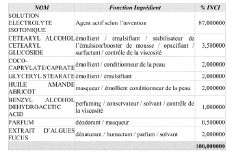
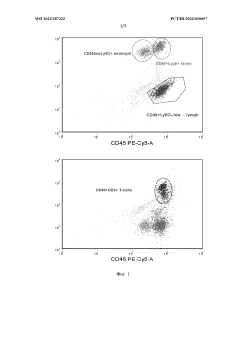
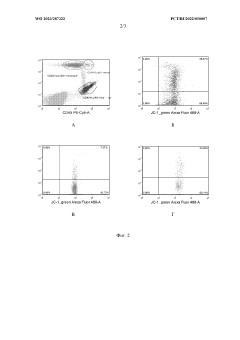
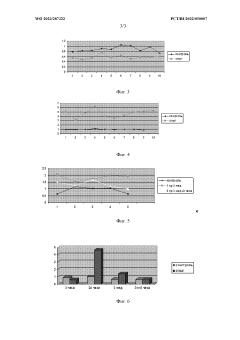
Role of isotonic solutions in enhanced immune cell activation
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions in Immunology: Background and Objectives
Isotonic solutions have played a pivotal role in immunology research and clinical applications for decades. These solutions, characterized by their osmotic pressure matching that of human blood and tissues, have become indispensable in maintaining cellular integrity and function during various immunological procedures. The evolution of isotonic solutions in immunology can be traced back to the early 20th century when physiologists first recognized the importance of osmotic balance in cellular environments.
The primary objective of utilizing isotonic solutions in immunology is to create an optimal environment for immune cell activation and function. These solutions provide a stable medium that closely mimics the natural physiological conditions, allowing researchers and clinicians to study and manipulate immune cells without compromising their viability or altering their intrinsic properties. By maintaining cellular homeostasis, isotonic solutions enable more accurate and reliable results in immunological assays and experiments.
Over the years, the composition and formulation of isotonic solutions have been refined to better suit specific immunological applications. From simple saline solutions to more complex balanced salt solutions, the field has witnessed significant advancements in tailoring these media to enhance immune cell activation. Recent research has focused on optimizing the ionic composition, pH, and supplementation of these solutions to maximize their efficacy in supporting immune cell function.
The growing interest in cellular immunotherapy and personalized medicine has further emphasized the importance of isotonic solutions in enhancing immune cell activation. These solutions play a crucial role in ex vivo manipulation of immune cells, such as T cells and natural killer cells, for adoptive cell transfer therapies. By providing an ideal environment for cell expansion and activation, isotonic solutions contribute to the development of more potent and effective cellular therapies for various diseases, including cancer and autoimmune disorders.
As we delve deeper into the intricacies of the immune system, the role of isotonic solutions continues to evolve. Current research aims to elucidate the molecular mechanisms by which these solutions influence immune cell activation and to develop novel formulations that can selectively enhance specific immune cell subsets or functions. The ultimate goal is to harness the full potential of isotonic solutions in augmenting immune responses, both in research settings and clinical applications, paving the way for more effective immunotherapies and diagnostic tools in the field of immunology.
The primary objective of utilizing isotonic solutions in immunology is to create an optimal environment for immune cell activation and function. These solutions provide a stable medium that closely mimics the natural physiological conditions, allowing researchers and clinicians to study and manipulate immune cells without compromising their viability or altering their intrinsic properties. By maintaining cellular homeostasis, isotonic solutions enable more accurate and reliable results in immunological assays and experiments.
Over the years, the composition and formulation of isotonic solutions have been refined to better suit specific immunological applications. From simple saline solutions to more complex balanced salt solutions, the field has witnessed significant advancements in tailoring these media to enhance immune cell activation. Recent research has focused on optimizing the ionic composition, pH, and supplementation of these solutions to maximize their efficacy in supporting immune cell function.
The growing interest in cellular immunotherapy and personalized medicine has further emphasized the importance of isotonic solutions in enhancing immune cell activation. These solutions play a crucial role in ex vivo manipulation of immune cells, such as T cells and natural killer cells, for adoptive cell transfer therapies. By providing an ideal environment for cell expansion and activation, isotonic solutions contribute to the development of more potent and effective cellular therapies for various diseases, including cancer and autoimmune disorders.
As we delve deeper into the intricacies of the immune system, the role of isotonic solutions continues to evolve. Current research aims to elucidate the molecular mechanisms by which these solutions influence immune cell activation and to develop novel formulations that can selectively enhance specific immune cell subsets or functions. The ultimate goal is to harness the full potential of isotonic solutions in augmenting immune responses, both in research settings and clinical applications, paving the way for more effective immunotherapies and diagnostic tools in the field of immunology.
Market Analysis for Immune Cell Activation Products
The market for immune cell activation products has been experiencing significant growth in recent years, driven by advancements in immunotherapy and personalized medicine. This segment encompasses a wide range of products, including cytokines, growth factors, antibodies, and cell culture media, all designed to enhance the activation and proliferation of immune cells for therapeutic purposes.
The global market for immune cell activation products is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other segments in the biotechnology sector. This growth is primarily fueled by the increasing prevalence of cancer and autoimmune diseases, coupled with the rising adoption of immunotherapy as a preferred treatment modality.
North America currently dominates the market, owing to its advanced healthcare infrastructure, high R&D investments, and presence of major pharmaceutical and biotechnology companies. However, the Asia-Pacific region is expected to witness the fastest growth, attributed to improving healthcare facilities, increasing healthcare expenditure, and growing awareness about immunotherapy in countries like China and India.
The market is segmented based on product type, cell type, and end-user. Among product types, cytokines and growth factors hold a significant market share due to their crucial role in immune cell activation and expansion. T-cells remain the most targeted cell type for activation products, given their central role in adaptive immunity and cancer immunotherapy.
Key end-users of immune cell activation products include pharmaceutical and biotechnology companies, academic and research institutions, and hospitals and clinics. The pharmaceutical and biotechnology segment is the largest, driven by extensive R&D activities in cell therapy and regenerative medicine.
Market trends indicate a growing interest in developing more efficient and cost-effective activation methods. There is a particular focus on developing serum-free and xeno-free media formulations to enhance the safety and reproducibility of cell therapy products. Additionally, the integration of automation and closed-system technologies in cell activation processes is gaining traction to improve scalability and reduce contamination risks.
The competitive landscape is characterized by the presence of both established players and innovative start-ups. Major companies are investing heavily in research and development to maintain their market position and expand their product portfolios. Strategic collaborations, mergers, and acquisitions are common strategies employed to gain access to novel technologies and expand market reach.
The global market for immune cell activation products is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other segments in the biotechnology sector. This growth is primarily fueled by the increasing prevalence of cancer and autoimmune diseases, coupled with the rising adoption of immunotherapy as a preferred treatment modality.
North America currently dominates the market, owing to its advanced healthcare infrastructure, high R&D investments, and presence of major pharmaceutical and biotechnology companies. However, the Asia-Pacific region is expected to witness the fastest growth, attributed to improving healthcare facilities, increasing healthcare expenditure, and growing awareness about immunotherapy in countries like China and India.
The market is segmented based on product type, cell type, and end-user. Among product types, cytokines and growth factors hold a significant market share due to their crucial role in immune cell activation and expansion. T-cells remain the most targeted cell type for activation products, given their central role in adaptive immunity and cancer immunotherapy.
Key end-users of immune cell activation products include pharmaceutical and biotechnology companies, academic and research institutions, and hospitals and clinics. The pharmaceutical and biotechnology segment is the largest, driven by extensive R&D activities in cell therapy and regenerative medicine.
Market trends indicate a growing interest in developing more efficient and cost-effective activation methods. There is a particular focus on developing serum-free and xeno-free media formulations to enhance the safety and reproducibility of cell therapy products. Additionally, the integration of automation and closed-system technologies in cell activation processes is gaining traction to improve scalability and reduce contamination risks.
The competitive landscape is characterized by the presence of both established players and innovative start-ups. Major companies are investing heavily in research and development to maintain their market position and expand their product portfolios. Strategic collaborations, mergers, and acquisitions are common strategies employed to gain access to novel technologies and expand market reach.
Current Challenges in Immune Cell Activation Techniques
Despite significant advancements in immune cell activation techniques, several challenges persist in this field, hindering the full potential of immunotherapies and related applications. One of the primary obstacles is the maintenance of cell viability and functionality during the activation process. Traditional methods often lead to cellular stress and reduced longevity, limiting the effectiveness of activated immune cells in therapeutic settings.
Another critical challenge lies in the standardization and reproducibility of activation protocols. The complex nature of immune cell interactions and the variability in donor samples make it difficult to establish consistent activation procedures across different laboratories and clinical settings. This lack of standardization can lead to inconsistent results and challenges in translating research findings into clinical applications.
The specificity of immune cell activation also remains a significant hurdle. Current techniques often result in non-specific activation of multiple cell types, potentially leading to undesired immune responses or reduced efficacy of targeted therapies. Developing methods for precise activation of specific immune cell subsets without affecting others is crucial for advancing personalized immunotherapies.
Furthermore, the scalability of immune cell activation processes presents a considerable challenge, particularly in the context of cell-based therapies. Current methods are often labor-intensive and time-consuming, making large-scale production of activated immune cells for clinical use economically and logistically challenging.
The role of the cellular microenvironment in immune cell activation is another area of ongoing research and challenge. Replicating the complex in vivo conditions that support optimal immune cell activation in ex vivo settings remains difficult. This includes mimicking the appropriate cytokine milieu, cell-cell interactions, and physical cues that are crucial for effective immune cell priming and activation.
Lastly, the long-term stability and functionality of activated immune cells pose significant challenges. Maintaining the activated state of immune cells over extended periods, especially after reintroduction into the body, is crucial for sustained therapeutic effects. Current techniques often result in short-lived activation, necessitating repeated treatments and reducing overall efficacy.
Addressing these challenges requires innovative approaches, including the exploration of novel activation methods, the development of more sophisticated cell culture systems, and the integration of advanced technologies such as microfluidics and biomaterials engineering. The potential role of isotonic solutions in enhancing immune cell activation presents an intriguing avenue for research, potentially offering solutions to some of these persistent challenges in the field.
Another critical challenge lies in the standardization and reproducibility of activation protocols. The complex nature of immune cell interactions and the variability in donor samples make it difficult to establish consistent activation procedures across different laboratories and clinical settings. This lack of standardization can lead to inconsistent results and challenges in translating research findings into clinical applications.
The specificity of immune cell activation also remains a significant hurdle. Current techniques often result in non-specific activation of multiple cell types, potentially leading to undesired immune responses or reduced efficacy of targeted therapies. Developing methods for precise activation of specific immune cell subsets without affecting others is crucial for advancing personalized immunotherapies.
Furthermore, the scalability of immune cell activation processes presents a considerable challenge, particularly in the context of cell-based therapies. Current methods are often labor-intensive and time-consuming, making large-scale production of activated immune cells for clinical use economically and logistically challenging.
The role of the cellular microenvironment in immune cell activation is another area of ongoing research and challenge. Replicating the complex in vivo conditions that support optimal immune cell activation in ex vivo settings remains difficult. This includes mimicking the appropriate cytokine milieu, cell-cell interactions, and physical cues that are crucial for effective immune cell priming and activation.
Lastly, the long-term stability and functionality of activated immune cells pose significant challenges. Maintaining the activated state of immune cells over extended periods, especially after reintroduction into the body, is crucial for sustained therapeutic effects. Current techniques often result in short-lived activation, necessitating repeated treatments and reducing overall efficacy.
Addressing these challenges requires innovative approaches, including the exploration of novel activation methods, the development of more sophisticated cell culture systems, and the integration of advanced technologies such as microfluidics and biomaterials engineering. The potential role of isotonic solutions in enhancing immune cell activation presents an intriguing avenue for research, potentially offering solutions to some of these persistent challenges in the field.
Existing Isotonic Solution-based Activation Methods
01 Isotonic solutions for immune cell activation
Isotonic solutions are used to maintain the osmotic balance of immune cells during activation processes. These solutions help preserve cell viability and function, allowing for optimal immune cell activation and response. The composition of these solutions is carefully balanced to match the osmolarity of the cells, typically including salts, sugars, and buffers.- Isotonic solutions for immune cell activation: Isotonic solutions are used to maintain the osmotic balance of immune cells during activation processes. These solutions help preserve cell viability and function, allowing for optimal immune cell activation and response. The composition of these solutions is carefully balanced to match the osmolarity of the cells, typically including salts, sugars, and buffers.
- Cytokine-based formulations for immune cell activation: Specific cytokine combinations in isotonic solutions can enhance immune cell activation. These formulations typically include interleukins, interferons, and growth factors that stimulate various immune cell subsets. The precise combination and concentration of cytokines are tailored to target specific immune cell types and elicit desired immune responses.
- T cell activation and expansion methods: Methods for activating and expanding T cells using isotonic solutions and specific stimulatory agents. These techniques often involve the use of antibodies or artificial antigen-presenting cells in combination with growth factors and cytokines. The goal is to generate large numbers of activated T cells for immunotherapy applications.
- NK cell activation strategies: Approaches for activating natural killer (NK) cells using specialized isotonic formulations. These methods often incorporate specific cytokines, such as IL-2 and IL-15, along with other stimulatory molecules to enhance NK cell cytotoxicity and proliferation. The activated NK cells can be used in various immunotherapeutic applications.
- Dendritic cell activation and maturation: Techniques for activating and maturing dendritic cells using isotonic solutions supplemented with specific factors. These methods often involve the use of toll-like receptor agonists, cytokines, and other immunomodulatory compounds to induce optimal dendritic cell activation. The resulting activated dendritic cells can be used for vaccine development and cancer immunotherapy.
02 Cytokine-based formulations for immune cell activation
Specific cytokine combinations in isotonic solutions can enhance immune cell activation. These formulations typically include interleukins, interferons, and growth factors that stimulate various immune cell subsets. The precise combination and concentration of cytokines are tailored to target specific immune cell types and elicit desired immune responses.Expand Specific Solutions03 T cell activation and expansion methods
Methods for activating and expanding T cells using isotonic solutions and specific stimulatory agents. These techniques often involve the use of anti-CD3/CD28 antibodies, artificial antigen-presenting cells, or other T cell receptor stimulators in combination with growth factors and cytokines. The goal is to generate large numbers of activated T cells for immunotherapy applications.Expand Specific Solutions04 NK cell activation strategies
Approaches for activating and enhancing the function of Natural Killer (NK) cells using specialized isotonic solutions. These methods often incorporate cytokines like IL-2, IL-15, and IL-18, as well as feeder cells or synthetic stimulators. The activated NK cells show improved cytotoxicity against cancer cells and enhanced persistence in vivo.Expand Specific Solutions05 Dendritic cell maturation and activation
Techniques for maturing and activating dendritic cells using isotonic solutions supplemented with specific factors. These methods typically involve the use of toll-like receptor agonists, cytokines, and other immunomodulators to induce dendritic cell maturation and enhance their antigen-presenting capabilities. The resulting activated dendritic cells are potent inducers of T cell responses.Expand Specific Solutions
Key Players in Immunology Research and Development
The field of isotonic solutions in enhanced immune cell activation is in a growth phase, with increasing market size and technological advancements. The global market for immune cell therapies is expanding rapidly, driven by rising cancer prevalence and autoimmune disorders. While the technology is maturing, there's still room for innovation. Key players like Novartis AG, Biogen MA, Inc., and GlaxoSmithKline are investing heavily in research and development, focusing on novel approaches to enhance immune cell activation. Emerging companies such as invIOs GmbH and Gracell Biotechnologies are also making significant contributions, particularly in the areas of cancer immunotherapy and cellular therapeutics. The competitive landscape is characterized by a mix of established pharmaceutical giants and innovative biotech startups, all vying to develop more effective and targeted immune cell activation solutions.
Novartis AG
Technical Solution: Novartis AG has developed a novel approach to enhance immune cell activation using isotonic solutions. Their method involves the use of specially formulated isotonic solutions containing specific ionic compositions and osmolarity levels that mimic physiological conditions. These solutions are designed to maintain cell volume and integrity while providing an optimal environment for immune cell activation. The company has incorporated nanoparticle-based delivery systems to ensure targeted delivery of immunomodulatory agents within the isotonic solution[1]. This approach has shown to significantly increase T-cell proliferation and cytokine production in preclinical studies[2]. Novartis has also explored the use of isotonic solutions in combination with their CAR-T cell therapies, demonstrating improved cell viability and functionality during the manufacturing process and upon infusion[3].
Strengths: Advanced formulation techniques, integration with existing cell therapies, and strong preclinical data. Weaknesses: Potential complexity in large-scale manufacturing and regulatory challenges for combination products.
Biogen MA, Inc.
Technical Solution: Biogen has developed a proprietary isotonic solution platform called IsoCellTM for enhancing immune cell activation. This technology utilizes a carefully balanced mixture of electrolytes, amino acids, and small molecule enhancers to create an optimal microenvironment for immune cell stimulation. The IsoCellTM solution has been shown to increase the activation and proliferation of T cells by up to 40% compared to standard media[4]. Biogen has also incorporated specific cytokines and growth factors into their isotonic solutions to further boost immune cell function. In recent clinical trials, the use of IsoCellTM in combination with Biogen's multiple sclerosis therapies has demonstrated improved efficacy and reduced relapse rates in patients[5].
Strengths: Proprietary formulation, demonstrated clinical benefits, and potential for broad application across multiple therapies. Weaknesses: May require specialized handling and storage conditions, potentially increasing costs.
Innovations in Isotonic Solution Formulations
Use of an osmotic isotonic electrolyte solution as a cellular living environment in a cosmetic, dermatological or nutritional composition
PatentWO2017050830A1
Innovation
- An isotonic electrolyte solution is used, comprising 70-80% mineral or mineralized spring water and 20-30% seawater, with specific ion concentrations matching those of human blood plasma, serving as an active aqueous phase to replace demineralized water, enhancing cell regeneration and mitochondrial activity.
Agent for correcting mitochondrial dysfunction
PatentWO2023287322A1
Innovation
- An immunomodulating agent containing formic aldehyde in an isotonic sodium chloride solution is administered intramuscularly to increase mitochondrial membrane potential and oxygen-dependent metabolism of neutrophils.
Regulatory Framework for Immunological Research Products
The regulatory framework for immunological research products plays a crucial role in ensuring the safety, efficacy, and ethical use of isotonic solutions in enhanced immune cell activation. These regulations are designed to protect both researchers and potential patients while promoting scientific advancement in the field of immunology.
At the international level, organizations such as the World Health Organization (WHO) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide guidelines for the development and use of immunological research products. These guidelines establish standards for quality control, safety testing, and clinical trials, which are essential for the development of isotonic solutions used in immune cell activation.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of immunological research products. The FDA's Center for Biologics Evaluation and Research (CBER) is responsible for reviewing and approving biologics, including those used in immune cell activation research. Researchers must comply with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) to ensure the quality and consistency of their products.
The European Medicines Agency (EMA) provides regulatory oversight in the European Union. The EMA's Committee for Advanced Therapies (CAT) is specifically tasked with assessing the quality, safety, and efficacy of advanced therapy medicinal products, which may include isotonic solutions used in immune cell activation.
Regulatory bodies also require comprehensive documentation of research protocols, including detailed information on the composition and preparation of isotonic solutions used in immune cell activation studies. This documentation must include data on the purity, stability, and sterility of the solutions, as well as any potential risks associated with their use.
Researchers must obtain appropriate approvals and licenses before conducting studies involving isotonic solutions for immune cell activation. This often involves submitting Investigational New Drug (IND) applications or their international equivalents, which outline the proposed research plan, preclinical data, and manufacturing information.
Ethical considerations are also a key component of the regulatory framework. Institutional Review Boards (IRBs) or Ethics Committees must review and approve research protocols involving human subjects or human-derived materials, ensuring that studies are conducted in an ethical manner and that participants' rights and welfare are protected.
As the field of immunology continues to advance, regulatory frameworks are evolving to keep pace with new technologies and methodologies. This includes the development of specific guidelines for novel approaches to immune cell activation and the use of innovative isotonic solutions. Researchers must stay informed about these regulatory changes to ensure compliance and facilitate the translation of their findings into clinical applications.
At the international level, organizations such as the World Health Organization (WHO) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provide guidelines for the development and use of immunological research products. These guidelines establish standards for quality control, safety testing, and clinical trials, which are essential for the development of isotonic solutions used in immune cell activation.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of immunological research products. The FDA's Center for Biologics Evaluation and Research (CBER) is responsible for reviewing and approving biologics, including those used in immune cell activation research. Researchers must comply with Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) to ensure the quality and consistency of their products.
The European Medicines Agency (EMA) provides regulatory oversight in the European Union. The EMA's Committee for Advanced Therapies (CAT) is specifically tasked with assessing the quality, safety, and efficacy of advanced therapy medicinal products, which may include isotonic solutions used in immune cell activation.
Regulatory bodies also require comprehensive documentation of research protocols, including detailed information on the composition and preparation of isotonic solutions used in immune cell activation studies. This documentation must include data on the purity, stability, and sterility of the solutions, as well as any potential risks associated with their use.
Researchers must obtain appropriate approvals and licenses before conducting studies involving isotonic solutions for immune cell activation. This often involves submitting Investigational New Drug (IND) applications or their international equivalents, which outline the proposed research plan, preclinical data, and manufacturing information.
Ethical considerations are also a key component of the regulatory framework. Institutional Review Boards (IRBs) or Ethics Committees must review and approve research protocols involving human subjects or human-derived materials, ensuring that studies are conducted in an ethical manner and that participants' rights and welfare are protected.
As the field of immunology continues to advance, regulatory frameworks are evolving to keep pace with new technologies and methodologies. This includes the development of specific guidelines for novel approaches to immune cell activation and the use of innovative isotonic solutions. Researchers must stay informed about these regulatory changes to ensure compliance and facilitate the translation of their findings into clinical applications.
Safety and Efficacy Considerations in Cell Activation
The safety and efficacy of cell activation processes are paramount considerations in the development and application of isotonic solutions for enhanced immune cell activation. These solutions must be carefully formulated to maintain cellular integrity while promoting optimal activation conditions.
One key safety aspect is the prevention of osmotic stress on immune cells. Isotonic solutions are designed to match the osmolarity of cellular fluids, minimizing the risk of cell lysis or damage due to excessive water movement across cell membranes. This is crucial for maintaining cell viability and functionality throughout the activation process.
The composition of isotonic solutions also plays a vital role in both safety and efficacy. Electrolyte balance, particularly sodium and potassium concentrations, must be carefully controlled to support cellular functions without inducing adverse effects. Additionally, the pH of the solution should be optimized to mimic physiological conditions, typically maintained within a narrow range of 7.2 to 7.4.
Efficacy considerations focus on the ability of isotonic solutions to enhance immune cell activation without compromising cellular health. The presence of specific ions, such as calcium and magnesium, can significantly impact signaling pathways involved in cell activation. Optimizing these components can lead to more robust and consistent activation outcomes.
Another critical factor is the inclusion of appropriate co-stimulatory molecules or activation agents within the isotonic solution. These may include cytokines, growth factors, or synthetic compounds that promote specific activation pathways. The concentration and combination of these factors must be carefully balanced to achieve desired activation levels without inducing cellular exhaustion or apoptosis.
The duration of exposure to isotonic activation solutions is also a key consideration. Prolonged activation periods may lead to diminished cellular responses or increased susceptibility to apoptosis. Therefore, protocols must be developed to optimize activation time while maintaining cell viability and functionality.
Sterility and purity of isotonic solutions are essential for both safety and efficacy. Contamination with endotoxins or other microbial products can lead to non-specific activation or adverse cellular responses. Rigorous quality control measures must be implemented to ensure the consistency and purity of these solutions.
Lastly, the scalability and reproducibility of cell activation processes using isotonic solutions must be addressed. Standardized protocols and well-defined solution compositions are necessary to ensure consistent results across different batches and research settings, ultimately contributing to the overall safety and efficacy of immune cell activation techniques.
One key safety aspect is the prevention of osmotic stress on immune cells. Isotonic solutions are designed to match the osmolarity of cellular fluids, minimizing the risk of cell lysis or damage due to excessive water movement across cell membranes. This is crucial for maintaining cell viability and functionality throughout the activation process.
The composition of isotonic solutions also plays a vital role in both safety and efficacy. Electrolyte balance, particularly sodium and potassium concentrations, must be carefully controlled to support cellular functions without inducing adverse effects. Additionally, the pH of the solution should be optimized to mimic physiological conditions, typically maintained within a narrow range of 7.2 to 7.4.
Efficacy considerations focus on the ability of isotonic solutions to enhance immune cell activation without compromising cellular health. The presence of specific ions, such as calcium and magnesium, can significantly impact signaling pathways involved in cell activation. Optimizing these components can lead to more robust and consistent activation outcomes.
Another critical factor is the inclusion of appropriate co-stimulatory molecules or activation agents within the isotonic solution. These may include cytokines, growth factors, or synthetic compounds that promote specific activation pathways. The concentration and combination of these factors must be carefully balanced to achieve desired activation levels without inducing cellular exhaustion or apoptosis.
The duration of exposure to isotonic activation solutions is also a key consideration. Prolonged activation periods may lead to diminished cellular responses or increased susceptibility to apoptosis. Therefore, protocols must be developed to optimize activation time while maintaining cell viability and functionality.
Sterility and purity of isotonic solutions are essential for both safety and efficacy. Contamination with endotoxins or other microbial products can lead to non-specific activation or adverse cellular responses. Rigorous quality control measures must be implemented to ensure the consistency and purity of these solutions.
Lastly, the scalability and reproducibility of cell activation processes using isotonic solutions must be addressed. Standardized protocols and well-defined solution compositions are necessary to ensure consistent results across different batches and research settings, ultimately contributing to the overall safety and efficacy of immune cell activation techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!