The effect of ionic variation in isotonic solutions on pharmacokinetics
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Variation Background and Objectives
Ionic variation in isotonic solutions plays a crucial role in pharmacokinetics, the study of how drugs move through the body. This field has evolved significantly over the past decades, driven by the need to optimize drug delivery and efficacy. The interplay between ionic composition and drug behavior has become a focal point for researchers and pharmaceutical companies alike.
The historical context of this research dates back to the early 20th century when scientists first began to understand the importance of electrolyte balance in biological systems. As our knowledge of cellular physiology advanced, so did our appreciation for the impact of ionic environments on drug absorption, distribution, metabolism, and excretion.
Recent technological advancements have allowed for more precise manipulation and measurement of ionic variations, leading to a renaissance in pharmacokinetic research. This has opened new avenues for tailoring drug formulations to specific ionic environments, potentially enhancing therapeutic outcomes across a wide range of medical conditions.
The primary objective of current research in this field is to elucidate the mechanisms by which ionic variations affect drug behavior at the molecular level. This includes understanding how changes in ion concentrations can alter drug solubility, membrane permeability, protein binding, and enzymatic activity. By gaining insights into these processes, researchers aim to develop more effective drug delivery systems and improve treatment strategies.
Another key goal is to establish predictive models that can accurately forecast how changes in ionic composition will impact pharmacokinetic parameters. Such models would be invaluable in drug development, allowing for more efficient screening of potential compounds and optimization of formulations before costly clinical trials.
Furthermore, there is a growing interest in exploring the potential of ionic variation as a tool for targeted drug delivery. By manipulating the ionic environment in specific tissues or organs, it may be possible to enhance drug accumulation in desired areas while minimizing systemic exposure and side effects.
The field also aims to address the challenges posed by individual variability in ionic composition, which can significantly affect drug response. This includes investigating how factors such as diet, hydration status, and certain medical conditions can alter the ionic milieu and, consequently, drug pharmacokinetics.
As we look to the future, the ultimate objective is to leverage our understanding of ionic variation to develop personalized medicine approaches. This could lead to tailored drug regimens that account for an individual's unique ionic profile, potentially revolutionizing treatment outcomes across various therapeutic areas.
The historical context of this research dates back to the early 20th century when scientists first began to understand the importance of electrolyte balance in biological systems. As our knowledge of cellular physiology advanced, so did our appreciation for the impact of ionic environments on drug absorption, distribution, metabolism, and excretion.
Recent technological advancements have allowed for more precise manipulation and measurement of ionic variations, leading to a renaissance in pharmacokinetic research. This has opened new avenues for tailoring drug formulations to specific ionic environments, potentially enhancing therapeutic outcomes across a wide range of medical conditions.
The primary objective of current research in this field is to elucidate the mechanisms by which ionic variations affect drug behavior at the molecular level. This includes understanding how changes in ion concentrations can alter drug solubility, membrane permeability, protein binding, and enzymatic activity. By gaining insights into these processes, researchers aim to develop more effective drug delivery systems and improve treatment strategies.
Another key goal is to establish predictive models that can accurately forecast how changes in ionic composition will impact pharmacokinetic parameters. Such models would be invaluable in drug development, allowing for more efficient screening of potential compounds and optimization of formulations before costly clinical trials.
Furthermore, there is a growing interest in exploring the potential of ionic variation as a tool for targeted drug delivery. By manipulating the ionic environment in specific tissues or organs, it may be possible to enhance drug accumulation in desired areas while minimizing systemic exposure and side effects.
The field also aims to address the challenges posed by individual variability in ionic composition, which can significantly affect drug response. This includes investigating how factors such as diet, hydration status, and certain medical conditions can alter the ionic milieu and, consequently, drug pharmacokinetics.
As we look to the future, the ultimate objective is to leverage our understanding of ionic variation to develop personalized medicine approaches. This could lead to tailored drug regimens that account for an individual's unique ionic profile, potentially revolutionizing treatment outcomes across various therapeutic areas.
Market Analysis for Isotonic Solutions
The market for isotonic solutions has experienced significant growth in recent years, driven by increasing demand in healthcare, sports nutrition, and pharmaceutical industries. These solutions, designed to maintain osmotic balance with bodily fluids, play a crucial role in various medical applications and are gaining popularity among athletes and fitness enthusiasts.
In the healthcare sector, isotonic solutions are widely used for intravenous fluid replacement, wound cleansing, and as a base for medication delivery. The aging population and rising prevalence of chronic diseases have contributed to the expanding market for these products. Hospitals, clinics, and home healthcare settings represent major consumers of isotonic solutions, with a steady increase in demand projected over the coming years.
The sports nutrition segment has emerged as a rapidly growing market for isotonic solutions. Athletes and active individuals are increasingly recognizing the importance of proper hydration and electrolyte balance for optimal performance and recovery. This has led to a surge in the development and consumption of isotonic sports drinks and supplements, with major beverage companies and specialized nutrition brands competing for market share.
In the pharmaceutical industry, isotonic solutions serve as essential components in drug formulation and delivery systems. The growing focus on personalized medicine and targeted drug delivery has further amplified the need for precisely formulated isotonic solutions. This trend is expected to continue as pharmaceutical companies invest in research and development of novel drug delivery methods.
Geographically, North America and Europe currently dominate the isotonic solutions market, owing to advanced healthcare infrastructure and high consumer awareness. However, the Asia-Pacific region is anticipated to witness the fastest growth rate in the coming years, fueled by improving healthcare access, rising disposable incomes, and increasing health consciousness among consumers.
The market is characterized by the presence of both established pharmaceutical companies and specialized manufacturers. Key players are investing in research and development to enhance product efficacy and expand their product portfolios. Innovation in packaging and delivery systems, such as ready-to-use bags and pre-filled syringes, is also driving market growth by improving convenience and reducing the risk of contamination.
As the understanding of ionic variation's impact on pharmacokinetics deepens, there is a growing demand for customized isotonic solutions tailored to specific medical conditions and patient needs. This trend presents both opportunities and challenges for market players, requiring continuous innovation and adaptation to evolving scientific knowledge and regulatory requirements.
In the healthcare sector, isotonic solutions are widely used for intravenous fluid replacement, wound cleansing, and as a base for medication delivery. The aging population and rising prevalence of chronic diseases have contributed to the expanding market for these products. Hospitals, clinics, and home healthcare settings represent major consumers of isotonic solutions, with a steady increase in demand projected over the coming years.
The sports nutrition segment has emerged as a rapidly growing market for isotonic solutions. Athletes and active individuals are increasingly recognizing the importance of proper hydration and electrolyte balance for optimal performance and recovery. This has led to a surge in the development and consumption of isotonic sports drinks and supplements, with major beverage companies and specialized nutrition brands competing for market share.
In the pharmaceutical industry, isotonic solutions serve as essential components in drug formulation and delivery systems. The growing focus on personalized medicine and targeted drug delivery has further amplified the need for precisely formulated isotonic solutions. This trend is expected to continue as pharmaceutical companies invest in research and development of novel drug delivery methods.
Geographically, North America and Europe currently dominate the isotonic solutions market, owing to advanced healthcare infrastructure and high consumer awareness. However, the Asia-Pacific region is anticipated to witness the fastest growth rate in the coming years, fueled by improving healthcare access, rising disposable incomes, and increasing health consciousness among consumers.
The market is characterized by the presence of both established pharmaceutical companies and specialized manufacturers. Key players are investing in research and development to enhance product efficacy and expand their product portfolios. Innovation in packaging and delivery systems, such as ready-to-use bags and pre-filled syringes, is also driving market growth by improving convenience and reducing the risk of contamination.
As the understanding of ionic variation's impact on pharmacokinetics deepens, there is a growing demand for customized isotonic solutions tailored to specific medical conditions and patient needs. This trend presents both opportunities and challenges for market players, requiring continuous innovation and adaptation to evolving scientific knowledge and regulatory requirements.
Current Challenges in Pharmacokinetics
Pharmacokinetics, the study of drug absorption, distribution, metabolism, and excretion, faces several significant challenges in the current scientific landscape. One of the primary issues is the complexity of biological systems and their interactions with drugs. The human body's intricate network of organs, tissues, and cellular processes makes it difficult to accurately predict and model drug behavior across diverse patient populations.
The variability in drug response among individuals poses another major challenge. Factors such as genetics, age, sex, diet, and overall health status can significantly influence how a drug is processed in the body. This inter-individual variability complicates the development of standardized dosing regimens and necessitates personalized medicine approaches, which are still in their infancy.
Another pressing challenge is the limited understanding of drug-drug interactions. As polypharmacy becomes increasingly common, especially in aging populations, the potential for adverse interactions between multiple medications grows exponentially. Predicting and managing these interactions remains a complex task, often requiring extensive clinical studies and post-marketing surveillance.
The blood-brain barrier presents a unique obstacle in pharmacokinetics, particularly for drugs targeting neurological disorders. Developing compounds that can effectively cross this barrier while maintaining their therapeutic properties is an ongoing challenge that demands innovative drug delivery strategies and formulation techniques.
The emergence of novel drug delivery systems, such as nanoparticles and targeted therapies, introduces new complexities in pharmacokinetic modeling. Traditional pharmacokinetic models may not adequately capture the behavior of these advanced formulations, necessitating the development of new mathematical and computational approaches.
Environmental factors and their impact on drug disposition represent another area of concern. Pollution, climate change, and exposure to various chemicals in daily life can potentially alter drug metabolism and efficacy. Understanding and accounting for these environmental influences on pharmacokinetics is becoming increasingly important.
Lastly, the challenge of translating in vitro and animal study results to human pharmacokinetics remains significant. Despite advances in in silico modeling and organoid technologies, accurately predicting human drug behavior based on preclinical data continues to be a major hurdle in drug development, often leading to costly failures in clinical trials.
The variability in drug response among individuals poses another major challenge. Factors such as genetics, age, sex, diet, and overall health status can significantly influence how a drug is processed in the body. This inter-individual variability complicates the development of standardized dosing regimens and necessitates personalized medicine approaches, which are still in their infancy.
Another pressing challenge is the limited understanding of drug-drug interactions. As polypharmacy becomes increasingly common, especially in aging populations, the potential for adverse interactions between multiple medications grows exponentially. Predicting and managing these interactions remains a complex task, often requiring extensive clinical studies and post-marketing surveillance.
The blood-brain barrier presents a unique obstacle in pharmacokinetics, particularly for drugs targeting neurological disorders. Developing compounds that can effectively cross this barrier while maintaining their therapeutic properties is an ongoing challenge that demands innovative drug delivery strategies and formulation techniques.
The emergence of novel drug delivery systems, such as nanoparticles and targeted therapies, introduces new complexities in pharmacokinetic modeling. Traditional pharmacokinetic models may not adequately capture the behavior of these advanced formulations, necessitating the development of new mathematical and computational approaches.
Environmental factors and their impact on drug disposition represent another area of concern. Pollution, climate change, and exposure to various chemicals in daily life can potentially alter drug metabolism and efficacy. Understanding and accounting for these environmental influences on pharmacokinetics is becoming increasingly important.
Lastly, the challenge of translating in vitro and animal study results to human pharmacokinetics remains significant. Despite advances in in silico modeling and organoid technologies, accurately predicting human drug behavior based on preclinical data continues to be a major hurdle in drug development, often leading to costly failures in clinical trials.
Existing Pharmacokinetic Models
01 Formulation of isotonic solutions for improved pharmacokinetics
Isotonic solutions are formulated to match the osmotic pressure of body fluids, enhancing drug absorption and distribution. These solutions can improve the pharmacokinetics of various drugs by maintaining physiological balance and reducing irritation at the site of administration.- Formulation of isotonic solutions for improved pharmacokinetics: Isotonic solutions are formulated to match the osmotic pressure of body fluids, enhancing drug absorption and distribution. These solutions can improve the pharmacokinetics of various drugs by maintaining physiological balance and reducing irritation at the site of administration.
- Use of isotonic solutions in ocular drug delivery: Isotonic solutions are particularly beneficial in ocular drug delivery systems. They help maintain the eye's natural environment, improving drug absorption and reducing discomfort. This application can lead to enhanced pharmacokinetics for ophthalmic medications.
- Isotonic solutions for parenteral administration: Parenteral administration of drugs using isotonic solutions can significantly impact pharmacokinetics. These solutions ensure proper drug distribution and absorption in the bloodstream, minimizing adverse effects and improving overall therapeutic efficacy.
- Development of novel isotonic formulations: Research focuses on developing new isotonic formulations to enhance drug pharmacokinetics. These may include the use of novel excipients, buffer systems, or combination of ingredients to optimize drug delivery and absorption while maintaining isotonicity.
- Isotonic solutions in controlled release systems: Incorporating isotonic solutions into controlled release drug delivery systems can modulate pharmacokinetics. This approach allows for sustained drug release while maintaining physiological compatibility, potentially improving bioavailability and reducing dosing frequency.
02 Isotonic solutions for ocular drug delivery
Isotonic solutions are particularly beneficial for ocular drug delivery, as they minimize discomfort and irritation to the eye. These formulations can enhance the pharmacokinetics of ophthalmic drugs by improving retention time and absorption through the cornea and conjunctiva.Expand Specific Solutions03 Parenteral isotonic solutions and their pharmacokinetic properties
Parenteral isotonic solutions are designed for intravenous or intramuscular administration, ensuring optimal drug distribution and absorption. These formulations can significantly impact the pharmacokinetics of drugs by maintaining proper osmolarity and pH, leading to improved bioavailability and therapeutic efficacy.Expand Specific Solutions04 Isotonic solutions for controlled release and sustained pharmacokinetics
Isotonic solutions can be formulated with polymers or other excipients to achieve controlled release of drugs, modifying their pharmacokinetic profiles. This approach can lead to sustained drug levels in the body, reducing dosing frequency and improving patient compliance.Expand Specific Solutions05 Influence of isotonicity on drug stability and pharmacokinetics
The isotonicity of a solution can affect drug stability and, consequently, its pharmacokinetics. Properly formulated isotonic solutions can enhance the shelf-life of drugs, maintain their chemical integrity, and ensure consistent pharmacokinetic behavior throughout the product's lifespan.Expand Specific Solutions
Key Players in Pharmaceutical Industry
The competitive landscape for research on ionic variation effects in isotonic solutions on pharmacokinetics is characterized by a mature market with established players and ongoing innovation. The field is in a growth phase, driven by increasing demand for improved drug delivery and efficacy. Major pharmaceutical companies like Merck, Bayer, AbbVie, and Novartis are actively involved, leveraging their extensive R&D capabilities. Smaller biotech firms such as Ryvu Therapeutics and Fochon Biosciences are also contributing to advancements. The technology's maturity is moderate, with continuous refinements in methodology and applications across various therapeutic areas. Collaborations between industry and academic institutions like Monash University and Queen's University Belfast are accelerating progress in this complex interdisciplinary field.
Bayer AG
Technical Solution: Bayer AG has developed a novel approach to study the effect of ionic variation in isotonic solutions on pharmacokinetics. Their method involves using advanced microfluidic devices to precisely control and manipulate ionic concentrations in real-time while monitoring drug absorption and distribution[1]. This technology allows for high-throughput screening of various ionic compositions and their impact on drug bioavailability. Additionally, Bayer has implemented machine learning algorithms to predict pharmacokinetic profiles based on ionic variations, enabling faster optimization of drug formulations[3]. The company has also invested in developing specialized in vitro models that closely mimic physiological conditions, including ion channels and transporters, to better understand the interplay between ionic environment and drug pharmacokinetics[5].
Strengths: Cutting-edge microfluidic technology, AI-driven predictive modeling, and advanced in vitro systems. Weaknesses: High cost of implementation and potential limitations in translating in vitro findings to in vivo scenarios.
AbbVie, Inc.
Technical Solution: AbbVie has focused on developing a comprehensive platform to investigate the impact of ionic variation on drug pharmacokinetics. Their approach combines high-resolution mass spectrometry with advanced bioinformatics to track minute changes in drug metabolism and distribution as a function of ionic composition[2]. The company has also pioneered the use of 3D-printed microfluidic organs-on-chips that incorporate ion-selective membranes, allowing for precise control and measurement of ionic gradients across cellular barriers[4]. Furthermore, AbbVie has developed proprietary software that integrates physiologically-based pharmacokinetic (PBPK) modeling with ionic variation data, enabling more accurate predictions of drug behavior in diverse physiological conditions[6].
Strengths: Integration of cutting-edge analytical techniques, innovative microfluidic models, and sophisticated PBPK modeling. Weaknesses: Complexity of the system may lead to challenges in data interpretation and validation.
Innovative Approaches in Ionic Studies
Pharmaceutical composition comprising mineralocorticoid receptor antagonist and use thereof
PatentActiveUS11806344B2
Innovation
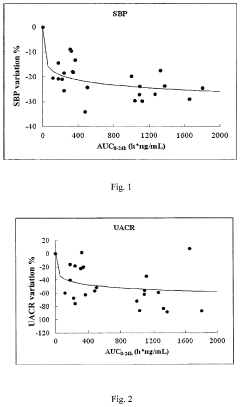
- A pharmaceutical composition containing Compound I, a non-steroid MRA, is developed with a bioavailability of 50% or more, achieved by reducing the particle size of Compound I to 25 μm or less and incorporating surfactants like benzalkonium chloride or sodium dodecyl sulfate, which allows for a safe and effective area under the plasma concentration-time curve (AUC) ranging from 188 ng*h/mL to 3173 ng*h/mL, thereby minimizing the risk of hyperkalemia and individual variation.
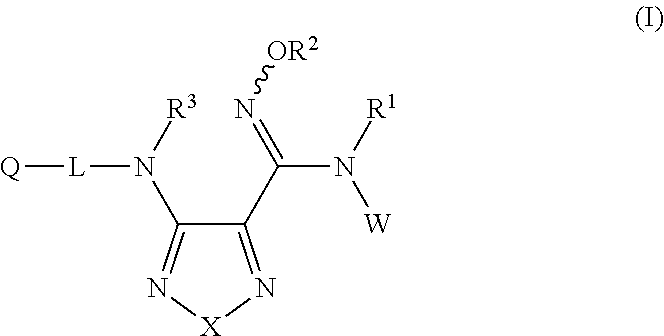
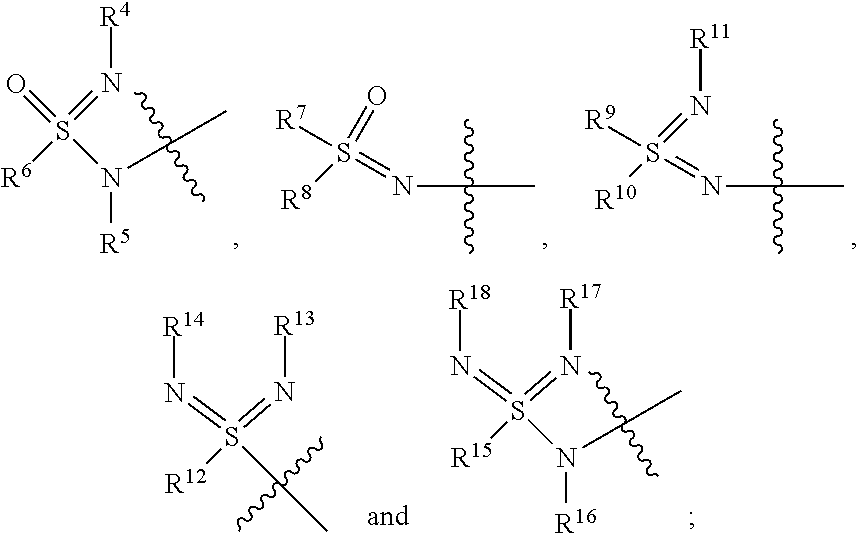
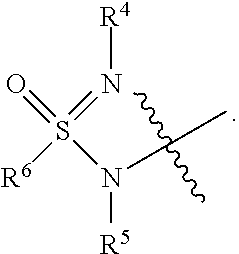
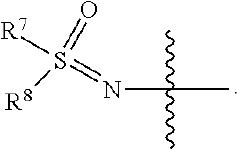
Sulfoximine, sulfonimidamide, sulfondiimine and diimidosulfonamide compounds as inhibitors of indoleamine 2,3-dioxygenase
PatentActiveUS20210070742A1
Innovation
- Development of novel sulfoximine, sulfonimidamide, and diimidosulfonamide compounds and their pharmaceutical compositions that inhibit indoleamine 2,3-dioxygenase (IDO) activity, offering enhanced properties over existing inhibitors.
Regulatory Considerations for Drug Development
The regulatory landscape for drug development is a critical consideration when studying the effect of ionic variation in isotonic solutions on pharmacokinetics. Regulatory bodies, such as the FDA and EMA, have established guidelines and requirements that must be adhered to throughout the drug development process.
One key aspect of regulatory compliance is the need for comprehensive documentation of all studies and experiments related to ionic variation and its impact on pharmacokinetics. This includes detailed protocols, raw data, and statistical analyses that demonstrate the reliability and reproducibility of results. Regulatory agencies will scrutinize this information to ensure the safety and efficacy of the drug under various ionic conditions.
Another important regulatory consideration is the selection of appropriate animal models and in vitro systems for studying the effects of ionic variation. These models must be validated and shown to be predictive of human pharmacokinetics. Regulatory bodies may require additional studies or justifications if the chosen models are not well-established or if there are significant interspecies differences in ion regulation.
The development of analytical methods for measuring drug concentrations and ionic compositions in biological fluids is also subject to regulatory oversight. These methods must be validated according to regulatory guidelines, demonstrating accuracy, precision, and robustness across the range of ionic conditions that may be encountered.
Regulatory agencies will also focus on the potential clinical implications of ionic variation on drug pharmacokinetics. This includes assessing the impact on drug absorption, distribution, metabolism, and excretion, as well as evaluating any potential drug-drug interactions that may be influenced by changes in ionic environment.
Safety considerations related to ionic variation must be thoroughly addressed to meet regulatory requirements. This includes evaluating the potential for adverse effects due to changes in drug exposure or altered pharmacodynamics under different ionic conditions. Risk mitigation strategies may need to be developed and incorporated into the drug's labeling and prescribing information.
Finally, regulatory bodies will expect manufacturers to implement appropriate quality control measures to ensure consistent ionic composition of drug formulations and delivery systems. This may involve setting specifications for ionic content and demonstrating batch-to-batch consistency in manufacturing processes.
One key aspect of regulatory compliance is the need for comprehensive documentation of all studies and experiments related to ionic variation and its impact on pharmacokinetics. This includes detailed protocols, raw data, and statistical analyses that demonstrate the reliability and reproducibility of results. Regulatory agencies will scrutinize this information to ensure the safety and efficacy of the drug under various ionic conditions.
Another important regulatory consideration is the selection of appropriate animal models and in vitro systems for studying the effects of ionic variation. These models must be validated and shown to be predictive of human pharmacokinetics. Regulatory bodies may require additional studies or justifications if the chosen models are not well-established or if there are significant interspecies differences in ion regulation.
The development of analytical methods for measuring drug concentrations and ionic compositions in biological fluids is also subject to regulatory oversight. These methods must be validated according to regulatory guidelines, demonstrating accuracy, precision, and robustness across the range of ionic conditions that may be encountered.
Regulatory agencies will also focus on the potential clinical implications of ionic variation on drug pharmacokinetics. This includes assessing the impact on drug absorption, distribution, metabolism, and excretion, as well as evaluating any potential drug-drug interactions that may be influenced by changes in ionic environment.
Safety considerations related to ionic variation must be thoroughly addressed to meet regulatory requirements. This includes evaluating the potential for adverse effects due to changes in drug exposure or altered pharmacodynamics under different ionic conditions. Risk mitigation strategies may need to be developed and incorporated into the drug's labeling and prescribing information.
Finally, regulatory bodies will expect manufacturers to implement appropriate quality control measures to ensure consistent ionic composition of drug formulations and delivery systems. This may involve setting specifications for ionic content and demonstrating batch-to-batch consistency in manufacturing processes.
Bioavailability and Drug Delivery Systems
Bioavailability and drug delivery systems play a crucial role in understanding the effect of ionic variation in isotonic solutions on pharmacokinetics. The bioavailability of a drug is significantly influenced by the ionic composition of the solution in which it is administered, affecting its absorption, distribution, and overall pharmacokinetic profile.
Isotonic solutions are designed to maintain osmotic balance with body fluids, but variations in their ionic composition can lead to changes in drug solubility, stability, and membrane permeability. These factors directly impact the bioavailability of the drug and its therapeutic efficacy. For instance, changes in pH or the presence of specific ions can alter the ionization state of a drug molecule, affecting its ability to cross biological membranes.
Drug delivery systems have been developed to optimize the bioavailability of pharmaceuticals in the presence of varying ionic environments. These systems can be tailored to respond to specific ionic conditions, ensuring controlled release and targeted delivery of the drug. For example, ion-exchange resins can be used to modulate drug release based on the ionic strength of the surrounding medium, providing a means to maintain consistent pharmacokinetics despite variations in the isotonic solution.
The development of novel drug delivery systems has focused on exploiting ionic interactions to enhance drug absorption and distribution. Nanocarriers and liposomal formulations can be designed with ionic-sensitive components that respond to changes in the local ionic environment, triggering drug release at specific sites or under particular conditions. This approach allows for more precise control over the pharmacokinetic profile of the drug, potentially improving its therapeutic index.
Furthermore, the impact of ionic variation on drug-protein binding must be considered when evaluating bioavailability. Changes in the ionic composition of isotonic solutions can affect the binding affinity of drugs to plasma proteins, influencing their free concentration and, consequently, their pharmacokinetic behavior. Advanced drug delivery systems can be engineered to account for these ionic effects, maintaining consistent drug-protein interactions across varying physiological conditions.
In conclusion, understanding the interplay between ionic variation in isotonic solutions, bioavailability, and drug delivery systems is essential for optimizing pharmacokinetics. This knowledge enables the development of more effective and robust pharmaceutical formulations that can maintain consistent therapeutic outcomes across diverse physiological environments.
Isotonic solutions are designed to maintain osmotic balance with body fluids, but variations in their ionic composition can lead to changes in drug solubility, stability, and membrane permeability. These factors directly impact the bioavailability of the drug and its therapeutic efficacy. For instance, changes in pH or the presence of specific ions can alter the ionization state of a drug molecule, affecting its ability to cross biological membranes.
Drug delivery systems have been developed to optimize the bioavailability of pharmaceuticals in the presence of varying ionic environments. These systems can be tailored to respond to specific ionic conditions, ensuring controlled release and targeted delivery of the drug. For example, ion-exchange resins can be used to modulate drug release based on the ionic strength of the surrounding medium, providing a means to maintain consistent pharmacokinetics despite variations in the isotonic solution.
The development of novel drug delivery systems has focused on exploiting ionic interactions to enhance drug absorption and distribution. Nanocarriers and liposomal formulations can be designed with ionic-sensitive components that respond to changes in the local ionic environment, triggering drug release at specific sites or under particular conditions. This approach allows for more precise control over the pharmacokinetic profile of the drug, potentially improving its therapeutic index.
Furthermore, the impact of ionic variation on drug-protein binding must be considered when evaluating bioavailability. Changes in the ionic composition of isotonic solutions can affect the binding affinity of drugs to plasma proteins, influencing their free concentration and, consequently, their pharmacokinetic behavior. Advanced drug delivery systems can be engineered to account for these ionic effects, maintaining consistent drug-protein interactions across varying physiological conditions.
In conclusion, understanding the interplay between ionic variation in isotonic solutions, bioavailability, and drug delivery systems is essential for optimizing pharmacokinetics. This knowledge enables the development of more effective and robust pharmaceutical formulations that can maintain consistent therapeutic outcomes across diverse physiological environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!