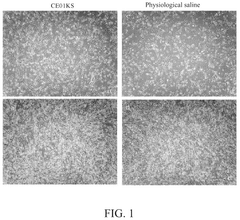
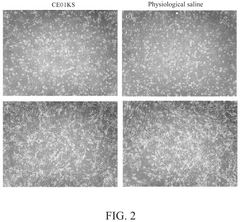
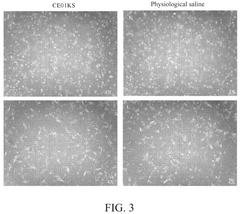
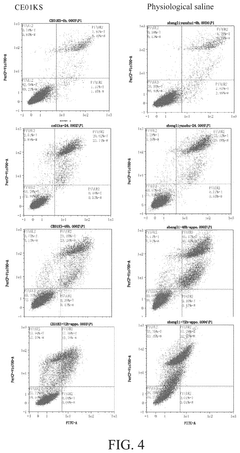
Isotonic solutions in stem cell preservation: a comparative study
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Stem Cell Preservation Background and Objectives
Stem cell preservation has emerged as a critical field in regenerative medicine and biomedical research. The ability to maintain the viability and functionality of stem cells over extended periods is paramount for their successful application in various therapeutic and research contexts. Isotonic solutions play a crucial role in this preservation process, serving as the medium in which stem cells are suspended during storage and transportation.
The evolution of stem cell preservation techniques has been closely tied to advancements in cryobiology and cell biology. Early methods often resulted in significant cell loss and reduced functionality upon thawing. However, the development of specialized isotonic solutions has markedly improved preservation outcomes. These solutions are designed to maintain osmotic balance, prevent cellular damage, and provide essential nutrients to support cell survival during the preservation period.
The primary objective of this comparative study on isotonic solutions in stem cell preservation is to evaluate the efficacy of different formulations in maintaining stem cell viability, functionality, and genetic stability. By analyzing various isotonic solutions, we aim to identify optimal preservation conditions that can enhance the long-term storage of stem cells while minimizing cellular damage and preserving their multipotency.
This research is driven by the increasing demand for high-quality stem cells in both clinical and research settings. As regenerative medicine continues to advance, the need for reliable and efficient preservation methods becomes more pressing. Improved preservation techniques can significantly impact the availability and quality of stem cells for transplantation, drug screening, and disease modeling.
Furthermore, this study seeks to address the challenges associated with current preservation methods, such as ice crystal formation, osmotic stress, and oxidative damage. By comparing different isotonic solutions, we aim to elucidate the mechanisms by which these solutions protect stem cells during the preservation process and identify key components that contribute to enhanced cell survival and functionality.
The outcomes of this comparative study are expected to contribute to the development of standardized protocols for stem cell preservation, potentially leading to improved clinical outcomes and more reliable research results. Additionally, insights gained from this research may inform the design of novel preservation solutions tailored to specific stem cell types or intended applications.
The evolution of stem cell preservation techniques has been closely tied to advancements in cryobiology and cell biology. Early methods often resulted in significant cell loss and reduced functionality upon thawing. However, the development of specialized isotonic solutions has markedly improved preservation outcomes. These solutions are designed to maintain osmotic balance, prevent cellular damage, and provide essential nutrients to support cell survival during the preservation period.
The primary objective of this comparative study on isotonic solutions in stem cell preservation is to evaluate the efficacy of different formulations in maintaining stem cell viability, functionality, and genetic stability. By analyzing various isotonic solutions, we aim to identify optimal preservation conditions that can enhance the long-term storage of stem cells while minimizing cellular damage and preserving their multipotency.
This research is driven by the increasing demand for high-quality stem cells in both clinical and research settings. As regenerative medicine continues to advance, the need for reliable and efficient preservation methods becomes more pressing. Improved preservation techniques can significantly impact the availability and quality of stem cells for transplantation, drug screening, and disease modeling.
Furthermore, this study seeks to address the challenges associated with current preservation methods, such as ice crystal formation, osmotic stress, and oxidative damage. By comparing different isotonic solutions, we aim to elucidate the mechanisms by which these solutions protect stem cells during the preservation process and identify key components that contribute to enhanced cell survival and functionality.
The outcomes of this comparative study are expected to contribute to the development of standardized protocols for stem cell preservation, potentially leading to improved clinical outcomes and more reliable research results. Additionally, insights gained from this research may inform the design of novel preservation solutions tailored to specific stem cell types or intended applications.
Market Analysis for Stem Cell Preservation Solutions
The stem cell preservation solutions market has been experiencing significant growth in recent years, driven by advancements in regenerative medicine and increasing awareness of the potential therapeutic applications of stem cells. The global market for stem cell preservation solutions is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the double digits over the next five years.
One of the key factors fueling market demand is the rising prevalence of chronic diseases and the growing aging population worldwide. As stem cell therapies show promise in treating various degenerative conditions and age-related disorders, the need for effective preservation solutions has become paramount. Additionally, the increasing number of stem cell banks and biorepositories has created a substantial demand for high-quality preservation media.
The market for isotonic solutions in stem cell preservation is particularly dynamic, with several key players competing to offer innovative products. These solutions play a crucial role in maintaining cell viability and functionality during cryopreservation and storage processes. The demand for isotonic solutions is driven by their ability to minimize cellular damage and preserve stem cell potency, which is essential for successful clinical applications.
Geographically, North America and Europe currently dominate the stem cell preservation solutions market, owing to their advanced healthcare infrastructure and significant investments in research and development. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing healthcare expenditure, rising awareness, and government initiatives to promote stem cell research and therapy.
In terms of end-users, research institutions and stem cell banks are the primary consumers of preservation solutions. However, there is a growing demand from hospitals and clinics as stem cell therapies become more widely adopted in clinical practice. This shift is expected to create new opportunities for market expansion and product diversification.
The competitive landscape of the stem cell preservation solutions market is characterized by the presence of both established players and innovative startups. Key market strategies include product development focused on improving preservation efficacy, strategic partnerships with research institutions, and expansion into emerging markets.
Looking ahead, the market for stem cell preservation solutions, particularly isotonic solutions, is poised for continued growth. Factors such as technological advancements in cryopreservation techniques, increasing investment in stem cell research, and the expanding applications of stem cell therapies are expected to drive market expansion in the coming years.
One of the key factors fueling market demand is the rising prevalence of chronic diseases and the growing aging population worldwide. As stem cell therapies show promise in treating various degenerative conditions and age-related disorders, the need for effective preservation solutions has become paramount. Additionally, the increasing number of stem cell banks and biorepositories has created a substantial demand for high-quality preservation media.
The market for isotonic solutions in stem cell preservation is particularly dynamic, with several key players competing to offer innovative products. These solutions play a crucial role in maintaining cell viability and functionality during cryopreservation and storage processes. The demand for isotonic solutions is driven by their ability to minimize cellular damage and preserve stem cell potency, which is essential for successful clinical applications.
Geographically, North America and Europe currently dominate the stem cell preservation solutions market, owing to their advanced healthcare infrastructure and significant investments in research and development. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing healthcare expenditure, rising awareness, and government initiatives to promote stem cell research and therapy.
In terms of end-users, research institutions and stem cell banks are the primary consumers of preservation solutions. However, there is a growing demand from hospitals and clinics as stem cell therapies become more widely adopted in clinical practice. This shift is expected to create new opportunities for market expansion and product diversification.
The competitive landscape of the stem cell preservation solutions market is characterized by the presence of both established players and innovative startups. Key market strategies include product development focused on improving preservation efficacy, strategic partnerships with research institutions, and expansion into emerging markets.
Looking ahead, the market for stem cell preservation solutions, particularly isotonic solutions, is poised for continued growth. Factors such as technological advancements in cryopreservation techniques, increasing investment in stem cell research, and the expanding applications of stem cell therapies are expected to drive market expansion in the coming years.
Current Challenges in Isotonic Solution Development
The development of isotonic solutions for stem cell preservation faces several significant challenges that researchers and bioengineers are actively working to overcome. One of the primary obstacles is maintaining the delicate balance between osmotic pressure and cellular integrity. Isotonic solutions must precisely match the osmolarity of stem cells to prevent cell shrinkage or swelling, which can lead to irreversible damage or loss of viability.
Another critical challenge lies in the formulation of isotonic solutions that can effectively protect stem cells from various stressors during preservation. These stressors include oxidative damage, temperature fluctuations, and mechanical stress during handling and storage. Developing solutions that can mitigate these factors while maintaining isotonicity requires a complex interplay of components and careful optimization.
The long-term stability of isotonic solutions presents an additional hurdle. As preservation times extend, the risk of solution degradation increases, potentially compromising its isotonic properties and protective capabilities. Researchers are exploring novel stabilizers and antioxidants to enhance the shelf-life and efficacy of these solutions over extended periods.
Furthermore, the heterogeneity of stem cell populations poses a significant challenge in isotonic solution development. Different types of stem cells, such as embryonic, adult, or induced pluripotent stem cells, may have varying osmotic tolerances and preservation requirements. Creating a universal isotonic solution that can effectively preserve diverse stem cell populations remains an elusive goal.
The scalability of isotonic solution production for clinical and commercial applications is another pressing issue. Ensuring consistent quality and isotonicity across large-scale batches while maintaining cost-effectiveness is crucial for widespread adoption in stem cell therapies and research.
Regulatory compliance and standardization of isotonic solutions for stem cell preservation also present ongoing challenges. Developing solutions that meet stringent quality control measures and regulatory requirements across different jurisdictions is essential for their use in clinical settings.
Lastly, the need for cryoprotectant-free or reduced cryoprotectant isotonic solutions is gaining attention. Traditional cryopreservation methods often rely on potentially toxic cryoprotectants, which can affect stem cell viability and functionality. Developing isotonic solutions that can preserve stem cells effectively without these agents or with minimal concentrations is a significant area of research and development in the field.
Another critical challenge lies in the formulation of isotonic solutions that can effectively protect stem cells from various stressors during preservation. These stressors include oxidative damage, temperature fluctuations, and mechanical stress during handling and storage. Developing solutions that can mitigate these factors while maintaining isotonicity requires a complex interplay of components and careful optimization.
The long-term stability of isotonic solutions presents an additional hurdle. As preservation times extend, the risk of solution degradation increases, potentially compromising its isotonic properties and protective capabilities. Researchers are exploring novel stabilizers and antioxidants to enhance the shelf-life and efficacy of these solutions over extended periods.
Furthermore, the heterogeneity of stem cell populations poses a significant challenge in isotonic solution development. Different types of stem cells, such as embryonic, adult, or induced pluripotent stem cells, may have varying osmotic tolerances and preservation requirements. Creating a universal isotonic solution that can effectively preserve diverse stem cell populations remains an elusive goal.
The scalability of isotonic solution production for clinical and commercial applications is another pressing issue. Ensuring consistent quality and isotonicity across large-scale batches while maintaining cost-effectiveness is crucial for widespread adoption in stem cell therapies and research.
Regulatory compliance and standardization of isotonic solutions for stem cell preservation also present ongoing challenges. Developing solutions that meet stringent quality control measures and regulatory requirements across different jurisdictions is essential for their use in clinical settings.
Lastly, the need for cryoprotectant-free or reduced cryoprotectant isotonic solutions is gaining attention. Traditional cryopreservation methods often rely on potentially toxic cryoprotectants, which can affect stem cell viability and functionality. Developing isotonic solutions that can preserve stem cells effectively without these agents or with minimal concentrations is a significant area of research and development in the field.
Existing Isotonic Solutions for Stem Cell Preservation
01 Isotonic solutions with antimicrobial agents
Incorporating antimicrobial agents into isotonic solutions enhances their preservation effectiveness. These agents inhibit microbial growth, extending the shelf life of the solution while maintaining its isotonicity. Common antimicrobial agents used include benzalkonium chloride, chlorhexidine, and sodium azide.- Isotonic solutions with antimicrobial agents: Incorporating antimicrobial agents into isotonic solutions enhances their preservation effectiveness. These agents inhibit microbial growth, extending the shelf life of the solution while maintaining its isotonicity. Common antimicrobial agents used include benzalkonium chloride, chlorhexidine, and sodium azide.
- pH adjustment for improved preservation: Optimizing the pH of isotonic solutions can significantly improve their preservation effectiveness. Adjusting the pH to a range that is unfavorable for microbial growth, typically between 4.5 and 7.0, helps maintain the solution's stability and extends its shelf life without compromising isotonicity.
- Use of natural preservatives in isotonic solutions: Natural preservatives, such as plant extracts or essential oils, can be incorporated into isotonic solutions to enhance their preservation effectiveness. These natural compounds often have antimicrobial properties and can help maintain the solution's stability while appealing to consumers seeking more natural product formulations.
- Packaging innovations for improved preservation: Innovative packaging solutions can enhance the preservation effectiveness of isotonic solutions. This includes using materials that provide better barrier properties against light, oxygen, and moisture, as well as incorporating dispensing systems that minimize contamination during use. Such packaging innovations help maintain the solution's integrity and extend its shelf life.
- Combination of preservatives for synergistic effects: Combining multiple preservatives in isotonic solutions can lead to synergistic effects, enhancing overall preservation effectiveness. This approach allows for lower concentrations of individual preservatives while achieving better antimicrobial activity and broader spectrum protection against various microorganisms.
02 pH adjustment for improved preservation
Adjusting the pH of isotonic solutions can significantly improve their preservation effectiveness. Maintaining an optimal pH range inhibits microbial growth and enhances the stability of active ingredients. Buffer systems are often employed to maintain the desired pH level over time.Expand Specific Solutions03 Use of natural preservatives in isotonic solutions
Natural preservatives derived from plant extracts or essential oils can be incorporated into isotonic solutions to enhance their preservation effectiveness. These natural alternatives offer antimicrobial properties while potentially reducing the risk of adverse reactions associated with synthetic preservatives.Expand Specific Solutions04 Combination of preservatives for synergistic effects
Combining multiple preservatives in isotonic solutions can lead to synergistic effects, enhancing overall preservation effectiveness. This approach allows for lower concentrations of individual preservatives while maintaining or improving antimicrobial activity, potentially reducing the risk of irritation or toxicity.Expand Specific Solutions05 Packaging and storage considerations for isotonic solutions
The choice of packaging materials and storage conditions plays a crucial role in maintaining the preservation effectiveness of isotonic solutions. Utilizing appropriate packaging materials, such as those with barrier properties against light and oxygen, along with proper storage temperatures, can significantly extend the shelf life and maintain the integrity of the solution.Expand Specific Solutions
Key Players in Stem Cell Preservation Industry
The field of isotonic solutions in stem cell preservation is in a growth phase, with increasing market size and technological advancements. The competitive landscape is diverse, featuring established medical companies like Terumo Corp. and Otsuka Pharmaceutical Factory, alongside specialized biotechnology firms such as Celularity, Inc. and Megakaryon Corp. The market is characterized by a mix of large corporations and innovative startups, indicating a dynamic environment for research and development. While the technology is progressing, it is not yet fully mature, as evidenced by ongoing research at institutions like Memorial Sloan Kettering Cancer Center and The Johns Hopkins University, suggesting potential for further improvements and market expansion in stem cell preservation techniques.
Celularity, Inc.
Technical Solution: Celularity has developed a proprietary isotonic solution for stem cell preservation, focusing on placental-derived stem cells. Their approach involves using a combination of antioxidants, osmolytes, and cryoprotectants to maintain cell viability during cryopreservation. The company's solution is designed to minimize ice crystal formation and reduce cellular stress during the freezing and thawing processes[1]. Celularity's method has shown promising results in preserving the functional properties of stem cells, including their differentiation potential and immunomodulatory capabilities[2].
Strengths: Specialized in placental-derived stem cells, potentially higher cell viability post-thaw. Weaknesses: May be less effective for other stem cell types, limited long-term clinical data available.
Memorial Sloan Kettering Cancer Center
Technical Solution: Memorial Sloan Kettering Cancer Center (MSKCC) has conducted extensive research on isotonic solutions for stem cell preservation, particularly in the context of hematopoietic stem cell transplantation. Their approach focuses on developing and optimizing cryopreservation media that maintain the functional properties of stem cells while minimizing toxicity. MSKCC researchers have investigated the use of alternative cryoprotectants to DMSO, such as trehalose and other sugar-based solutions[7]. They have also explored the impact of controlled-rate freezing and rapid thawing techniques on stem cell viability and function. MSKCC's work has contributed to improved protocols for preserving both autologous and allogeneic stem cell grafts[8].
Strengths: Strong focus on clinical applications, particularly in cancer treatment. Weaknesses: Research may be more focused on hematopoietic stem cells, potentially limiting applicability to other stem cell types.
Innovative Approaches in Isotonic Solution Formulation
Preservation solution for cryopreservation of adipose-derived mesenchymal stem cells
PatentPendingUS20250154474A1
Innovation
- A preservation solution comprising a compound electrolyte, glucose, sodium chloride, a vitamin, and an amino acid, which is specifically formulated to maintain the viability of adipose-derived mesenchymal stem cells at low temperatures.
Preservative solution for live cells or composition comprising live cells
PatentWO2018097228A1
Innovation
- A preservation solution containing vitamins C and/or E in a physiological isotonic solution, specifically Hank's balanced salt solution, is used to enhance the shelf life and viability of sheet-shaped cell cultures by immersing them in a low-nutrient environment at 2-8°C for 24 to 120 hours.
Regulatory Framework for Stem Cell Preservation Solutions
The regulatory framework for stem cell preservation solutions is a critical aspect of the biotechnology and healthcare industries. It encompasses a complex set of guidelines, standards, and legal requirements that govern the development, production, and use of isotonic solutions for stem cell preservation.
At the international level, organizations such as the International Society for Stem Cell Research (ISSCR) and the World Health Organization (WHO) provide overarching guidelines for stem cell research and preservation. These guidelines often serve as a foundation for national regulatory bodies to develop more specific regulations.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating stem cell preservation solutions. The FDA classifies these solutions as medical devices and requires manufacturers to comply with Good Manufacturing Practices (GMP) and obtain premarket approval or clearance before commercialization. The Center for Biologics Evaluation and Research (CBER) within the FDA is responsible for overseeing the regulation of cellular and gene therapy products, including stem cell preservation solutions.
The European Union has established a comprehensive regulatory framework through the European Medicines Agency (EMA). The Advanced Therapy Medicinal Products (ATMP) Regulation governs stem cell-based products and their preservation methods. This regulation ensures that stem cell preservation solutions meet stringent quality, safety, and efficacy standards before they can be marketed within the EU.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of stem cell preservation solutions. The country has implemented a fast-track approval system for regenerative medicine products, which includes provisions for the preservation and storage of stem cells.
Regulatory bodies worldwide emphasize the importance of quality control and assurance in the production of stem cell preservation solutions. Manufacturers must demonstrate that their products maintain stem cell viability, prevent contamination, and ensure long-term stability. This often involves extensive testing and validation processes, including sterility testing, endotoxin testing, and stability studies.
The regulatory landscape also addresses ethical considerations surrounding stem cell research and preservation. Many countries have established specific guidelines for the sourcing and use of stem cells, particularly those derived from embryonic sources. These ethical guidelines often influence the development and approval of preservation solutions.
As the field of stem cell research continues to advance, regulatory frameworks are evolving to keep pace with new technologies and applications. This dynamic regulatory environment requires ongoing collaboration between researchers, industry stakeholders, and regulatory agencies to ensure that stem cell preservation solutions meet the highest standards of safety and efficacy while facilitating scientific progress and innovation in regenerative medicine.
At the international level, organizations such as the International Society for Stem Cell Research (ISSCR) and the World Health Organization (WHO) provide overarching guidelines for stem cell research and preservation. These guidelines often serve as a foundation for national regulatory bodies to develop more specific regulations.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating stem cell preservation solutions. The FDA classifies these solutions as medical devices and requires manufacturers to comply with Good Manufacturing Practices (GMP) and obtain premarket approval or clearance before commercialization. The Center for Biologics Evaluation and Research (CBER) within the FDA is responsible for overseeing the regulation of cellular and gene therapy products, including stem cell preservation solutions.
The European Union has established a comprehensive regulatory framework through the European Medicines Agency (EMA). The Advanced Therapy Medicinal Products (ATMP) Regulation governs stem cell-based products and their preservation methods. This regulation ensures that stem cell preservation solutions meet stringent quality, safety, and efficacy standards before they can be marketed within the EU.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of stem cell preservation solutions. The country has implemented a fast-track approval system for regenerative medicine products, which includes provisions for the preservation and storage of stem cells.
Regulatory bodies worldwide emphasize the importance of quality control and assurance in the production of stem cell preservation solutions. Manufacturers must demonstrate that their products maintain stem cell viability, prevent contamination, and ensure long-term stability. This often involves extensive testing and validation processes, including sterility testing, endotoxin testing, and stability studies.
The regulatory landscape also addresses ethical considerations surrounding stem cell research and preservation. Many countries have established specific guidelines for the sourcing and use of stem cells, particularly those derived from embryonic sources. These ethical guidelines often influence the development and approval of preservation solutions.
As the field of stem cell research continues to advance, regulatory frameworks are evolving to keep pace with new technologies and applications. This dynamic regulatory environment requires ongoing collaboration between researchers, industry stakeholders, and regulatory agencies to ensure that stem cell preservation solutions meet the highest standards of safety and efficacy while facilitating scientific progress and innovation in regenerative medicine.
Bioethical Considerations in Stem Cell Research and Preservation
The ethical considerations surrounding stem cell research and preservation are complex and multifaceted, requiring careful examination of various perspectives. One primary concern is the source of stem cells, particularly when derived from human embryos. This raises questions about the moral status of embryos and the potential exploitation of human life for scientific purposes. Balancing the potential medical benefits with respect for human dignity is a central challenge.
Another critical aspect is informed consent in stem cell donation and research. Ensuring that donors fully understand the implications of their contribution, including potential future uses of their genetic material, is essential. This becomes particularly complex when considering the long-term storage of stem cells and the possibility of unforeseen future applications.
Privacy and confidentiality issues also come to the forefront in stem cell preservation. As stem cells contain an individual's complete genetic information, protecting this sensitive data from misuse or unauthorized access is paramount. Establishing robust safeguards and clear guidelines for data handling is crucial to maintain public trust and protect individual rights.
The equitable distribution of stem cell therapies and technologies is another significant ethical consideration. As these treatments become more advanced, ensuring fair access across different socioeconomic groups and geographical regions becomes increasingly important. This includes addressing potential disparities in both research focus and treatment availability.
Ethical concerns also arise regarding the commercialization of stem cell technologies. The potential for profit-driven research to influence scientific integrity or lead to the exploitation of vulnerable populations must be carefully monitored and regulated. Striking a balance between incentivizing innovation and protecting public interests is a delicate task.
In the context of isotonic solutions for stem cell preservation, ethical considerations extend to the development and testing of these solutions. Ensuring that research protocols adhere to strict ethical standards, including minimizing harm to donors and respecting cultural sensitivities, is crucial. Additionally, the long-term implications of using various preservation methods on stem cell viability and potential applications must be thoroughly evaluated from an ethical standpoint.
Another critical aspect is informed consent in stem cell donation and research. Ensuring that donors fully understand the implications of their contribution, including potential future uses of their genetic material, is essential. This becomes particularly complex when considering the long-term storage of stem cells and the possibility of unforeseen future applications.
Privacy and confidentiality issues also come to the forefront in stem cell preservation. As stem cells contain an individual's complete genetic information, protecting this sensitive data from misuse or unauthorized access is paramount. Establishing robust safeguards and clear guidelines for data handling is crucial to maintain public trust and protect individual rights.
The equitable distribution of stem cell therapies and technologies is another significant ethical consideration. As these treatments become more advanced, ensuring fair access across different socioeconomic groups and geographical regions becomes increasingly important. This includes addressing potential disparities in both research focus and treatment availability.
Ethical concerns also arise regarding the commercialization of stem cell technologies. The potential for profit-driven research to influence scientific integrity or lead to the exploitation of vulnerable populations must be carefully monitored and regulated. Striking a balance between incentivizing innovation and protecting public interests is a delicate task.
In the context of isotonic solutions for stem cell preservation, ethical considerations extend to the development and testing of these solutions. Ensuring that research protocols adhere to strict ethical standards, including minimizing harm to donors and respecting cultural sensitivities, is crucial. Additionally, the long-term implications of using various preservation methods on stem cell viability and potential applications must be thoroughly evaluated from an ethical standpoint.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!