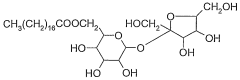
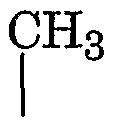How isotonic solutions stabilize emulsions in pharmaceutical products
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Emulsion Stabilization Background and Objectives
Emulsion stabilization has been a critical area of research in pharmaceutical product development for decades. The use of isotonic solutions to stabilize emulsions represents a significant advancement in this field, addressing key challenges in drug delivery and formulation stability. Historically, emulsions have been widely used in pharmaceuticals due to their ability to encapsulate both hydrophilic and lipophilic compounds, enhancing bioavailability and controlled release of active ingredients.
The primary objective of utilizing isotonic solutions in emulsion stabilization is to create a stable, homogeneous, and biocompatible formulation that maintains its integrity throughout its shelf life and during administration. Isotonic solutions, which have the same osmotic pressure as body fluids, play a crucial role in preventing osmotic stress on emulsion droplets, thereby reducing the risk of coalescence and phase separation.
The evolution of emulsion stabilization techniques has been driven by the increasing complexity of pharmaceutical formulations and the demand for improved drug efficacy and patient compliance. Early approaches relied heavily on surfactants and emulsifiers, which often led to issues with toxicity and long-term stability. The introduction of isotonic solutions as stabilizers marks a shift towards more biocompatible and physiologically relevant formulation strategies.
Recent technological advancements have enabled a deeper understanding of the physicochemical properties governing emulsion stability. This has led to the development of sophisticated isotonic formulations that not only stabilize emulsions but also enhance their functional properties. These innovations aim to address specific challenges in drug delivery, such as targeted release, extended shelf life, and improved absorption of poorly soluble drugs.
The current research landscape focuses on optimizing the composition and properties of isotonic solutions to achieve superior emulsion stability. This includes investigating the role of various electrolytes, non-electrolytes, and buffer systems in maintaining the osmotic balance and interfacial properties of emulsions. Additionally, there is growing interest in combining isotonic stabilization with other advanced technologies, such as nanoparticle engineering and smart polymer systems, to create next-generation pharmaceutical emulsions.
As the field progresses, the objectives of emulsion stabilization research are expanding beyond mere physical stability. There is an increasing emphasis on developing isotonic emulsion systems that can respond to physiological stimuli, enhance drug targeting, and improve the overall therapeutic index of pharmaceutical products. This multifaceted approach aims to revolutionize drug delivery systems, potentially leading to more effective treatments and improved patient outcomes.
The primary objective of utilizing isotonic solutions in emulsion stabilization is to create a stable, homogeneous, and biocompatible formulation that maintains its integrity throughout its shelf life and during administration. Isotonic solutions, which have the same osmotic pressure as body fluids, play a crucial role in preventing osmotic stress on emulsion droplets, thereby reducing the risk of coalescence and phase separation.
The evolution of emulsion stabilization techniques has been driven by the increasing complexity of pharmaceutical formulations and the demand for improved drug efficacy and patient compliance. Early approaches relied heavily on surfactants and emulsifiers, which often led to issues with toxicity and long-term stability. The introduction of isotonic solutions as stabilizers marks a shift towards more biocompatible and physiologically relevant formulation strategies.
Recent technological advancements have enabled a deeper understanding of the physicochemical properties governing emulsion stability. This has led to the development of sophisticated isotonic formulations that not only stabilize emulsions but also enhance their functional properties. These innovations aim to address specific challenges in drug delivery, such as targeted release, extended shelf life, and improved absorption of poorly soluble drugs.
The current research landscape focuses on optimizing the composition and properties of isotonic solutions to achieve superior emulsion stability. This includes investigating the role of various electrolytes, non-electrolytes, and buffer systems in maintaining the osmotic balance and interfacial properties of emulsions. Additionally, there is growing interest in combining isotonic stabilization with other advanced technologies, such as nanoparticle engineering and smart polymer systems, to create next-generation pharmaceutical emulsions.
As the field progresses, the objectives of emulsion stabilization research are expanding beyond mere physical stability. There is an increasing emphasis on developing isotonic emulsion systems that can respond to physiological stimuli, enhance drug targeting, and improve the overall therapeutic index of pharmaceutical products. This multifaceted approach aims to revolutionize drug delivery systems, potentially leading to more effective treatments and improved patient outcomes.
Pharmaceutical Market Demand for Stable Emulsions
The pharmaceutical industry has witnessed a growing demand for stable emulsions in recent years, driven by the need for improved drug delivery systems and enhanced product efficacy. Emulsions play a crucial role in various pharmaceutical formulations, including topical creams, injectable medications, and oral suspensions. The market for stable emulsions in pharmaceuticals is expected to continue its upward trajectory due to several key factors.
Firstly, the increasing prevalence of chronic diseases and the aging population have led to a surge in demand for pharmaceutical products that require stable emulsion formulations. These formulations offer better bioavailability and controlled release of active ingredients, making them particularly valuable for treating conditions such as cardiovascular diseases, cancer, and autoimmune disorders.
Moreover, the rise of personalized medicine and targeted drug delivery has further fueled the need for advanced emulsion technologies. Pharmaceutical companies are investing heavily in research and development to create innovative emulsion-based formulations that can precisely deliver drugs to specific sites in the body, minimizing side effects and maximizing therapeutic outcomes.
The cosmeceutical sector, which bridges pharmaceuticals and cosmetics, has also contributed significantly to the growing demand for stable emulsions. As consumers become more health-conscious and seek multifunctional products, there is an increased interest in emulsion-based formulations that offer both therapeutic and cosmetic benefits.
In addition, the biotechnology and biopharmaceutical industries have emerged as major drivers of emulsion technology adoption. Many biological drugs, including proteins and peptides, require stable emulsion systems to maintain their efficacy and extend their shelf life. This has led to a surge in demand for advanced emulsion stabilization techniques in the production of biopharmaceuticals.
Furthermore, the global push towards more sustainable and environmentally friendly pharmaceutical products has created new opportunities for emulsion technology. Manufacturers are exploring novel, plant-based emulsifiers and stabilizers to replace synthetic alternatives, aligning with the growing consumer preference for natural and eco-friendly formulations.
The COVID-19 pandemic has also highlighted the importance of stable emulsions in vaccine development and production. The successful development of mRNA-based vaccines, which rely on lipid nanoparticle emulsions for delivery, has underscored the critical role of emulsion technology in addressing global health challenges.
Firstly, the increasing prevalence of chronic diseases and the aging population have led to a surge in demand for pharmaceutical products that require stable emulsion formulations. These formulations offer better bioavailability and controlled release of active ingredients, making them particularly valuable for treating conditions such as cardiovascular diseases, cancer, and autoimmune disorders.
Moreover, the rise of personalized medicine and targeted drug delivery has further fueled the need for advanced emulsion technologies. Pharmaceutical companies are investing heavily in research and development to create innovative emulsion-based formulations that can precisely deliver drugs to specific sites in the body, minimizing side effects and maximizing therapeutic outcomes.
The cosmeceutical sector, which bridges pharmaceuticals and cosmetics, has also contributed significantly to the growing demand for stable emulsions. As consumers become more health-conscious and seek multifunctional products, there is an increased interest in emulsion-based formulations that offer both therapeutic and cosmetic benefits.
In addition, the biotechnology and biopharmaceutical industries have emerged as major drivers of emulsion technology adoption. Many biological drugs, including proteins and peptides, require stable emulsion systems to maintain their efficacy and extend their shelf life. This has led to a surge in demand for advanced emulsion stabilization techniques in the production of biopharmaceuticals.
Furthermore, the global push towards more sustainable and environmentally friendly pharmaceutical products has created new opportunities for emulsion technology. Manufacturers are exploring novel, plant-based emulsifiers and stabilizers to replace synthetic alternatives, aligning with the growing consumer preference for natural and eco-friendly formulations.
The COVID-19 pandemic has also highlighted the importance of stable emulsions in vaccine development and production. The successful development of mRNA-based vaccines, which rely on lipid nanoparticle emulsions for delivery, has underscored the critical role of emulsion technology in addressing global health challenges.
Current Challenges in Emulsion Stability
Emulsion stability remains a critical challenge in pharmaceutical product development, particularly in the context of isotonic solutions. The primary issue lies in maintaining the long-term stability of emulsions, which are inherently thermodynamically unstable systems. Pharmaceutical emulsions often contain a complex mixture of active ingredients, excipients, and stabilizers, making them susceptible to various destabilization mechanisms.
One of the key challenges is preventing coalescence, where dispersed droplets merge to form larger ones, eventually leading to phase separation. This process is accelerated by factors such as temperature fluctuations, pH changes, and the presence of electrolytes in isotonic solutions. The Ostwald ripening phenomenon, where smaller droplets dissolve and redeposit onto larger ones, further complicates stability maintenance.
Another significant hurdle is the control of interfacial tension between the oil and water phases. Isotonic solutions, while necessary for physiological compatibility, can interfere with the effectiveness of surfactants and emulsifiers. This interference can lead to reduced emulsion stability over time, potentially compromising the product's shelf life and therapeutic efficacy.
The presence of proteins and other biomolecules in pharmaceutical emulsions adds another layer of complexity. These components can adsorb at the oil-water interface, potentially displacing emulsifiers or altering the interfacial properties. This phenomenon, known as competitive adsorption, can significantly impact emulsion stability and is particularly challenging to control in isotonic environments.
Oxidative stability is another critical concern, especially for emulsions containing unsaturated lipids or sensitive active ingredients. The presence of dissolved oxygen and metal ions in isotonic solutions can catalyze oxidation reactions, leading to rancidity, loss of potency, and potential formation of harmful byproducts. Developing effective antioxidant strategies that remain stable and active in isotonic conditions is an ongoing challenge.
Furthermore, the viscosity and rheological properties of emulsions in isotonic solutions pose additional stability challenges. Maintaining the desired flow characteristics while ensuring long-term stability is crucial for proper dosing and administration of pharmaceutical products. Balancing these properties with the need for isotonicity often requires complex formulation strategies and extensive stability testing.
Lastly, the scalability of stable emulsion production remains a significant hurdle. Techniques that work well at laboratory scale may not translate effectively to industrial production, necessitating careful process optimization and quality control measures to ensure consistent emulsion stability across different batch sizes and manufacturing conditions.
One of the key challenges is preventing coalescence, where dispersed droplets merge to form larger ones, eventually leading to phase separation. This process is accelerated by factors such as temperature fluctuations, pH changes, and the presence of electrolytes in isotonic solutions. The Ostwald ripening phenomenon, where smaller droplets dissolve and redeposit onto larger ones, further complicates stability maintenance.
Another significant hurdle is the control of interfacial tension between the oil and water phases. Isotonic solutions, while necessary for physiological compatibility, can interfere with the effectiveness of surfactants and emulsifiers. This interference can lead to reduced emulsion stability over time, potentially compromising the product's shelf life and therapeutic efficacy.
The presence of proteins and other biomolecules in pharmaceutical emulsions adds another layer of complexity. These components can adsorb at the oil-water interface, potentially displacing emulsifiers or altering the interfacial properties. This phenomenon, known as competitive adsorption, can significantly impact emulsion stability and is particularly challenging to control in isotonic environments.
Oxidative stability is another critical concern, especially for emulsions containing unsaturated lipids or sensitive active ingredients. The presence of dissolved oxygen and metal ions in isotonic solutions can catalyze oxidation reactions, leading to rancidity, loss of potency, and potential formation of harmful byproducts. Developing effective antioxidant strategies that remain stable and active in isotonic conditions is an ongoing challenge.
Furthermore, the viscosity and rheological properties of emulsions in isotonic solutions pose additional stability challenges. Maintaining the desired flow characteristics while ensuring long-term stability is crucial for proper dosing and administration of pharmaceutical products. Balancing these properties with the need for isotonicity often requires complex formulation strategies and extensive stability testing.
Lastly, the scalability of stable emulsion production remains a significant hurdle. Techniques that work well at laboratory scale may not translate effectively to industrial production, necessitating careful process optimization and quality control measures to ensure consistent emulsion stability across different batch sizes and manufacturing conditions.
Isotonic Solutions as Emulsion Stabilizers
01 Stabilization of isotonic solutions using buffer systems
Buffer systems are employed to maintain the pH and stability of isotonic solutions. These systems help prevent degradation of active ingredients and ensure the solution remains isotonic over time. Common buffer components include phosphates, citrates, and acetates, which work together to resist changes in pH and maintain the osmotic balance of the solution.- Stabilization of isotonic solutions using buffer systems: Buffer systems are employed to maintain the pH and stability of isotonic solutions. These systems help prevent degradation of active ingredients and ensure the solution remains isotonic over time. Common buffer components include phosphates, citrates, and acetates, which work together to resist changes in pH and maintain the osmotic balance of the solution.
- Antioxidants for enhancing stability of isotonic solutions: Antioxidants are added to isotonic solutions to prevent oxidation of sensitive components and improve overall stability. These compounds, such as ascorbic acid, tocopherols, or butylated hydroxyanisole (BHA), scavenge free radicals and protect the solution from degradation caused by oxidative stress, thereby extending the shelf life of the product.
- Preservatives for microbial control in isotonic solutions: Preservatives are incorporated into isotonic solutions to prevent microbial growth and maintain sterility. Commonly used preservatives include benzalkonium chloride, chlorhexidine, and parabens. These agents help ensure the long-term stability of the solution by inhibiting the growth of bacteria, fungi, and other microorganisms that could compromise the product's integrity.
- Packaging and storage considerations for isotonic solution stability: The choice of packaging materials and storage conditions plays a crucial role in maintaining the stability of isotonic solutions. Factors such as light protection, oxygen permeability, and temperature control are considered when selecting appropriate packaging. Additionally, proper storage conditions, including temperature and humidity control, are essential for preserving the stability and efficacy of the solution over time.
- Stabilization through complexation and chelation: Complexing agents and chelators are used to enhance the stability of isotonic solutions by binding to metal ions that can catalyze degradation reactions. These agents, such as EDTA or citric acid, form stable complexes with metal ions, preventing them from interacting with other components of the solution and potentially causing instability or precipitation.
02 Use of antioxidants to enhance stability
Antioxidants are added to isotonic solutions to prevent oxidation and degradation of sensitive components. These additives help maintain the efficacy and shelf life of the solution by neutralizing free radicals and inhibiting oxidative processes. Common antioxidants used include ascorbic acid, tocopherols, and butylated hydroxyanisole (BHA).Expand Specific Solutions03 Temperature control for stability enhancement
Proper temperature control during manufacturing, storage, and transportation is crucial for maintaining the stability of isotonic solutions. Cold chain management and temperature-controlled environments help prevent degradation of heat-sensitive components and maintain the solution's isotonicity. Some formulations may require specific storage conditions to ensure long-term stability.Expand Specific Solutions04 Packaging innovations for improved stability
Advanced packaging technologies are utilized to enhance the stability of isotonic solutions. These may include multi-layer packaging materials, oxygen-barrier containers, or light-protective packaging to prevent degradation caused by environmental factors. Some packaging solutions incorporate desiccants or oxygen scavengers to maintain optimal conditions within the container.Expand Specific Solutions05 Stabilization through chelating agents
Chelating agents are incorporated into isotonic solutions to improve stability by binding metal ions that could catalyze degradation reactions. These agents, such as EDTA or citric acid, help prevent oxidation, discoloration, and precipitation in the solution. By sequestering metal ions, chelating agents contribute to maintaining the clarity and efficacy of the isotonic solution over time.Expand Specific Solutions
Key Players in Pharmaceutical Emulsion Industry
The competitive landscape for isotonic solutions in pharmaceutical emulsion stabilization is characterized by a mature market with established players and ongoing innovation. The global market for pharmaceutical emulsions is substantial, driven by increasing demand for advanced drug delivery systems. Technologically, the field is well-developed but continues to evolve, with companies like Takeda Pharmaceutical, Henkel, and Baxter International leading in research and development. These firms, along with others such as L'Oréal and Bayer AG, are investing in novel formulation techniques to enhance emulsion stability and efficacy. The industry is seeing a trend towards more sophisticated, multifunctional isotonic solutions that offer improved performance and patient outcomes.
Baxter International, Inc.
Technical Solution: Baxter International has developed a proprietary emulsion stabilization technology for pharmaceutical products using isotonic solutions. Their approach involves the use of carefully selected emulsifiers and stabilizers in combination with isotonic agents such as glycerin or sorbitol. The company employs a multi-step emulsification process that includes high-pressure homogenization to create nano-sized droplets, enhancing the stability of the emulsion[1]. Additionally, Baxter has implemented a novel temperature-controlled mixing technique that allows for better control of particle size distribution and improved long-term stability of the emulsion[3]. The company has also incorporated antioxidants and pH buffers to prevent oxidation and maintain the optimal pH range for maximum stability[5].
Strengths: Advanced emulsification technology, precise control over particle size, and enhanced long-term stability. Weaknesses: Potentially higher production costs due to complex processes and specialized equipment requirements.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim has developed an innovative approach to stabilizing emulsions in pharmaceutical products using isotonic solutions. Their method involves the use of a combination of natural and synthetic emulsifiers, carefully selected for their compatibility with various active pharmaceutical ingredients. The company has patented a unique process that utilizes a controlled temperature gradient during emulsification, which results in a more uniform droplet size distribution[2]. Additionally, Boehringer Ingelheim has incorporated a novel surfactant system that provides both steric and electrostatic stabilization, enhancing the overall stability of the emulsion[4]. The company also employs a proprietary blend of osmolytes to maintain isotonicity while contributing to the emulsion's stability through preferential hydration effects[6].
Strengths: Versatile emulsion system suitable for a wide range of APIs, enhanced stability through multiple mechanisms. Weaknesses: May require specialized manufacturing equipment and processes, potentially limiting production flexibility.
Core Mechanisms of Isotonic Stabilization
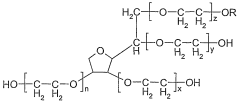
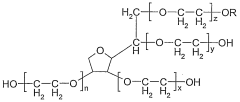
Emulsifier system, emulsion and the use thereof
PatentWO2007003565A1
Innovation
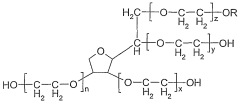
- An emulsifier system comprising ascorbic acid esters, ethoxylated sorbitan fatty acid esters, and sugar fatty acid esters, which provides a synergistic effect to stabilize emulsions, allowing for high concentrations of fat-soluble substances, maintaining stability at various temperatures, and ensuring color strength and bacteriostatic properties.
Emulsion containing an oppositely-charged surfactant and polymer and the production method therefor
PatentWO2003037496A1
Innovation
- An emulsion is stabilized by combining surfactants and polymers of opposite charges, where the surfactant and polymer concentrations are optimized to form a stable complex at the interface, reducing the required surfactant level below 5*10^-3 mol/1, and the polymer concentration is determined based on its charge and ionic strength, using conventional anionic and cationic surfactants and polymers.
Regulatory Considerations for Pharmaceutical Emulsions
The regulatory landscape for pharmaceutical emulsions is complex and multifaceted, requiring careful consideration throughout the product development and manufacturing processes. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established stringent guidelines to ensure the safety, efficacy, and quality of pharmaceutical emulsions.
One of the primary regulatory considerations is the selection of excipients used in emulsion formulations. Manufacturers must ensure that all ingredients are approved for pharmaceutical use and comply with relevant pharmacopoeial standards. This includes the isotonic agents used to stabilize emulsions, which must be thoroughly evaluated for their safety profile and potential interactions with other components.
Stability testing is another critical aspect of regulatory compliance for pharmaceutical emulsions. Regulatory agencies require comprehensive stability studies to demonstrate that the emulsion remains stable throughout its intended shelf life. This includes evaluating physical, chemical, and microbiological stability under various environmental conditions. The use of isotonic solutions to stabilize emulsions must be validated through these stability studies to ensure consistent product performance.
Manufacturing processes for pharmaceutical emulsions are subject to strict regulatory oversight. Good Manufacturing Practices (GMP) must be followed to ensure product quality and consistency. This includes validated procedures for emulsion preparation, sterilization (if required), and packaging. The role of isotonic solutions in maintaining emulsion stability during manufacturing and storage must be thoroughly documented and supported by scientific evidence.
Quality control measures are essential for regulatory compliance. Manufacturers must implement robust analytical methods to assess emulsion characteristics, including droplet size distribution, zeta potential, and rheological properties. The impact of isotonic solutions on these parameters must be well-understood and controlled within specified limits.
Regulatory submissions for pharmaceutical emulsions require comprehensive documentation of formulation development, manufacturing processes, and analytical methods. This includes detailed information on the mechanism by which isotonic solutions contribute to emulsion stability. Regulatory agencies may request additional studies or clarifications to ensure that the stabilization approach is well-characterized and reliable.
Post-market surveillance is an ongoing regulatory requirement for pharmaceutical emulsions. Manufacturers must monitor and report any adverse events or quality issues related to their products. This includes evaluating the long-term performance of isotonic solutions in maintaining emulsion stability under real-world conditions.
One of the primary regulatory considerations is the selection of excipients used in emulsion formulations. Manufacturers must ensure that all ingredients are approved for pharmaceutical use and comply with relevant pharmacopoeial standards. This includes the isotonic agents used to stabilize emulsions, which must be thoroughly evaluated for their safety profile and potential interactions with other components.
Stability testing is another critical aspect of regulatory compliance for pharmaceutical emulsions. Regulatory agencies require comprehensive stability studies to demonstrate that the emulsion remains stable throughout its intended shelf life. This includes evaluating physical, chemical, and microbiological stability under various environmental conditions. The use of isotonic solutions to stabilize emulsions must be validated through these stability studies to ensure consistent product performance.
Manufacturing processes for pharmaceutical emulsions are subject to strict regulatory oversight. Good Manufacturing Practices (GMP) must be followed to ensure product quality and consistency. This includes validated procedures for emulsion preparation, sterilization (if required), and packaging. The role of isotonic solutions in maintaining emulsion stability during manufacturing and storage must be thoroughly documented and supported by scientific evidence.
Quality control measures are essential for regulatory compliance. Manufacturers must implement robust analytical methods to assess emulsion characteristics, including droplet size distribution, zeta potential, and rheological properties. The impact of isotonic solutions on these parameters must be well-understood and controlled within specified limits.
Regulatory submissions for pharmaceutical emulsions require comprehensive documentation of formulation development, manufacturing processes, and analytical methods. This includes detailed information on the mechanism by which isotonic solutions contribute to emulsion stability. Regulatory agencies may request additional studies or clarifications to ensure that the stabilization approach is well-characterized and reliable.
Post-market surveillance is an ongoing regulatory requirement for pharmaceutical emulsions. Manufacturers must monitor and report any adverse events or quality issues related to their products. This includes evaluating the long-term performance of isotonic solutions in maintaining emulsion stability under real-world conditions.
Environmental Impact of Emulsion Stabilizers
The environmental impact of emulsion stabilizers used in pharmaceutical products is a growing concern in the industry. Isotonic solutions, while effective in stabilizing emulsions, can have both positive and negative effects on the environment throughout their lifecycle.
One of the primary environmental benefits of using isotonic solutions as emulsion stabilizers is their potential biodegradability. Many isotonic solutions are composed of naturally occurring compounds, such as sugars or salts, which can be more easily broken down in the environment compared to synthetic stabilizers. This characteristic reduces the long-term accumulation of these substances in ecosystems and minimizes their impact on aquatic and terrestrial life.
However, the production and disposal of isotonic solutions can still contribute to environmental issues. The manufacturing process often requires significant energy inputs and may involve the use of chemical precursors, leading to greenhouse gas emissions and potential chemical waste. Additionally, the packaging and transportation of these solutions contribute to carbon footprints and plastic waste, particularly if single-use containers are employed.
Water consumption is another environmental consideration. The production of isotonic solutions requires purified water, which can strain local water resources, especially in water-scarce regions. Furthermore, the disposal of unused or expired solutions may lead to water pollution if not properly managed, potentially affecting aquatic ecosystems and drinking water sources.
The pharmaceutical industry's increasing focus on sustainability has led to efforts to mitigate these environmental impacts. Some companies are exploring the use of renewable energy sources in production facilities and implementing closed-loop water systems to reduce water consumption. There is also a growing trend towards developing "green" isotonic solutions using plant-based ingredients and biodegradable packaging materials.
Regulatory bodies are also playing a role in addressing the environmental impact of emulsion stabilizers. Many countries have implemented stricter guidelines for pharmaceutical waste disposal and are encouraging the adoption of more environmentally friendly practices in drug formulation and packaging.
Research into the long-term environmental effects of isotonic solutions is ongoing. Studies are being conducted to assess their persistence in the environment, potential for bioaccumulation, and effects on non-target organisms. This research is crucial for developing more sustainable stabilization methods and improving environmental risk assessments for pharmaceutical products.
In conclusion, while isotonic solutions offer advantages in emulsion stabilization, their environmental impact remains a complex issue. The pharmaceutical industry faces the challenge of balancing the need for effective and stable drug formulations with the imperative to minimize environmental harm. Continued innovation in green chemistry, sustainable manufacturing processes, and responsible disposal methods will be key to addressing these environmental concerns in the future.
One of the primary environmental benefits of using isotonic solutions as emulsion stabilizers is their potential biodegradability. Many isotonic solutions are composed of naturally occurring compounds, such as sugars or salts, which can be more easily broken down in the environment compared to synthetic stabilizers. This characteristic reduces the long-term accumulation of these substances in ecosystems and minimizes their impact on aquatic and terrestrial life.
However, the production and disposal of isotonic solutions can still contribute to environmental issues. The manufacturing process often requires significant energy inputs and may involve the use of chemical precursors, leading to greenhouse gas emissions and potential chemical waste. Additionally, the packaging and transportation of these solutions contribute to carbon footprints and plastic waste, particularly if single-use containers are employed.
Water consumption is another environmental consideration. The production of isotonic solutions requires purified water, which can strain local water resources, especially in water-scarce regions. Furthermore, the disposal of unused or expired solutions may lead to water pollution if not properly managed, potentially affecting aquatic ecosystems and drinking water sources.
The pharmaceutical industry's increasing focus on sustainability has led to efforts to mitigate these environmental impacts. Some companies are exploring the use of renewable energy sources in production facilities and implementing closed-loop water systems to reduce water consumption. There is also a growing trend towards developing "green" isotonic solutions using plant-based ingredients and biodegradable packaging materials.
Regulatory bodies are also playing a role in addressing the environmental impact of emulsion stabilizers. Many countries have implemented stricter guidelines for pharmaceutical waste disposal and are encouraging the adoption of more environmentally friendly practices in drug formulation and packaging.
Research into the long-term environmental effects of isotonic solutions is ongoing. Studies are being conducted to assess their persistence in the environment, potential for bioaccumulation, and effects on non-target organisms. This research is crucial for developing more sustainable stabilization methods and improving environmental risk assessments for pharmaceutical products.
In conclusion, while isotonic solutions offer advantages in emulsion stabilization, their environmental impact remains a complex issue. The pharmaceutical industry faces the challenge of balancing the need for effective and stable drug formulations with the imperative to minimize environmental harm. Continued innovation in green chemistry, sustainable manufacturing processes, and responsible disposal methods will be key to addressing these environmental concerns in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!