How isotonic solutions optimize biopharmaceutical fermentation processes
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions in Biopharma: Background and Objectives
Isotonic solutions have played a pivotal role in optimizing biopharmaceutical fermentation processes, marking a significant milestone in the evolution of biotechnology. The concept of isotonicity, which refers to the equal osmotic pressure between two solutions, has been fundamental in maintaining cellular integrity and enhancing productivity in fermentation systems.
The journey of isotonic solutions in biopharmaceutical applications can be traced back to the early days of cell culture techniques. As researchers sought to improve the yield and quality of biologics, they recognized the critical importance of creating an environment that closely mimics physiological conditions. This realization led to the development and refinement of isotonic media formulations specifically tailored for biopharmaceutical production.
Over the years, the use of isotonic solutions in fermentation processes has expanded from simple salt-based buffers to complex, nutrient-rich media. These advancements have been driven by a deeper understanding of cellular metabolism, osmotic stress responses, and the specific requirements of different cell lines used in biopharmaceutical production.
The primary objective of incorporating isotonic solutions in fermentation processes is to maximize cell viability, growth, and product yield while minimizing stress-induced cellular responses. By maintaining an optimal osmotic balance, these solutions help prevent cell lysis, reduce metabolic burden, and enhance the overall efficiency of the bioprocess.
Recent technological developments have further refined the application of isotonic solutions. The advent of high-throughput screening methods and advanced analytical techniques has enabled researchers to fine-tune media compositions for specific cell lines and products. This tailored approach has led to significant improvements in process robustness and product quality.
The biopharmaceutical industry's growing focus on continuous manufacturing and perfusion culture systems has also influenced the evolution of isotonic solutions. These advanced production methods require carefully balanced media that can support prolonged cell growth and protein expression without compromising product quality or cellular health.
Looking ahead, the field of isotonic solutions in biopharmaceutical fermentation is poised for further innovation. Emerging trends include the development of chemically defined media, the integration of real-time monitoring and control systems, and the exploration of novel osmolytes and protective agents. These advancements aim to push the boundaries of what is possible in terms of product yield, quality, and consistency in biopharmaceutical manufacturing.
The journey of isotonic solutions in biopharmaceutical applications can be traced back to the early days of cell culture techniques. As researchers sought to improve the yield and quality of biologics, they recognized the critical importance of creating an environment that closely mimics physiological conditions. This realization led to the development and refinement of isotonic media formulations specifically tailored for biopharmaceutical production.
Over the years, the use of isotonic solutions in fermentation processes has expanded from simple salt-based buffers to complex, nutrient-rich media. These advancements have been driven by a deeper understanding of cellular metabolism, osmotic stress responses, and the specific requirements of different cell lines used in biopharmaceutical production.
The primary objective of incorporating isotonic solutions in fermentation processes is to maximize cell viability, growth, and product yield while minimizing stress-induced cellular responses. By maintaining an optimal osmotic balance, these solutions help prevent cell lysis, reduce metabolic burden, and enhance the overall efficiency of the bioprocess.
Recent technological developments have further refined the application of isotonic solutions. The advent of high-throughput screening methods and advanced analytical techniques has enabled researchers to fine-tune media compositions for specific cell lines and products. This tailored approach has led to significant improvements in process robustness and product quality.
The biopharmaceutical industry's growing focus on continuous manufacturing and perfusion culture systems has also influenced the evolution of isotonic solutions. These advanced production methods require carefully balanced media that can support prolonged cell growth and protein expression without compromising product quality or cellular health.
Looking ahead, the field of isotonic solutions in biopharmaceutical fermentation is poised for further innovation. Emerging trends include the development of chemically defined media, the integration of real-time monitoring and control systems, and the exploration of novel osmolytes and protective agents. These advancements aim to push the boundaries of what is possible in terms of product yield, quality, and consistency in biopharmaceutical manufacturing.
Market Demand for Optimized Fermentation Processes
The biopharmaceutical industry has witnessed a growing demand for optimized fermentation processes, driven by the need for increased productivity, cost-effectiveness, and product quality. Isotonic solutions play a crucial role in this optimization, as they help maintain cellular integrity and enhance metabolic efficiency during fermentation.
Market research indicates that the global biopharmaceutical fermentation market is experiencing significant growth, with a projected compound annual growth rate (CAGR) of over 9% from 2021 to 2026. This growth is primarily attributed to the rising demand for biologics, including monoclonal antibodies, vaccines, and recombinant proteins. As biopharmaceutical companies strive to meet this increasing demand, there is a pressing need for advanced fermentation technologies that can improve yields and reduce production costs.
The adoption of isotonic solutions in fermentation processes has gained traction due to their ability to optimize cellular performance and product quality. These solutions help maintain osmotic balance, prevent cell lysis, and enhance nutrient uptake, resulting in higher product titers and improved consistency. As a result, biopharmaceutical manufacturers are increasingly seeking isotonic solution-based fermentation technologies to gain a competitive edge in the market.
The demand for optimized fermentation processes is particularly strong in emerging markets, such as Asia-Pacific and Latin America, where the biopharmaceutical industry is rapidly expanding. These regions are witnessing increased investments in biomanufacturing facilities and a growing focus on developing biosimilars and novel biologics. Consequently, there is a heightened interest in advanced fermentation technologies that can support efficient and cost-effective production.
Furthermore, the COVID-19 pandemic has accelerated the demand for optimized fermentation processes, especially in vaccine production. The urgent need for large-scale vaccine manufacturing has highlighted the importance of efficient and scalable fermentation technologies. Isotonic solutions have emerged as a valuable tool in this context, enabling faster development and production of vaccine candidates.
The market demand for optimized fermentation processes extends beyond traditional biopharmaceuticals to include emerging therapeutic modalities such as cell and gene therapies. These advanced therapies often require specialized fermentation conditions to ensure the viability and functionality of cellular products. Isotonic solutions play a critical role in maintaining optimal conditions for these sensitive cellular systems, driving further demand for advanced fermentation technologies.
As sustainability becomes an increasingly important consideration in biopharmaceutical manufacturing, there is a growing interest in fermentation processes that minimize resource consumption and environmental impact. Isotonic solutions contribute to this goal by improving process efficiency and reducing waste generation, aligning with the industry's sustainability objectives and regulatory requirements.
Market research indicates that the global biopharmaceutical fermentation market is experiencing significant growth, with a projected compound annual growth rate (CAGR) of over 9% from 2021 to 2026. This growth is primarily attributed to the rising demand for biologics, including monoclonal antibodies, vaccines, and recombinant proteins. As biopharmaceutical companies strive to meet this increasing demand, there is a pressing need for advanced fermentation technologies that can improve yields and reduce production costs.
The adoption of isotonic solutions in fermentation processes has gained traction due to their ability to optimize cellular performance and product quality. These solutions help maintain osmotic balance, prevent cell lysis, and enhance nutrient uptake, resulting in higher product titers and improved consistency. As a result, biopharmaceutical manufacturers are increasingly seeking isotonic solution-based fermentation technologies to gain a competitive edge in the market.
The demand for optimized fermentation processes is particularly strong in emerging markets, such as Asia-Pacific and Latin America, where the biopharmaceutical industry is rapidly expanding. These regions are witnessing increased investments in biomanufacturing facilities and a growing focus on developing biosimilars and novel biologics. Consequently, there is a heightened interest in advanced fermentation technologies that can support efficient and cost-effective production.
Furthermore, the COVID-19 pandemic has accelerated the demand for optimized fermentation processes, especially in vaccine production. The urgent need for large-scale vaccine manufacturing has highlighted the importance of efficient and scalable fermentation technologies. Isotonic solutions have emerged as a valuable tool in this context, enabling faster development and production of vaccine candidates.
The market demand for optimized fermentation processes extends beyond traditional biopharmaceuticals to include emerging therapeutic modalities such as cell and gene therapies. These advanced therapies often require specialized fermentation conditions to ensure the viability and functionality of cellular products. Isotonic solutions play a critical role in maintaining optimal conditions for these sensitive cellular systems, driving further demand for advanced fermentation technologies.
As sustainability becomes an increasingly important consideration in biopharmaceutical manufacturing, there is a growing interest in fermentation processes that minimize resource consumption and environmental impact. Isotonic solutions contribute to this goal by improving process efficiency and reducing waste generation, aligning with the industry's sustainability objectives and regulatory requirements.
Current Challenges in Biopharmaceutical Fermentation
Biopharmaceutical fermentation processes face several significant challenges that hinder optimal production and efficiency. One of the primary issues is maintaining consistent cell growth and productivity throughout the fermentation process. Fluctuations in nutrient availability, pH levels, and osmotic pressure can lead to suboptimal cell performance and reduced product yield.
Another major challenge is the accumulation of metabolic byproducts during fermentation. As cells grow and produce the desired biopharmaceutical, they also generate waste products that can inhibit further growth and production. These byproducts can alter the culture environment, potentially leading to cellular stress and decreased viability.
Scalability remains a persistent challenge in biopharmaceutical fermentation. Processes that work well at laboratory scale often encounter difficulties when scaled up to industrial production levels. This can result in reduced efficiency, inconsistent product quality, and increased production costs.
Contamination risk is an ever-present concern in biopharmaceutical fermentation. Despite stringent clean room practices and sterilization protocols, the risk of microbial contamination persists, potentially leading to batch failures and significant economic losses.
Oxygen transfer limitations pose another significant challenge, particularly in large-scale fermentations. Ensuring adequate oxygen supply to cells throughout the culture volume is crucial for maintaining cell viability and productivity. Insufficient oxygen transfer can result in reduced growth rates and compromised product quality.
The heterogeneity of cell populations within bioreactors presents an additional challenge. Variations in local microenvironments can lead to differences in cell behavior and metabolism, resulting in inconsistent product quality and reduced overall yield.
Optimizing media composition and feeding strategies remains a complex task. Balancing nutrient availability to support cell growth and product formation while minimizing waste accumulation requires careful consideration and often involves trade-offs between different process parameters.
Finally, the increasing demand for higher product titers and improved quality attributes puts pressure on fermentation processes to continually evolve and improve. Meeting these demands while maintaining cost-effectiveness and regulatory compliance presents an ongoing challenge for the biopharmaceutical industry.
Another major challenge is the accumulation of metabolic byproducts during fermentation. As cells grow and produce the desired biopharmaceutical, they also generate waste products that can inhibit further growth and production. These byproducts can alter the culture environment, potentially leading to cellular stress and decreased viability.
Scalability remains a persistent challenge in biopharmaceutical fermentation. Processes that work well at laboratory scale often encounter difficulties when scaled up to industrial production levels. This can result in reduced efficiency, inconsistent product quality, and increased production costs.
Contamination risk is an ever-present concern in biopharmaceutical fermentation. Despite stringent clean room practices and sterilization protocols, the risk of microbial contamination persists, potentially leading to batch failures and significant economic losses.
Oxygen transfer limitations pose another significant challenge, particularly in large-scale fermentations. Ensuring adequate oxygen supply to cells throughout the culture volume is crucial for maintaining cell viability and productivity. Insufficient oxygen transfer can result in reduced growth rates and compromised product quality.
The heterogeneity of cell populations within bioreactors presents an additional challenge. Variations in local microenvironments can lead to differences in cell behavior and metabolism, resulting in inconsistent product quality and reduced overall yield.
Optimizing media composition and feeding strategies remains a complex task. Balancing nutrient availability to support cell growth and product formation while minimizing waste accumulation requires careful consideration and often involves trade-offs between different process parameters.
Finally, the increasing demand for higher product titers and improved quality attributes puts pressure on fermentation processes to continually evolve and improve. Meeting these demands while maintaining cost-effectiveness and regulatory compliance presents an ongoing challenge for the biopharmaceutical industry.
Existing Isotonic Solution Formulations and Applications
01 Composition optimization for isotonic solutions
Optimization of isotonic solutions involves carefully adjusting the composition of electrolytes, minerals, and other components to match the osmotic pressure of body fluids. This process may include fine-tuning the concentrations of sodium, potassium, chloride, and other ions to ensure optimal physiological compatibility and effectiveness for various medical and pharmaceutical applications.- Composition optimization for isotonic solutions: Optimization of isotonic solutions involves carefully adjusting the composition of electrolytes, sugars, and other solutes to match the osmolarity of body fluids. This process may include fine-tuning the concentrations of sodium, potassium, chloride, and glucose to ensure optimal physiological compatibility and effectiveness for various medical applications.
- pH and buffer system optimization: Optimizing the pH and buffer system of isotonic solutions is crucial for maintaining stability and effectiveness. This may involve selecting appropriate buffer compounds and adjusting their concentrations to achieve the desired pH range, which is typically close to physiological pH. The optimization process aims to enhance the solution's performance and shelf life.
- Osmolarity adjustment techniques: Various techniques can be employed to adjust the osmolarity of isotonic solutions. These may include using osmolarity-adjusting agents, implementing precise measurement methods, and developing mathematical models to predict and control osmolarity. The goal is to achieve and maintain isotonicity with body fluids for optimal therapeutic efficacy.
- Stability and preservation methods: Optimizing the stability and preservation of isotonic solutions involves developing methods to extend shelf life and maintain efficacy. This may include the use of preservatives, antioxidants, or specialized packaging materials. Additionally, techniques such as sterile filtration or aseptic processing may be employed to ensure product integrity and safety.
- Application-specific formulation optimization: Isotonic solutions can be optimized for specific medical applications, such as intravenous therapy, wound irrigation, or ophthalmic use. This involves tailoring the composition, pH, and other parameters to meet the unique requirements of each application. The optimization process may include in vitro and in vivo testing to ensure optimal performance and safety for the intended use.
02 pH and buffer system optimization
Optimizing the pH and buffer system of isotonic solutions is crucial for maintaining stability and effectiveness. This involves selecting appropriate buffer components and adjusting their concentrations to achieve the desired pH range, which can vary depending on the specific application of the isotonic solution. Proper pH control ensures optimal performance and minimizes potential adverse effects on the body.Expand Specific Solutions03 Osmolality adjustment techniques
Various techniques can be employed to adjust the osmolality of isotonic solutions to match that of body fluids. These may include the use of osmotic agents, such as glucose or mannitol, or the precise balancing of electrolyte concentrations. Advanced methods for measuring and controlling osmolality during the manufacturing process can help achieve optimal isotonicity for different applications.Expand Specific Solutions04 Stability and shelf-life enhancement
Optimizing the stability and shelf-life of isotonic solutions involves careful selection of ingredients, packaging materials, and storage conditions. This may include the use of preservatives, antioxidants, or other stabilizing agents to prevent degradation and maintain the solution's efficacy over time. Advanced packaging technologies and sterilization methods can also contribute to extended shelf-life and improved product quality.Expand Specific Solutions05 Application-specific formulation optimization
Tailoring isotonic solutions for specific applications, such as intravenous therapy, wound irrigation, or ophthalmic use, requires optimization of various parameters. This may involve adjusting the composition, viscosity, or adding specific active ingredients to enhance the solution's effectiveness for its intended use. Consideration of factors such as biocompatibility, drug delivery efficiency, and patient comfort guide the optimization process for different medical and pharmaceutical applications.Expand Specific Solutions
Key Players in Biopharmaceutical Fermentation Industry
The biopharmaceutical fermentation process optimization using isotonic solutions is in a growth phase, with increasing market size and technological advancements. The industry is characterized by a mix of established players and innovative startups, indicating a maturing but still evolving technology landscape. Companies like DSM IP Assets BV, LanzaTech NZ Ltd., and Gevo, Inc. are at the forefront, leveraging their expertise in biotechnology and fermentation processes. Academic institutions such as the University of Campinas and Zhejiang University of Technology are contributing to research and development, fostering innovation in this field. The involvement of major pharmaceutical companies like Amgen, Inc. and Baxter International, Inc. suggests growing commercial interest and potential for widespread application in biopharmaceutical production.
Amgen, Inc.
Technical Solution: Amgen has developed a proprietary isotonic solution optimization system for biopharmaceutical fermentation. Their approach involves using a combination of osmolytes and electrolytes to maintain optimal cellular osmotic pressure throughout the fermentation process. The company employs advanced bioreactors with real-time monitoring capabilities to adjust the isotonic environment dynamically. This system includes automated feedback loops that continuously measure and adjust osmolality, pH, and nutrient concentrations[1]. Amgen's method also incorporates machine learning algorithms to predict and preemptively address osmotic stress conditions, potentially improving product yield and quality[3].
Strengths: Advanced real-time monitoring and adjustment capabilities, integration of machine learning for predictive optimization. Weaknesses: Potentially high implementation costs, may require significant staff training for optimal use.
Baxter International, Inc.
Technical Solution: Baxter International has developed a novel isotonic solution formulation specifically designed for mammalian cell culture in biopharmaceutical production. Their approach focuses on a balanced mixture of inorganic salts and organic compounds that closely mimics the intracellular environment of target cells. The company utilizes a proprietary blend of amino acids and carbohydrates to maintain osmotic balance while simultaneously providing essential nutrients[2]. Baxter's system also incorporates a gradual osmolality shift strategy during the fermentation process, which has been shown to enhance protein production in certain cell lines[4]. Additionally, they have implemented a controlled release mechanism for key osmolytes, ensuring a more stable isotonic environment throughout extended fermentation runs.
Strengths: Tailored formulation for mammalian cell cultures, innovative osmolality shift strategy. Weaknesses: May be less adaptable to non-mammalian cell types, potential for increased complexity in media preparation.
Core Innovations in Isotonic Solution Technology
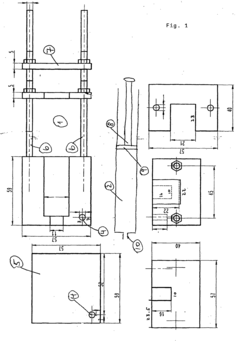
Sampling method and device
PatentInactiveEP1719997A2
Innovation
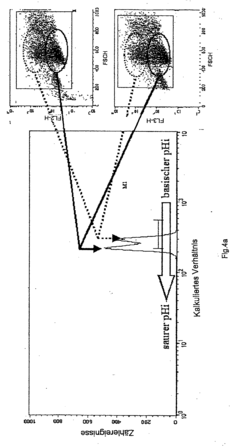
- An isobaric sampling method using a specialized sampling device that maintains constant pressure during sample collection and dye introduction, ensuring that pH and gas concentrations are measured under conditions similar to those in the fermenter, reducing apoptosis and improving measurement accuracy.
Pharmaceutical composition comprising CD34+ cells
PatentInactiveEP2817014A2
Innovation
- A pharmaceutical composition comprising CD34+ cells, plasma protein, and an isotonic solution, specifically formulated for short-term storage and direct administration, which maintains cell viability and functionality, eliminating the need for separate storage and administration solutions and ensuring a stable environment for CD34+ cells.
Regulatory Considerations for Biopharmaceutical Processes
Regulatory considerations play a crucial role in the development and implementation of biopharmaceutical fermentation processes, including those utilizing isotonic solutions. These regulations are designed to ensure product safety, efficacy, and quality throughout the manufacturing process.
The primary regulatory bodies overseeing biopharmaceutical production include the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and similar organizations in other regions. These agencies have established guidelines and requirements that manufacturers must adhere to when developing and implementing fermentation processes.
One key aspect of regulatory compliance is the implementation of Good Manufacturing Practices (GMP). GMP guidelines cover various aspects of production, including facility design, equipment maintenance, personnel training, and documentation. When optimizing fermentation processes with isotonic solutions, manufacturers must ensure that all modifications align with GMP standards.
Process validation is another critical regulatory requirement. Manufacturers must demonstrate that their fermentation processes, including the use of isotonic solutions, consistently produce a product that meets predetermined specifications. This involves conducting thorough studies to establish the reproducibility and reliability of the process.
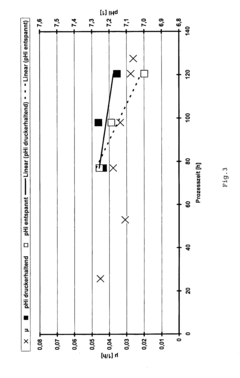
Risk assessment and management are integral components of regulatory compliance. Manufacturers must identify potential risks associated with the use of isotonic solutions in fermentation processes and implement appropriate control measures. This may include monitoring osmolality, pH, and other critical parameters throughout the fermentation process.
Raw material control is also a significant regulatory consideration. Manufacturers must ensure that all components used in the preparation of isotonic solutions meet specified quality standards. This includes implementing robust supplier qualification programs and conducting regular quality checks on incoming materials.
Documentation and traceability are essential for regulatory compliance. Manufacturers must maintain detailed records of all aspects of the fermentation process, including the preparation and use of isotonic solutions. This documentation should be readily available for regulatory inspections and audits.
Regulatory agencies also require manufacturers to establish and maintain a robust quality management system. This system should encompass all aspects of production, including the use of isotonic solutions in fermentation processes. Regular internal audits and continuous improvement initiatives are typically expected as part of this system.
As biopharmaceutical manufacturing technologies evolve, regulatory frameworks must adapt accordingly. Manufacturers working with isotonic solutions in fermentation processes should stay informed about emerging regulatory trends and engage in open dialogue with regulatory authorities to ensure compliance with current and future requirements.
The primary regulatory bodies overseeing biopharmaceutical production include the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and similar organizations in other regions. These agencies have established guidelines and requirements that manufacturers must adhere to when developing and implementing fermentation processes.
One key aspect of regulatory compliance is the implementation of Good Manufacturing Practices (GMP). GMP guidelines cover various aspects of production, including facility design, equipment maintenance, personnel training, and documentation. When optimizing fermentation processes with isotonic solutions, manufacturers must ensure that all modifications align with GMP standards.
Process validation is another critical regulatory requirement. Manufacturers must demonstrate that their fermentation processes, including the use of isotonic solutions, consistently produce a product that meets predetermined specifications. This involves conducting thorough studies to establish the reproducibility and reliability of the process.
Risk assessment and management are integral components of regulatory compliance. Manufacturers must identify potential risks associated with the use of isotonic solutions in fermentation processes and implement appropriate control measures. This may include monitoring osmolality, pH, and other critical parameters throughout the fermentation process.
Raw material control is also a significant regulatory consideration. Manufacturers must ensure that all components used in the preparation of isotonic solutions meet specified quality standards. This includes implementing robust supplier qualification programs and conducting regular quality checks on incoming materials.
Documentation and traceability are essential for regulatory compliance. Manufacturers must maintain detailed records of all aspects of the fermentation process, including the preparation and use of isotonic solutions. This documentation should be readily available for regulatory inspections and audits.
Regulatory agencies also require manufacturers to establish and maintain a robust quality management system. This system should encompass all aspects of production, including the use of isotonic solutions in fermentation processes. Regular internal audits and continuous improvement initiatives are typically expected as part of this system.
As biopharmaceutical manufacturing technologies evolve, regulatory frameworks must adapt accordingly. Manufacturers working with isotonic solutions in fermentation processes should stay informed about emerging regulatory trends and engage in open dialogue with regulatory authorities to ensure compliance with current and future requirements.
Economic Impact of Optimized Fermentation Processes
The optimization of biopharmaceutical fermentation processes through isotonic solutions has significant economic implications for the industry. By enhancing the efficiency and productivity of fermentation, companies can achieve substantial cost savings and increased revenue potential.
One of the primary economic benefits is the reduction in production costs. Optimized fermentation processes require fewer raw materials and consume less energy, leading to lower operational expenses. This cost-effectiveness is particularly crucial in an industry where production costs can be a significant portion of the overall expenses.
Improved yield is another key economic advantage. Isotonic solutions help maintain optimal conditions for microorganisms, resulting in higher product titers and increased biomass production. This translates to greater output per batch, effectively boosting the overall production capacity without the need for additional capital investment in equipment or facilities.
The enhanced consistency and quality of the final product also contribute to economic gains. By maintaining a stable osmotic environment, isotonic solutions reduce stress on cells, leading to more predictable and reproducible fermentation outcomes. This consistency reduces the likelihood of batch failures and product rejections, minimizing costly waste and rework.
Furthermore, optimized fermentation processes can lead to faster production cycles. The improved conditions allow for more rapid cell growth and product formation, potentially reducing the time required for each batch. This increased throughput enables manufacturers to respond more quickly to market demands and potentially capture a larger market share.
The economic impact extends to downstream processing as well. With higher product concentrations and improved quality from the fermentation stage, subsequent purification and formulation steps become more efficient. This can lead to reduced processing times and lower costs in these later stages of production.
From a broader perspective, the adoption of optimized fermentation processes can enhance a company's competitiveness in the global biopharmaceutical market. The ability to produce high-quality products more efficiently can lead to improved profit margins and potentially lower prices for consumers, making treatments more accessible and expanding market reach.
In conclusion, the economic impact of optimizing biopharmaceutical fermentation processes through isotonic solutions is multifaceted and significant. It encompasses cost reduction, increased productivity, improved product quality, and enhanced market competitiveness, all of which contribute to the overall economic success of biopharmaceutical companies in an increasingly competitive industry landscape.
One of the primary economic benefits is the reduction in production costs. Optimized fermentation processes require fewer raw materials and consume less energy, leading to lower operational expenses. This cost-effectiveness is particularly crucial in an industry where production costs can be a significant portion of the overall expenses.
Improved yield is another key economic advantage. Isotonic solutions help maintain optimal conditions for microorganisms, resulting in higher product titers and increased biomass production. This translates to greater output per batch, effectively boosting the overall production capacity without the need for additional capital investment in equipment or facilities.
The enhanced consistency and quality of the final product also contribute to economic gains. By maintaining a stable osmotic environment, isotonic solutions reduce stress on cells, leading to more predictable and reproducible fermentation outcomes. This consistency reduces the likelihood of batch failures and product rejections, minimizing costly waste and rework.
Furthermore, optimized fermentation processes can lead to faster production cycles. The improved conditions allow for more rapid cell growth and product formation, potentially reducing the time required for each batch. This increased throughput enables manufacturers to respond more quickly to market demands and potentially capture a larger market share.
The economic impact extends to downstream processing as well. With higher product concentrations and improved quality from the fermentation stage, subsequent purification and formulation steps become more efficient. This can lead to reduced processing times and lower costs in these later stages of production.
From a broader perspective, the adoption of optimized fermentation processes can enhance a company's competitiveness in the global biopharmaceutical market. The ability to produce high-quality products more efficiently can lead to improved profit margins and potentially lower prices for consumers, making treatments more accessible and expanding market reach.
In conclusion, the economic impact of optimizing biopharmaceutical fermentation processes through isotonic solutions is multifaceted and significant. It encompasses cost reduction, increased productivity, improved product quality, and enhanced market competitiveness, all of which contribute to the overall economic success of biopharmaceutical companies in an increasingly competitive industry landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!