How isotonic solutions stabilize cytokine treatments
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cytokine Stabilization Goals
Cytokine stabilization in isotonic solutions represents a critical goal in the development and application of cytokine-based therapies. The primary objective is to maintain the structural integrity and biological activity of cytokines during storage, transportation, and administration. This is particularly challenging due to the inherent instability of many cytokines, which are prone to degradation, aggregation, and loss of function under various environmental conditions.
One of the key aims is to develop isotonic formulations that mimic physiological conditions, thereby minimizing stress on the cytokine molecules. These solutions typically have an osmolality similar to that of blood and extracellular fluids, which helps prevent osmotic shock to cells and tissues upon administration. The isotonicity also plays a crucial role in maintaining the native conformation of cytokines, which is essential for their biological activity.
Another important goal is to optimize the pH and ionic composition of the stabilizing solutions. Cytokines often have specific pH ranges where they exhibit maximum stability and activity. By carefully adjusting the pH and incorporating appropriate buffer systems, researchers aim to create an environment that minimizes chemical degradation pathways such as deamidation, oxidation, and hydrolysis.
The inclusion of specific excipients in isotonic solutions is another target for cytokine stabilization. These may include sugars, amino acids, or polymers that can act as cryoprotectants, antioxidants, or stabilizing agents. The goal is to identify combinations of excipients that can effectively protect cytokines from various stressors, including temperature fluctuations, mechanical stress, and exposure to light or oxygen.
Long-term stability is a critical objective in cytokine stabilization efforts. Researchers aim to develop formulations that can maintain cytokine potency over extended periods, ideally at room temperature or under refrigerated conditions. This is particularly important for improving the shelf life of cytokine-based products and reducing the need for cold chain logistics in their distribution.
Lastly, there is a focus on developing stabilization methods that are compatible with various delivery systems and administration routes. This includes ensuring that the stabilized cytokines remain effective when incorporated into advanced drug delivery platforms such as nanoparticles, liposomes, or sustained-release formulations. The ultimate goal is to create robust, versatile stabilization strategies that can be applied across a wide range of cytokine therapies, enhancing their clinical efficacy and accessibility.
One of the key aims is to develop isotonic formulations that mimic physiological conditions, thereby minimizing stress on the cytokine molecules. These solutions typically have an osmolality similar to that of blood and extracellular fluids, which helps prevent osmotic shock to cells and tissues upon administration. The isotonicity also plays a crucial role in maintaining the native conformation of cytokines, which is essential for their biological activity.
Another important goal is to optimize the pH and ionic composition of the stabilizing solutions. Cytokines often have specific pH ranges where they exhibit maximum stability and activity. By carefully adjusting the pH and incorporating appropriate buffer systems, researchers aim to create an environment that minimizes chemical degradation pathways such as deamidation, oxidation, and hydrolysis.
The inclusion of specific excipients in isotonic solutions is another target for cytokine stabilization. These may include sugars, amino acids, or polymers that can act as cryoprotectants, antioxidants, or stabilizing agents. The goal is to identify combinations of excipients that can effectively protect cytokines from various stressors, including temperature fluctuations, mechanical stress, and exposure to light or oxygen.
Long-term stability is a critical objective in cytokine stabilization efforts. Researchers aim to develop formulations that can maintain cytokine potency over extended periods, ideally at room temperature or under refrigerated conditions. This is particularly important for improving the shelf life of cytokine-based products and reducing the need for cold chain logistics in their distribution.
Lastly, there is a focus on developing stabilization methods that are compatible with various delivery systems and administration routes. This includes ensuring that the stabilized cytokines remain effective when incorporated into advanced drug delivery platforms such as nanoparticles, liposomes, or sustained-release formulations. The ultimate goal is to create robust, versatile stabilization strategies that can be applied across a wide range of cytokine therapies, enhancing their clinical efficacy and accessibility.
Market Demand Analysis
The market demand for cytokine treatments has been steadily growing, driven by the increasing prevalence of chronic diseases, autoimmune disorders, and cancer. Cytokines play a crucial role in modulating immune responses and have shown promising results in various therapeutic applications. However, the stability of cytokine-based treatments remains a significant challenge, limiting their widespread adoption and effectiveness.
Isotonic solutions have emerged as a potential stabilizer for cytokine treatments, addressing the critical need for improved shelf-life and efficacy. This development has sparked considerable interest from pharmaceutical companies and healthcare providers, as it could potentially revolutionize the delivery and storage of cytokine-based therapies.
The global cytokine market is projected to experience substantial growth in the coming years. Factors contributing to this growth include the rising incidence of inflammatory diseases, advancements in biotechnology, and increased research and development activities in the field of immunotherapy. The stabilization of cytokine treatments using isotonic solutions is expected to further accelerate market expansion by overcoming existing limitations in product stability and delivery.
Healthcare providers and patients alike are seeking more effective and convenient treatment options, particularly for chronic conditions that require long-term management. Stabilized cytokine treatments offer the potential for improved patient outcomes, reduced treatment frequency, and enhanced quality of life. This aligns with the growing trend towards personalized medicine and targeted therapies, driving demand for innovative cytokine-based products.
The biotechnology and pharmaceutical industries are investing heavily in research and development efforts focused on cytokine stabilization techniques. This investment reflects the recognition of the significant market potential for stable cytokine treatments across various therapeutic areas, including oncology, autoimmune disorders, and infectious diseases.
Regulatory agencies are also showing increased interest in novel stabilization methods for biological products, including cytokine treatments. This regulatory focus is likely to facilitate the development and approval of new stabilized cytokine therapies, further stimulating market growth and innovation in this field.
As healthcare systems worldwide strive to reduce costs and improve treatment outcomes, the demand for more stable and effective cytokine treatments is expected to rise. Isotonic solution-stabilized cytokines have the potential to reduce waste, improve supply chain efficiency, and ultimately lower healthcare costs, making them an attractive option for healthcare providers and payers.
In conclusion, the market demand for stabilized cytokine treatments using isotonic solutions is poised for significant growth. This demand is driven by the need for more effective and stable biological therapies, the increasing prevalence of chronic diseases, and the potential for improved patient outcomes and healthcare cost reduction. As research in this area progresses, we can expect to see a surge in innovative products and applications, further expanding the market for stabilized cytokine treatments.
Isotonic solutions have emerged as a potential stabilizer for cytokine treatments, addressing the critical need for improved shelf-life and efficacy. This development has sparked considerable interest from pharmaceutical companies and healthcare providers, as it could potentially revolutionize the delivery and storage of cytokine-based therapies.
The global cytokine market is projected to experience substantial growth in the coming years. Factors contributing to this growth include the rising incidence of inflammatory diseases, advancements in biotechnology, and increased research and development activities in the field of immunotherapy. The stabilization of cytokine treatments using isotonic solutions is expected to further accelerate market expansion by overcoming existing limitations in product stability and delivery.
Healthcare providers and patients alike are seeking more effective and convenient treatment options, particularly for chronic conditions that require long-term management. Stabilized cytokine treatments offer the potential for improved patient outcomes, reduced treatment frequency, and enhanced quality of life. This aligns with the growing trend towards personalized medicine and targeted therapies, driving demand for innovative cytokine-based products.
The biotechnology and pharmaceutical industries are investing heavily in research and development efforts focused on cytokine stabilization techniques. This investment reflects the recognition of the significant market potential for stable cytokine treatments across various therapeutic areas, including oncology, autoimmune disorders, and infectious diseases.
Regulatory agencies are also showing increased interest in novel stabilization methods for biological products, including cytokine treatments. This regulatory focus is likely to facilitate the development and approval of new stabilized cytokine therapies, further stimulating market growth and innovation in this field.
As healthcare systems worldwide strive to reduce costs and improve treatment outcomes, the demand for more stable and effective cytokine treatments is expected to rise. Isotonic solution-stabilized cytokines have the potential to reduce waste, improve supply chain efficiency, and ultimately lower healthcare costs, making them an attractive option for healthcare providers and payers.
In conclusion, the market demand for stabilized cytokine treatments using isotonic solutions is poised for significant growth. This demand is driven by the need for more effective and stable biological therapies, the increasing prevalence of chronic diseases, and the potential for improved patient outcomes and healthcare cost reduction. As research in this area progresses, we can expect to see a surge in innovative products and applications, further expanding the market for stabilized cytokine treatments.
Isotonic Solution Challenges
Isotonic solutions play a crucial role in stabilizing cytokine treatments, yet they face several challenges that need to be addressed for optimal performance. One of the primary issues is maintaining the delicate balance of osmotic pressure between the solution and cellular environment. Even slight deviations from isotonicity can lead to cell damage or altered cytokine activity, compromising the effectiveness of the treatment.
Another significant challenge lies in the formulation of isotonic solutions that can accommodate a wide range of cytokines with diverse physicochemical properties. Different cytokines may require specific pH ranges, ionic strengths, or additional stabilizing agents, making it difficult to develop a universal isotonic solution suitable for all cytokine treatments.
The stability of cytokines within isotonic solutions over extended periods poses another hurdle. Many cytokines are prone to degradation, aggregation, or loss of biological activity during storage or administration. Developing isotonic solutions that can maintain cytokine stability without compromising their therapeutic efficacy remains a complex task.
Furthermore, the interaction between isotonic solutions and various materials used in storage containers, delivery devices, or administration systems presents additional challenges. Adsorption of cytokines to surfaces, leaching of materials into the solution, or chemical reactions between solution components and container materials can all impact the stability and efficacy of cytokine treatments.
The scalability of isotonic solution production for cytokine treatments is another critical challenge. Ensuring consistent quality, sterility, and stability across large-scale manufacturing processes while maintaining cost-effectiveness is essential for widespread clinical application.
Regulatory compliance and quality control present additional hurdles in the development and use of isotonic solutions for cytokine treatments. Meeting stringent regulatory requirements for pharmaceutical-grade products, including stability testing, sterility assurance, and documentation of manufacturing processes, adds complexity to the development pipeline.
Lastly, the potential for immunogenicity or adverse reactions to components of isotonic solutions must be carefully considered. Even seemingly inert excipients or stabilizing agents may trigger immune responses in some patients, necessitating thorough safety evaluations and potentially limiting the use of certain formulations.
Addressing these challenges requires a multidisciplinary approach, combining expertise in biochemistry, formulation science, materials engineering, and clinical pharmacology. Ongoing research and development efforts are focused on innovative strategies to overcome these obstacles and improve the stability and efficacy of cytokine treatments using advanced isotonic solution technologies.
Another significant challenge lies in the formulation of isotonic solutions that can accommodate a wide range of cytokines with diverse physicochemical properties. Different cytokines may require specific pH ranges, ionic strengths, or additional stabilizing agents, making it difficult to develop a universal isotonic solution suitable for all cytokine treatments.
The stability of cytokines within isotonic solutions over extended periods poses another hurdle. Many cytokines are prone to degradation, aggregation, or loss of biological activity during storage or administration. Developing isotonic solutions that can maintain cytokine stability without compromising their therapeutic efficacy remains a complex task.
Furthermore, the interaction between isotonic solutions and various materials used in storage containers, delivery devices, or administration systems presents additional challenges. Adsorption of cytokines to surfaces, leaching of materials into the solution, or chemical reactions between solution components and container materials can all impact the stability and efficacy of cytokine treatments.
The scalability of isotonic solution production for cytokine treatments is another critical challenge. Ensuring consistent quality, sterility, and stability across large-scale manufacturing processes while maintaining cost-effectiveness is essential for widespread clinical application.
Regulatory compliance and quality control present additional hurdles in the development and use of isotonic solutions for cytokine treatments. Meeting stringent regulatory requirements for pharmaceutical-grade products, including stability testing, sterility assurance, and documentation of manufacturing processes, adds complexity to the development pipeline.
Lastly, the potential for immunogenicity or adverse reactions to components of isotonic solutions must be carefully considered. Even seemingly inert excipients or stabilizing agents may trigger immune responses in some patients, necessitating thorough safety evaluations and potentially limiting the use of certain formulations.
Addressing these challenges requires a multidisciplinary approach, combining expertise in biochemistry, formulation science, materials engineering, and clinical pharmacology. Ongoing research and development efforts are focused on innovative strategies to overcome these obstacles and improve the stability and efficacy of cytokine treatments using advanced isotonic solution technologies.
Current Stabilization Methods
01 Stabilization of isotonic solutions using buffer systems
Buffer systems are employed to maintain the pH and stability of isotonic solutions. These systems help prevent degradation of active ingredients and ensure the solution remains isotonic over time. Common buffer components include phosphates, citrates, and acetates, which work together to resist changes in pH and maintain the solution's stability.- Stabilization of isotonic solutions using buffer systems: Buffer systems are employed to maintain the pH and stability of isotonic solutions. These systems help prevent degradation of active ingredients and ensure the solution remains isotonic over time. Common buffer components include phosphates, citrates, and acetates, which work together to resist changes in pH and maintain the osmotic balance of the solution.
- Antioxidants for enhancing stability of isotonic solutions: Antioxidants are added to isotonic solutions to prevent oxidation and degradation of sensitive components. These additives help maintain the stability and efficacy of the solution during storage and use. Common antioxidants used include ascorbic acid, tocopherols, and butylated hydroxyanisole (BHA), which scavenge free radicals and protect the solution from oxidative stress.
- Temperature control for isotonic solution stability: Proper temperature control during manufacturing, storage, and transportation is crucial for maintaining the stability of isotonic solutions. Specific temperature ranges are established to prevent degradation of active ingredients and maintain the solution's isotonicity. Temperature-controlled environments and specialized packaging are used to ensure the solution remains stable throughout its shelf life.
- Packaging innovations for improved isotonic solution stability: Advanced packaging technologies are developed to enhance the stability of isotonic solutions. These innovations include barrier materials, modified atmosphere packaging, and multi-layer containers that protect the solution from light, oxygen, and moisture. Specialized closures and sealing methods are also employed to maintain the integrity of the package and prevent contamination.
- Stabilizing agents for preserving isotonic solution integrity: Various stabilizing agents are incorporated into isotonic solutions to maintain their physical and chemical properties. These agents help prevent precipitation, aggregation, and degradation of active ingredients. Common stabilizers include cyclodextrins, surfactants, and chelating agents, which work synergistically to enhance the overall stability of the solution and extend its shelf life.
02 Antioxidants for enhancing stability of isotonic solutions
Antioxidants are added to isotonic solutions to prevent oxidation and degradation of sensitive components. These additives help maintain the stability and efficacy of the solution by neutralizing free radicals and inhibiting oxidative processes. Common antioxidants used include ascorbic acid, tocopherols, and butylated hydroxyanisole (BHA).Expand Specific Solutions03 Temperature control for isotonic solution stability
Proper temperature control during manufacturing, storage, and transportation is crucial for maintaining the stability of isotonic solutions. Specific temperature ranges are established to prevent degradation of active ingredients and maintain the solution's isotonicity. Cold chain management and temperature-controlled packaging are often employed to ensure stability throughout the product lifecycle.Expand Specific Solutions04 Packaging innovations for improved stability
Advanced packaging technologies are developed to enhance the stability of isotonic solutions. These innovations include barrier materials to prevent moisture and oxygen permeation, light-protective containers to shield from UV radiation, and single-dose packaging to maintain sterility and prevent contamination. Such packaging solutions help extend the shelf life and maintain the efficacy of isotonic formulations.Expand Specific Solutions05 Stabilizing agents for complex isotonic formulations
Specific stabilizing agents are incorporated into complex isotonic formulations to maintain their stability. These agents can include chelating compounds, surfactants, and polymers that help prevent interactions between ingredients, reduce particle aggregation, and maintain the homogeneity of the solution. The choice of stabilizing agents depends on the specific composition and intended use of the isotonic solution.Expand Specific Solutions
Key Industry Players
The market for isotonic solutions in cytokine treatments is in a growth phase, driven by increasing demand for advanced biopharmaceutical therapies. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with companies like Bayer AG, Boehringer Ingelheim, and Amgen leading innovation. These firms are investing heavily in R&D to improve the stability and efficacy of cytokine treatments using isotonic solutions. Emerging players such as Xeris Pharmaceuticals are also making strides with novel formulation technologies. The competitive landscape is characterized by a mix of established pharmaceutical giants and specialized biotech firms, each striving to develop more effective and stable cytokine treatments.
Bayer AG
Technical Solution: Bayer has developed an innovative isotonic solution for cytokine stabilization using a combination of trehalose and recombinant human albumin. This formulation provides a protective matrix that prevents cytokine aggregation and maintains their native conformation. Bayer's research has demonstrated that their isotonic solution can preserve cytokine activity for up to 36 months when stored at 2-8°C[5]. The company has also implemented a lyophilization process that allows for the production of stable, dry cytokine formulations that can be easily reconstituted with their isotonic diluent. This approach has been particularly successful in stabilizing growth factors and colony-stimulating factors[6].
Strengths: Long-term stability, versatility in both liquid and lyophilized forms, and compatibility with a wide range of cytokines. Weaknesses: Potential allergenicity concerns due to the use of human albumin, which may limit application in certain patient populations.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim has developed a proprietary isotonic formulation for cytokine stabilization using a combination of non-reducing sugars and amino acids. Their approach focuses on creating a stabilizing environment that minimizes chemical degradation and physical instability of cytokines. The company's research has shown that their isotonic solutions can maintain cytokine stability for up to 24 months at 2-8°C and up to 6 months at room temperature[7]. Boehringer Ingelheim has also incorporated polysorbate surfactants into their formulations to prevent adsorption of cytokines to container surfaces, further enhancing stability during storage and administration[8].
Strengths: Dual temperature stability options, prevention of surface adsorption, and minimized chemical degradation. Weaknesses: Potential for increased viscosity in some formulations, which may affect ease of administration.
Isotonic Solution Innovations
Stable sustained release therapeutic compositions in aprotic polar solvents and methods of manufacturing the same
PatentPendingUS20240238381A1
Innovation
- Development of a storage stable sustained release (SR) glucagon formulation using an aprotic polar solvent system with a divalent zinc-containing compound as a sustained release modifier, stabilizing glucagon in solution to create a depot that gradually releases glucagon upon administration, maintaining stability and solubility.
Protein formulation, preparation method therefor and use thereof
PatentPendingUS20250108091A1
Innovation
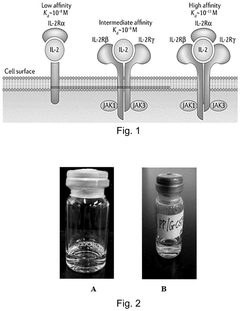
- A novel formulation comprising cytokine proteins, polyethylene glycol-distearoyl phosphatidylethanolamine (PEG-DSPE), and a pH regulator, which adjusts the pH to 5.5-6.0, enhances solubility and stability, and selectively binds to high-affinity receptors, reducing low-affinity interactions.
Regulatory Considerations
The regulatory landscape for cytokine treatments stabilized with isotonic solutions is complex and multifaceted. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established stringent guidelines for the development, manufacturing, and marketing of these biopharmaceutical products. These regulations aim to ensure the safety, efficacy, and quality of cytokine treatments throughout their lifecycle.
One of the primary regulatory considerations is the classification of cytokine treatments. Depending on their intended use and mechanism of action, they may be categorized as biologics, drugs, or combination products. This classification determines the specific regulatory pathway and requirements for approval. For instance, biologics typically follow the Biologics License Application (BLA) process in the US, while drugs may require a New Drug Application (NDA).
The use of isotonic solutions as stabilizers introduces additional regulatory complexities. Manufacturers must demonstrate that these solutions effectively maintain the stability and potency of cytokines without introducing new safety risks. This requires comprehensive stability studies, including accelerated and long-term testing under various environmental conditions. Regulatory agencies expect detailed data on the physicochemical properties, biological activity, and degradation profiles of cytokine products in isotonic solutions.
Quality control and assurance measures are crucial regulatory considerations. Manufacturers must implement robust quality management systems that comply with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP). These systems should include validated analytical methods for assessing the stability, purity, and potency of cytokine treatments in isotonic solutions. Regulatory bodies may conduct inspections to ensure compliance with these standards.
The selection and sourcing of isotonic solution components also fall under regulatory scrutiny. Manufacturers must provide detailed information on the composition, purity, and sourcing of these materials. Any changes in the formulation or manufacturing process, including modifications to the isotonic solution, may require regulatory approval through supplement submissions or comparability studies.
Pharmacovigilance and post-market surveillance are ongoing regulatory requirements for cytokine treatments. Manufacturers must have systems in place to monitor and report adverse events, particularly those that may be related to the use of isotonic solutions as stabilizers. This includes conducting post-marketing studies to gather long-term safety and efficacy data in real-world settings.
Lastly, international harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), play a significant role in shaping regulatory considerations. These initiatives aim to standardize requirements across different regions, potentially streamlining the global development and approval process for cytokine treatments stabilized with isotonic solutions.
One of the primary regulatory considerations is the classification of cytokine treatments. Depending on their intended use and mechanism of action, they may be categorized as biologics, drugs, or combination products. This classification determines the specific regulatory pathway and requirements for approval. For instance, biologics typically follow the Biologics License Application (BLA) process in the US, while drugs may require a New Drug Application (NDA).
The use of isotonic solutions as stabilizers introduces additional regulatory complexities. Manufacturers must demonstrate that these solutions effectively maintain the stability and potency of cytokines without introducing new safety risks. This requires comprehensive stability studies, including accelerated and long-term testing under various environmental conditions. Regulatory agencies expect detailed data on the physicochemical properties, biological activity, and degradation profiles of cytokine products in isotonic solutions.
Quality control and assurance measures are crucial regulatory considerations. Manufacturers must implement robust quality management systems that comply with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP). These systems should include validated analytical methods for assessing the stability, purity, and potency of cytokine treatments in isotonic solutions. Regulatory bodies may conduct inspections to ensure compliance with these standards.
The selection and sourcing of isotonic solution components also fall under regulatory scrutiny. Manufacturers must provide detailed information on the composition, purity, and sourcing of these materials. Any changes in the formulation or manufacturing process, including modifications to the isotonic solution, may require regulatory approval through supplement submissions or comparability studies.
Pharmacovigilance and post-market surveillance are ongoing regulatory requirements for cytokine treatments. Manufacturers must have systems in place to monitor and report adverse events, particularly those that may be related to the use of isotonic solutions as stabilizers. This includes conducting post-marketing studies to gather long-term safety and efficacy data in real-world settings.
Lastly, international harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), play a significant role in shaping regulatory considerations. These initiatives aim to standardize requirements across different regions, potentially streamlining the global development and approval process for cytokine treatments stabilized with isotonic solutions.
Biocompatibility Assessment
Biocompatibility assessment is a critical aspect of developing isotonic solutions for cytokine stabilization. This evaluation ensures that the formulated solutions do not elicit adverse reactions when introduced into biological systems, particularly in the context of therapeutic applications.
The primary focus of biocompatibility assessment for isotonic cytokine solutions is to examine their interaction with various cellular components and physiological systems. This includes evaluating potential cytotoxicity, immunogenicity, and inflammatory responses. In vitro studies using relevant cell lines are typically the first step in this assessment, providing insights into the direct effects of the solution on cell viability and function.
Hemocompatibility testing is another crucial component, given that many cytokine treatments are administered intravenously. This involves assessing the solution's impact on blood components, including red blood cells, white blood cells, and platelets. Hemolysis assays and platelet activation tests are commonly employed to evaluate potential adverse effects on blood constituents.
Furthermore, the biocompatibility assessment extends to examining the solution's impact on protein stability and functionality. This is particularly important for cytokine treatments, as the therapeutic efficacy relies on maintaining the structural integrity and biological activity of these proteins. Techniques such as circular dichroism spectroscopy and enzyme-linked immunosorbent assays (ELISA) are utilized to assess protein conformation and activity in the presence of the isotonic solution.
Long-term stability studies are also conducted to ensure that the biocompatibility of the isotonic solution remains consistent over time. This involves subjecting the formulation to various environmental conditions, such as temperature fluctuations and light exposure, and subsequently evaluating its biocompatibility profile.
In vivo studies, typically conducted in animal models, provide a more comprehensive assessment of the solution's biocompatibility in a complex biological system. These studies evaluate systemic responses, tissue distribution, and potential organ-specific effects. Histopathological examinations and biomarker analyses are often employed to detect any subtle changes in tissue architecture or physiological parameters.
Regulatory considerations play a significant role in shaping the biocompatibility assessment process. Adherence to guidelines set forth by regulatory bodies, such as the FDA and EMA, is essential for ensuring the safety and efficacy of isotonic solutions used in cytokine treatments. These guidelines often dictate specific testing protocols and acceptance criteria for biocompatibility assessments.
The primary focus of biocompatibility assessment for isotonic cytokine solutions is to examine their interaction with various cellular components and physiological systems. This includes evaluating potential cytotoxicity, immunogenicity, and inflammatory responses. In vitro studies using relevant cell lines are typically the first step in this assessment, providing insights into the direct effects of the solution on cell viability and function.
Hemocompatibility testing is another crucial component, given that many cytokine treatments are administered intravenously. This involves assessing the solution's impact on blood components, including red blood cells, white blood cells, and platelets. Hemolysis assays and platelet activation tests are commonly employed to evaluate potential adverse effects on blood constituents.
Furthermore, the biocompatibility assessment extends to examining the solution's impact on protein stability and functionality. This is particularly important for cytokine treatments, as the therapeutic efficacy relies on maintaining the structural integrity and biological activity of these proteins. Techniques such as circular dichroism spectroscopy and enzyme-linked immunosorbent assays (ELISA) are utilized to assess protein conformation and activity in the presence of the isotonic solution.
Long-term stability studies are also conducted to ensure that the biocompatibility of the isotonic solution remains consistent over time. This involves subjecting the formulation to various environmental conditions, such as temperature fluctuations and light exposure, and subsequently evaluating its biocompatibility profile.
In vivo studies, typically conducted in animal models, provide a more comprehensive assessment of the solution's biocompatibility in a complex biological system. These studies evaluate systemic responses, tissue distribution, and potential organ-specific effects. Histopathological examinations and biomarker analyses are often employed to detect any subtle changes in tissue architecture or physiological parameters.
Regulatory considerations play a significant role in shaping the biocompatibility assessment process. Adherence to guidelines set forth by regulatory bodies, such as the FDA and EMA, is essential for ensuring the safety and efficacy of isotonic solutions used in cytokine treatments. These guidelines often dictate specific testing protocols and acceptance criteria for biocompatibility assessments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!