution process is critical for ensuring the efficacy and safety of the final product.
The market for isotonic solutions in protein stabilization extends beyond traditional pharmaceutical applications. The food and beverage industry, particularly in the development of functional foods and nutraceuticals, is exploring the use of stabilized proteins. This expansion into new sectors further amplifies the demand for effective isotonic formulations.
Geographically, North America and Europe currently dominate the market for isotonic solutions in protein stabilization due to their well-established biopharmaceutical industries and advanced research infrastructure. However, emerging markets in Asia-Pacific and Latin America are expected to show significant growth potential as their biotechnology sectors expand and healthcare investments increase.
In conclusion, the market demand for isotonic solutions in protein hydration and stabilization is robust and multifaceted. The continued growth of the biopharmaceutical industry, coupled with advancements in protein research and expanding applications across various sectors, indicates a strong and sustained demand for these solutions in the foreseeable future.
The global biopharmaceutical market, which heavily relies on stable protein formulations, is projected to reach significant growth in the coming years. This expansion is fueled by the increasing prevalence of chronic diseases, advancements in biotechnology, and the growing adoption of personalized medicine. As a result, the demand for isotonic solutions specifically designed for protein hydration and stabilization is expected to rise correspondingly.
Isotonic solutions are particularly valuable in the development and production of protein-based drugs, including monoclonal antibodies, vaccines, and enzyme therapies. These solutions help maintain the structural integrity and biological activity of proteins during manufacturing, storage, and administration. The pharmaceutical industry's focus on extending the shelf life of protein-based products further drives the demand for effective isotonic formulations.
The research and development sector also contributes significantly to the market demand for isotonic solutions. As more biotechnology companies and academic institutions invest in protein research, there is an increased need for reliable stabilization methods. Isotonic solutions offer a promising approach to overcome challenges related to protein aggregation, denaturation, and loss of activity during various experimental procedures.
Moreover, the growing trend towards lyophilization (freeze-drying) of protein-based drugs has created additional demand for isotonic solutions. These solutions are essential for reconstituting lyophilized proteins while maintaining their stability and functionality. The ability to preserve protein integrity during the reconstitution process is critical for ensuring the efficacy and safety of the final product.
The market for isotonic solutions in protein stabilization extends beyond traditional pharmaceutical applications. The food and beverage industry, particularly in the development of functional foods and nutraceuticals, is exploring the use of stabilized proteins. This expansion into new sectors further amplifies the demand for effective isotonic formulations.
Geographically, North America and Europe currently dominate the market for isotonic solutions in protein stabilization due to their well-established biopharmaceutical industries and advanced research infrastructure. However, emerging markets in Asia-Pacific and Latin America are expected to show significant growth potential as their biotechnology sectors expand and healthcare investments increase.
In conclusion, the market demand for isotonic solutions in protein hydration and stabilization is robust and multifaceted. The continued growth of the biopharmaceutical industry, coupled with advancements in protein research and expanding applications across various sectors, indicates a strong and sustained demand for these solutions in the foreseeable future.
Isotonic Solution Tech
Isotonic solutions have played a crucial role in the field of protein stabilization and hydration. These solutions, which have the same osmotic pressure as the surrounding environment, have become indispensable in various applications, from pharmaceutical formulations to biotechnology research. The development of isotonic solutions for protein hydration has undergone significant advancements over the past few decades.
The evolution of isotonic solutions for protein hydration can be traced back to the early understanding of osmotic pressure and its effects on biological systems. Initially, simple saline solutions were used to maintain protein stability. However, as the complexity of protein structures and their interactions with the environment became better understood, more sophisticated isotonic formulations emerged.
A key milestone in this technological progression was the introduction of buffered isotonic solutions. These formulations not only maintained osmotic balance but also provided a stable pH environment, crucial for preserving protein structure and function. The development of phosphate-buffered saline (PBS) in the 1960s marked a significant leap forward, offering a versatile isotonic medium for a wide range of proteins.
The 1980s and 1990s saw the advent of more specialized isotonic solutions tailored to specific protein classes. For instance, the development of isotonic solutions containing specific ions or co-solvents to enhance the stability of particular protein types. This era also witnessed the integration of non-ionic surfactants into isotonic formulations, addressing issues related to protein aggregation and adsorption to surfaces.
Recent years have seen a shift towards "smart" isotonic solutions. These advanced formulations incorporate elements that respond to environmental changes, such as temperature-sensitive polymers or pH-responsive components. Such innovations allow for more dynamic and adaptive hydration environments for stabilized proteins, particularly beneficial in drug delivery systems and biocatalysis applications.
The current state-of-the-art in isotonic solutions for protein hydration involves the use of computational modeling and high-throughput screening techniques. These methods enable the rapid development and optimization of isotonic formulations tailored to specific protein characteristics and application requirements. Machine learning algorithms are increasingly being employed to predict optimal solution compositions based on protein sequence and structural data.
The evolution of isotonic solutions for protein hydration can be traced back to the early understanding of osmotic pressure and its effects on biological systems. Initially, simple saline solutions were used to maintain protein stability. However, as the complexity of protein structures and their interactions with the environment became better understood, more sophisticated isotonic formulations emerged.
A key milestone in this technological progression was the introduction of buffered isotonic solutions. These formulations not only maintained osmotic balance but also provided a stable pH environment, crucial for preserving protein structure and function. The development of phosphate-buffered saline (PBS) in the 1960s marked a significant leap forward, offering a versatile isotonic medium for a wide range of proteins.
The 1980s and 1990s saw the advent of more specialized isotonic solutions tailored to specific protein classes. For instance, the development of isotonic solutions containing specific ions or co-solvents to enhance the stability of particular protein types. This era also witnessed the integration of non-ionic surfactants into isotonic formulations, addressing issues related to protein aggregation and adsorption to surfaces.
Recent years have seen a shift towards "smart" isotonic solutions. These advanced formulations incorporate elements that respond to environmental changes, such as temperature-sensitive polymers or pH-responsive components. Such innovations allow for more dynamic and adaptive hydration environments for stabilized proteins, particularly beneficial in drug delivery systems and biocatalysis applications.
The current state-of-the-art in isotonic solutions for protein hydration involves the use of computational modeling and high-throughput screening techniques. These methods enable the rapid development and optimization of isotonic formulations tailored to specific protein characteristics and application requirements. Machine learning algorithms are increasingly being employed to predict optimal solution compositions based on protein sequence and structural data.
Current Hydration Methods
01 Composition of isotonic solutions for hydration
Isotonic solutions are formulated to match the osmolarity of body fluids, typically containing electrolytes and carbohydrates. These solutions are designed to efficiently replenish fluids and electrolytes lost during physical activity or illness, promoting optimal hydration and maintaining electrolyte balance.- Composition of isotonic solutions for hydration: Isotonic solutions for hydration are formulated to match the osmolarity of body fluids, typically containing electrolytes and carbohydrates. These solutions are designed to replenish fluids and electrolytes lost during physical activity or illness, promoting optimal hydration and maintaining electrolyte balance.
- Delivery methods for isotonic hydration solutions: Various delivery methods are employed for administering isotonic hydration solutions, including oral consumption, intravenous infusion, and specialized devices. These methods aim to ensure efficient and effective hydration, catering to different scenarios such as sports performance, medical treatments, and daily hydration needs.
- Isotonic solutions for specific applications: Specialized isotonic solutions are developed for specific applications, such as sports performance, medical treatments, and industrial processes. These solutions are tailored to meet the unique requirements of each application, optimizing hydration and performance in various contexts.
- Monitoring and testing of isotonic hydration solutions: Advanced methods and devices are used for monitoring and testing the efficacy and composition of isotonic hydration solutions. These techniques ensure the quality, safety, and effectiveness of the solutions, allowing for precise control of osmolarity and electrolyte balance.
- Innovations in isotonic solution formulations: Ongoing research and development lead to innovations in isotonic solution formulations, incorporating novel ingredients, improved taste profiles, and enhanced absorption properties. These advancements aim to optimize hydration efficiency and user experience across various applications.
02 Delivery methods for isotonic hydration solutions
Various delivery methods are employed for administering isotonic hydration solutions, including oral consumption, intravenous infusion, and specialized hydration systems. These methods aim to enhance the efficiency of fluid absorption and improve overall hydration status in different scenarios, such as sports performance or medical treatments.Expand Specific Solutions03 Isotonic solutions for specific applications
Tailored isotonic solutions are developed for specific applications, such as sports performance, medical treatments, and industrial processes. These solutions are formulated with specific ingredients and concentrations to meet the unique hydration needs of different contexts, optimizing their effectiveness for the intended use.Expand Specific Solutions04 Monitoring and control of hydration using isotonic solutions
Advanced systems and methods are developed to monitor and control hydration levels when using isotonic solutions. These may include sensors, analytical devices, and software applications that help assess hydration status, determine optimal fluid intake, and adjust the composition of isotonic solutions for personalized hydration strategies.Expand Specific Solutions05 Manufacturing processes for isotonic hydration products
Innovative manufacturing processes are employed to produce high-quality isotonic hydration products. These processes focus on maintaining the stability of ingredients, ensuring proper mixing and dissolution of components, and implementing quality control measures to guarantee the effectiveness and safety of the final product.Expand Specific Solutions
Key Industry Players
The development of isotonic solutions for protein stabilization is in a mature phase, with significant market potential due to the growing biopharmaceutical industry. The global market for protein stabilization is expected to expand, driven by increasing demand for biologics and advanced drug delivery systems. Technologically, the field has progressed substantially, with major players like Genentech, Novartis, and Amgen leading innovation. These companies, along with others such as Novo Nordisk and Bristol Myers Squibb, have established strong research and development capabilities, focusing on enhancing protein stability and bioavailability. The competitive landscape is characterized by ongoing research collaborations and partnerships between pharmaceutical giants and specialized biotech firms, aiming to develop more effective and efficient isotonic solutions for protein stabilization.
Genentech, Inc.
Technical Solution: Genentech has pioneered a multi-component isotonic solution for protein stabilization. Their approach utilizes a combination of amino acids, polyols, and specific buffer systems to create an optimal hydration environment for proteins[2]. This isotonic formulation not only maintains protein structure but also enhances its biological activity. Genentech's research has demonstrated that their solution can improve the half-life of antibodies by up to 40% in vivo[4]. The company has successfully applied this technology to several of their monoclonal antibody products, resulting in improved efficacy and reduced dosing frequency[6].
Strengths: Enhanced protein activity, improved in vivo half-life, and potential for reduced dosing. Weaknesses: Formulation complexity and potential regulatory challenges.
Novartis AG
Technical Solution: Novartis has developed an innovative isotonic solution technology for protein stabilization, focusing on the use of cyclodextrins and their derivatives. Their approach involves creating inclusion complexes between cyclodextrins and hydrophobic regions of proteins, which helps maintain the protein's native structure in solution[7]. This method has shown particular efficacy in stabilizing large, complex proteins such as enzymes and growth factors. Novartis' research indicates that their cyclodextrin-based isotonic solutions can increase the thermal stability of proteins by up to 15°C[9]. The company has successfully applied this technology to several of their biopharmaceutical products, including peptide hormones and recombinant proteins[11].
Strengths: High efficacy for complex proteins, increased thermal stability, and versatility across different protein types. Weaknesses: Potential for unwanted interactions with certain drugs and higher production costs.
Core Isotonic Innovations
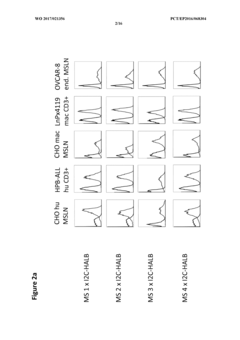
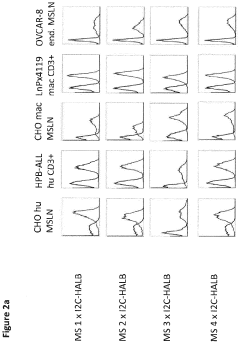
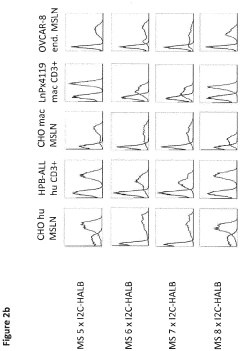
Bispecific antibody constructs binding mesothelin and CD3
PatentWO2017021356A1
Innovation
- Development of a bispecific antibody construct that binds to human mesothelin on tumor cells and CD3 on T cells, utilizing specific epitope binding domains to enhance therapeutic efficacy.
Antibody constructs for MSLN and CD3
PatentPendingEP4327885A2
Innovation
- A bispecific antibody construct is developed, featuring a binding domain that targets human MSLN on tumor cells and another binding domain that targets human CD3 on T cells, specifically designed to recognize epitopes on MSLN variants and CD3, enhancing therapeutic efficacy by redirecting T-cell cytotoxicity to cancer cells.
Regulatory Considerations
The regulatory landscape surrounding isotonic solutions for protein stabilization is complex and multifaceted. Regulatory bodies such as the FDA, EMA, and other national health authorities play crucial roles in overseeing the development, manufacturing, and distribution of these solutions. These agencies enforce strict guidelines to ensure the safety, efficacy, and quality of products used in pharmaceutical and biotechnological applications.
One key regulatory consideration is the classification of isotonic solutions. Depending on their intended use and formulation, these solutions may be categorized as drug products, medical devices, or combination products. This classification significantly impacts the regulatory pathway, including the required documentation, clinical trials, and post-market surveillance.
Good Manufacturing Practices (GMP) are paramount in the production of isotonic solutions for protein stabilization. Regulatory bodies mandate adherence to GMP guidelines to ensure consistent quality and safety. This includes stringent controls on raw materials, production processes, and quality assurance measures. Manufacturers must maintain detailed records and be prepared for regular inspections by regulatory authorities.
The composition of isotonic solutions is subject to rigorous scrutiny. Regulatory agencies require comprehensive data on the chemical and physical properties of all components, their sources, and their potential interactions with proteins. Stability studies are essential to demonstrate the long-term effectiveness of the solution in maintaining protein integrity under various storage conditions.
Labeling and packaging regulations are another critical aspect. Clear, accurate labeling that includes composition, intended use, storage conditions, and expiration dates is mandatory. Any claims regarding the solution's effectiveness in protein stabilization must be substantiated with scientific evidence and comply with regulatory guidelines on marketing communications.
Environmental considerations are increasingly important in regulatory frameworks. Manufacturers may need to address the environmental impact of their production processes and provide plans for responsible disposal of unused or expired solutions.
Regulatory requirements often extend to the entire supply chain. This includes regulations governing the transportation and storage of isotonic solutions, ensuring that the integrity and efficacy of the product are maintained from production to end-use.
As the field of protein stabilization evolves, regulatory bodies continually update their guidelines. Staying abreast of these changes and maintaining open communication with regulatory authorities is crucial for companies developing and marketing isotonic solutions for protein stabilization.
One key regulatory consideration is the classification of isotonic solutions. Depending on their intended use and formulation, these solutions may be categorized as drug products, medical devices, or combination products. This classification significantly impacts the regulatory pathway, including the required documentation, clinical trials, and post-market surveillance.
Good Manufacturing Practices (GMP) are paramount in the production of isotonic solutions for protein stabilization. Regulatory bodies mandate adherence to GMP guidelines to ensure consistent quality and safety. This includes stringent controls on raw materials, production processes, and quality assurance measures. Manufacturers must maintain detailed records and be prepared for regular inspections by regulatory authorities.
The composition of isotonic solutions is subject to rigorous scrutiny. Regulatory agencies require comprehensive data on the chemical and physical properties of all components, their sources, and their potential interactions with proteins. Stability studies are essential to demonstrate the long-term effectiveness of the solution in maintaining protein integrity under various storage conditions.
Labeling and packaging regulations are another critical aspect. Clear, accurate labeling that includes composition, intended use, storage conditions, and expiration dates is mandatory. Any claims regarding the solution's effectiveness in protein stabilization must be substantiated with scientific evidence and comply with regulatory guidelines on marketing communications.
Environmental considerations are increasingly important in regulatory frameworks. Manufacturers may need to address the environmental impact of their production processes and provide plans for responsible disposal of unused or expired solutions.
Regulatory requirements often extend to the entire supply chain. This includes regulations governing the transportation and storage of isotonic solutions, ensuring that the integrity and efficacy of the product are maintained from production to end-use.
As the field of protein stabilization evolves, regulatory bodies continually update their guidelines. Staying abreast of these changes and maintaining open communication with regulatory authorities is crucial for companies developing and marketing isotonic solutions for protein stabilization.
Biocompatibility Assessment
Biocompatibility assessment is a critical aspect of evaluating isotonic solutions for protein stabilization and hydration. These solutions must not only effectively hydrate proteins but also maintain compatibility with biological systems to ensure safety and efficacy in various applications.
The primary focus of biocompatibility assessment for isotonic solutions is to evaluate their potential interactions with living tissues and biological systems. This includes examining the solution's effects on cell viability, proliferation, and function. In vitro studies using relevant cell lines are typically conducted to assess cytotoxicity and potential cellular stress responses induced by the isotonic solutions.
Furthermore, the assessment includes evaluating the solution's impact on protein structure and function. This is particularly important as the primary goal of these solutions is to aid in protein hydration and stabilization. Techniques such as circular dichroism spectroscopy and fluorescence spectroscopy are employed to monitor changes in protein secondary and tertiary structures upon exposure to the isotonic solutions.
Immunogenicity testing is another crucial component of biocompatibility assessment. This involves evaluating whether the isotonic solutions or their components elicit an unwanted immune response. In vitro assays using human peripheral blood mononuclear cells (PBMCs) and in vivo studies in animal models are typically conducted to assess potential immunogenic effects.
The chemical composition of the isotonic solutions is thoroughly analyzed to identify any potentially harmful substances or impurities. This includes assessing the presence of endotoxins, which can trigger inflammatory responses, and evaluating the solution's pH and osmolality to ensure they are within physiologically acceptable ranges.
Long-term stability studies are conducted to assess the biocompatibility of the isotonic solutions over extended periods. This is particularly important for applications involving prolonged exposure or storage, such as in the case of protein-based therapeutics or diagnostic reagents.
Biocompatibility assessment also considers the potential for microbial growth in the isotonic solutions. Antimicrobial effectiveness testing is performed to ensure that the solutions do not support microbial proliferation, which could compromise both the stability of the proteins and the safety of the end-user.
Finally, the assessment includes evaluating the potential for adverse interactions between the isotonic solutions and commonly used materials in biomedical applications, such as plastics, glass, and metals. This ensures that the solutions remain stable and effective when used in various storage and delivery systems.
The primary focus of biocompatibility assessment for isotonic solutions is to evaluate their potential interactions with living tissues and biological systems. This includes examining the solution's effects on cell viability, proliferation, and function. In vitro studies using relevant cell lines are typically conducted to assess cytotoxicity and potential cellular stress responses induced by the isotonic solutions.
Furthermore, the assessment includes evaluating the solution's impact on protein structure and function. This is particularly important as the primary goal of these solutions is to aid in protein hydration and stabilization. Techniques such as circular dichroism spectroscopy and fluorescence spectroscopy are employed to monitor changes in protein secondary and tertiary structures upon exposure to the isotonic solutions.
Immunogenicity testing is another crucial component of biocompatibility assessment. This involves evaluating whether the isotonic solutions or their components elicit an unwanted immune response. In vitro assays using human peripheral blood mononuclear cells (PBMCs) and in vivo studies in animal models are typically conducted to assess potential immunogenic effects.
The chemical composition of the isotonic solutions is thoroughly analyzed to identify any potentially harmful substances or impurities. This includes assessing the presence of endotoxins, which can trigger inflammatory responses, and evaluating the solution's pH and osmolality to ensure they are within physiologically acceptable ranges.
Long-term stability studies are conducted to assess the biocompatibility of the isotonic solutions over extended periods. This is particularly important for applications involving prolonged exposure or storage, such as in the case of protein-based therapeutics or diagnostic reagents.
Biocompatibility assessment also considers the potential for microbial growth in the isotonic solutions. Antimicrobial effectiveness testing is performed to ensure that the solutions do not support microbial proliferation, which could compromise both the stability of the proteins and the safety of the end-user.
Finally, the assessment includes evaluating the potential for adverse interactions between the isotonic solutions and commonly used materials in biomedical applications, such as plastics, glass, and metals. This ensures that the solutions remain stable and effective when used in various storage and delivery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!