Isotonic solutions and their role in clinical diagnostics
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isotonic Solutions Background and Objectives
Isotonic solutions have played a pivotal role in clinical diagnostics and medical treatments for decades. These solutions, characterized by their osmotic pressure being equal to that of human blood, have become indispensable in various medical applications. The development of isotonic solutions can be traced back to the early 20th century when physiologists began to understand the importance of maintaining electrolyte balance in biological systems.
The primary objective of isotonic solutions in clinical diagnostics is to provide a medium that closely mimics the body's natural environment. This similarity allows for accurate testing and analysis of biological samples without altering their composition or cellular integrity. As diagnostic techniques have evolved, the demand for specialized isotonic solutions has grown, leading to continuous refinement and diversification of these products.
In the realm of clinical diagnostics, isotonic solutions serve multiple purposes. They are extensively used in hematology for blood cell counting and analysis, ensuring that cells maintain their shape and size during examination. In microbiology, these solutions are crucial for preparing and diluting samples, preserving microorganisms for further study. Additionally, isotonic solutions play a vital role in immunology, serving as diluents for antibodies and antigens in various diagnostic assays.
The evolution of isotonic solutions has been closely tied to advancements in medical science. As our understanding of cellular physiology and biochemistry has deepened, the composition of these solutions has been fine-tuned to better replicate physiological conditions. This ongoing refinement has led to the development of specialized isotonic solutions for specific diagnostic applications, each tailored to meet the unique requirements of different tests and procedures.
The technological progress in the field of clinical diagnostics has also driven the evolution of isotonic solutions. The advent of automated diagnostic systems and point-of-care testing devices has necessitated the creation of isotonic solutions with enhanced stability and compatibility. These advancements have not only improved the accuracy and reliability of diagnostic tests but have also expanded the range of tests that can be performed in various clinical settings.
Looking ahead, the future of isotonic solutions in clinical diagnostics is poised for further innovation. Emerging trends such as personalized medicine and molecular diagnostics are likely to drive the development of more sophisticated isotonic solutions. These may include solutions with enhanced preservation capabilities for genetic material or those designed to maintain the integrity of complex biomarkers.
The primary objective of isotonic solutions in clinical diagnostics is to provide a medium that closely mimics the body's natural environment. This similarity allows for accurate testing and analysis of biological samples without altering their composition or cellular integrity. As diagnostic techniques have evolved, the demand for specialized isotonic solutions has grown, leading to continuous refinement and diversification of these products.
In the realm of clinical diagnostics, isotonic solutions serve multiple purposes. They are extensively used in hematology for blood cell counting and analysis, ensuring that cells maintain their shape and size during examination. In microbiology, these solutions are crucial for preparing and diluting samples, preserving microorganisms for further study. Additionally, isotonic solutions play a vital role in immunology, serving as diluents for antibodies and antigens in various diagnostic assays.
The evolution of isotonic solutions has been closely tied to advancements in medical science. As our understanding of cellular physiology and biochemistry has deepened, the composition of these solutions has been fine-tuned to better replicate physiological conditions. This ongoing refinement has led to the development of specialized isotonic solutions for specific diagnostic applications, each tailored to meet the unique requirements of different tests and procedures.
The technological progress in the field of clinical diagnostics has also driven the evolution of isotonic solutions. The advent of automated diagnostic systems and point-of-care testing devices has necessitated the creation of isotonic solutions with enhanced stability and compatibility. These advancements have not only improved the accuracy and reliability of diagnostic tests but have also expanded the range of tests that can be performed in various clinical settings.
Looking ahead, the future of isotonic solutions in clinical diagnostics is poised for further innovation. Emerging trends such as personalized medicine and molecular diagnostics are likely to drive the development of more sophisticated isotonic solutions. These may include solutions with enhanced preservation capabilities for genetic material or those designed to maintain the integrity of complex biomarkers.
Clinical Diagnostics Market Analysis
The clinical diagnostics market has been experiencing significant growth in recent years, driven by factors such as the increasing prevalence of chronic and infectious diseases, growing awareness about early disease detection, and technological advancements in diagnostic techniques. The global clinical diagnostics market was valued at approximately $70 billion in 2020 and is projected to reach over $100 billion by 2026, with a compound annual growth rate (CAGR) of around 6-7% during the forecast period.
Isotonic solutions play a crucial role in this expanding market, particularly in areas such as hematology, immunology, and microbiology. These solutions are essential for maintaining cell integrity and providing accurate diagnostic results. The demand for isotonic solutions in clinical diagnostics is closely tied to the overall growth of the in vitro diagnostics (IVD) market, which accounts for a substantial portion of the clinical diagnostics sector.
Within the clinical diagnostics market, the segment for reagents and consumables, including isotonic solutions, represents a significant share. This segment is expected to grow at a faster rate compared to the overall market due to the recurring nature of reagent consumption and the increasing adoption of automated diagnostic systems that require specialized solutions.
Geographically, North America and Europe dominate the clinical diagnostics market, accounting for over 60% of the global market share. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, rising disposable incomes, and increasing healthcare awareness in countries like China and India.
The market for isotonic solutions in clinical diagnostics is characterized by a mix of large multinational corporations and specialized manufacturers. Key players in this space include companies like Thermo Fisher Scientific, Abbott Laboratories, and Becton, Dickinson and Company, which offer a wide range of diagnostic products and solutions.
Technological advancements, such as the development of novel isotonic formulations with enhanced stability and compatibility with various diagnostic platforms, are expected to drive market growth. Additionally, the increasing focus on personalized medicine and point-of-care testing is creating new opportunities for isotonic solutions in clinical diagnostics.
However, the market also faces challenges, including stringent regulatory requirements for diagnostic products and the pressure to reduce healthcare costs. These factors may impact the pricing and adoption of isotonic solutions in certain regions. Despite these challenges, the overall outlook for isotonic solutions in the clinical diagnostics market remains positive, supported by the growing demand for accurate and reliable diagnostic tests across various medical specialties.
Isotonic solutions play a crucial role in this expanding market, particularly in areas such as hematology, immunology, and microbiology. These solutions are essential for maintaining cell integrity and providing accurate diagnostic results. The demand for isotonic solutions in clinical diagnostics is closely tied to the overall growth of the in vitro diagnostics (IVD) market, which accounts for a substantial portion of the clinical diagnostics sector.
Within the clinical diagnostics market, the segment for reagents and consumables, including isotonic solutions, represents a significant share. This segment is expected to grow at a faster rate compared to the overall market due to the recurring nature of reagent consumption and the increasing adoption of automated diagnostic systems that require specialized solutions.
Geographically, North America and Europe dominate the clinical diagnostics market, accounting for over 60% of the global market share. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, rising disposable incomes, and increasing healthcare awareness in countries like China and India.
The market for isotonic solutions in clinical diagnostics is characterized by a mix of large multinational corporations and specialized manufacturers. Key players in this space include companies like Thermo Fisher Scientific, Abbott Laboratories, and Becton, Dickinson and Company, which offer a wide range of diagnostic products and solutions.
Technological advancements, such as the development of novel isotonic formulations with enhanced stability and compatibility with various diagnostic platforms, are expected to drive market growth. Additionally, the increasing focus on personalized medicine and point-of-care testing is creating new opportunities for isotonic solutions in clinical diagnostics.
However, the market also faces challenges, including stringent regulatory requirements for diagnostic products and the pressure to reduce healthcare costs. These factors may impact the pricing and adoption of isotonic solutions in certain regions. Despite these challenges, the overall outlook for isotonic solutions in the clinical diagnostics market remains positive, supported by the growing demand for accurate and reliable diagnostic tests across various medical specialties.
Current Challenges in Isotonic Solution Development
The development of isotonic solutions for clinical diagnostics faces several significant challenges that researchers and manufacturers must address to improve their efficacy and applicability. One of the primary obstacles is maintaining the delicate balance of osmolarity and pH in these solutions. As biological samples are highly sensitive to changes in their environment, even slight deviations can lead to inaccurate diagnostic results or compromised sample integrity.
Another critical challenge lies in the formulation of isotonic solutions that are compatible with a wide range of diagnostic tests and analytical instruments. Different diagnostic procedures may require specific solution properties, making it difficult to create a universal isotonic medium. This necessitates the development of specialized formulations, which can be both time-consuming and costly.
The stability of isotonic solutions over time presents yet another hurdle. Many of these solutions contain components that may degrade or interact with each other during storage, potentially altering their isotonic properties. Ensuring long-term stability without compromising the solution's effectiveness is crucial for reliable clinical diagnostics, especially in settings where frequent replenishment is not feasible.
Contamination control is an ongoing concern in isotonic solution development. These solutions must remain sterile to prevent interference with diagnostic tests and to ensure patient safety when used in clinical procedures. Developing robust sterilization methods that do not affect the solution's isotonic properties or introduce harmful byproducts is a complex task that requires continuous innovation.
The increasing demand for point-of-care diagnostics has introduced new challenges in isotonic solution development. These solutions must be adaptable to miniaturized and portable diagnostic devices, which often have different requirements compared to traditional laboratory equipment. Formulating isotonic solutions that maintain their properties in these compact systems while ensuring accurate and reliable results is a significant technical challenge.
Environmental concerns and sustainability issues also play a role in the development of isotonic solutions. There is growing pressure to create eco-friendly formulations that minimize the use of harmful chemicals and reduce waste. This shift towards green chemistry in isotonic solution production requires novel approaches and alternative ingredients that do not compromise the solution's performance or safety.
Lastly, the regulatory landscape for isotonic solutions used in clinical diagnostics is becoming increasingly complex. Manufacturers must navigate stringent quality control measures and comply with evolving international standards. This regulatory burden can slow down innovation and increase development costs, presenting a significant challenge to the advancement of isotonic solution technology in the clinical diagnostics field.
Another critical challenge lies in the formulation of isotonic solutions that are compatible with a wide range of diagnostic tests and analytical instruments. Different diagnostic procedures may require specific solution properties, making it difficult to create a universal isotonic medium. This necessitates the development of specialized formulations, which can be both time-consuming and costly.
The stability of isotonic solutions over time presents yet another hurdle. Many of these solutions contain components that may degrade or interact with each other during storage, potentially altering their isotonic properties. Ensuring long-term stability without compromising the solution's effectiveness is crucial for reliable clinical diagnostics, especially in settings where frequent replenishment is not feasible.
Contamination control is an ongoing concern in isotonic solution development. These solutions must remain sterile to prevent interference with diagnostic tests and to ensure patient safety when used in clinical procedures. Developing robust sterilization methods that do not affect the solution's isotonic properties or introduce harmful byproducts is a complex task that requires continuous innovation.
The increasing demand for point-of-care diagnostics has introduced new challenges in isotonic solution development. These solutions must be adaptable to miniaturized and portable diagnostic devices, which often have different requirements compared to traditional laboratory equipment. Formulating isotonic solutions that maintain their properties in these compact systems while ensuring accurate and reliable results is a significant technical challenge.
Environmental concerns and sustainability issues also play a role in the development of isotonic solutions. There is growing pressure to create eco-friendly formulations that minimize the use of harmful chemicals and reduce waste. This shift towards green chemistry in isotonic solution production requires novel approaches and alternative ingredients that do not compromise the solution's performance or safety.
Lastly, the regulatory landscape for isotonic solutions used in clinical diagnostics is becoming increasingly complex. Manufacturers must navigate stringent quality control measures and comply with evolving international standards. This regulatory burden can slow down innovation and increase development costs, presenting a significant challenge to the advancement of isotonic solution technology in the clinical diagnostics field.
Existing Isotonic Solution Formulations
01 Composition of isotonic solutions
Isotonic solutions are formulated to have the same osmotic pressure as body fluids, typically containing a balance of electrolytes and other solutes. These solutions are crucial in medical applications, including intravenous therapy and cell culture media. The composition often includes sodium chloride, potassium chloride, and other essential ions to maintain physiological balance.- Composition of isotonic solutions: Isotonic solutions are formulated to have the same osmotic pressure as body fluids, typically containing a balance of electrolytes and other solutes. These solutions are crucial in medical applications, including intravenous therapy and cell culture media, as they maintain cellular integrity and prevent osmotic shock.
- Medical applications of isotonic solutions: Isotonic solutions are widely used in various medical procedures, such as wound irrigation, eye care, and intravenous fluid replacement. They help maintain proper hydration, electrolyte balance, and cellular function in patients, particularly during surgery or in cases of dehydration.
- Isotonic solutions in dialysis and blood processing: Specialized isotonic solutions are used in dialysis and blood processing procedures to ensure the safe and effective treatment of patients with kidney disorders. These solutions help maintain the proper balance of electrolytes and other essential components during the filtration process.
- Isotonic solutions for cell culture and preservation: In biotechnology and research applications, isotonic solutions play a crucial role in maintaining cell viability during culture, cryopreservation, and other experimental procedures. These solutions provide an optimal environment for cells, tissues, and organs outside the body.
- Development of novel isotonic formulations: Ongoing research focuses on developing new isotonic formulations with enhanced properties, such as improved stability, extended shelf life, or targeted delivery of therapeutic agents. These advancements aim to expand the applications of isotonic solutions in various medical and scientific fields.
02 Medical applications of isotonic solutions
Isotonic solutions have various medical applications, including use in intravenous fluids, dialysis, wound irrigation, and ophthalmic preparations. These solutions help maintain proper hydration, electrolyte balance, and cellular function in patients. They are also used in medical devices and diagnostic equipment to ensure compatibility with biological systems.Expand Specific Solutions03 Isotonic solutions in sports and exercise
Isotonic sports drinks and solutions are designed to replenish fluids and electrolytes lost during physical activity. These beverages help maintain hydration and electrolyte balance, potentially improving athletic performance and recovery. The formulation often includes carbohydrates for energy in addition to electrolytes.Expand Specific Solutions04 Production and quality control of isotonic solutions
Manufacturing isotonic solutions requires precise control of osmolality and pH. Advanced production techniques and quality control measures ensure the consistency and safety of these solutions. This includes sterile processing, accurate measurement of solute concentrations, and rigorous testing protocols to meet regulatory standards for medical and consumer use.Expand Specific Solutions05 Novel applications and delivery systems for isotonic solutions
Innovative approaches to isotonic solution delivery and application are being developed. These include advanced packaging systems, controlled release mechanisms, and integration with smart devices for monitoring and administration. Such innovations aim to improve the efficacy, convenience, and safety of isotonic solutions in various fields, from healthcare to consumer products.Expand Specific Solutions
Key Players in Clinical Diagnostics Industry
The field of isotonic solutions in clinical diagnostics is in a mature stage of development, with a well-established market and widespread application. The global market size for isotonic solutions is substantial, driven by their essential role in various medical procedures and diagnostic tests. Technologically, the field is relatively mature, with major pharmaceutical companies like Pfizer, Novartis, and Novo Nordisk leading the way in research and development. These companies, along with others such as Janssen Pharmaceutica and Genentech, have made significant advancements in formulating and optimizing isotonic solutions for specific clinical applications. The competitive landscape is characterized by ongoing innovation in formulation techniques and the development of specialized isotonic solutions for emerging diagnostic technologies.
Pfizer Inc.
Technical Solution: Pfizer has developed advanced isotonic solutions for clinical diagnostics, focusing on improving the accuracy and reliability of diagnostic tests. Their approach involves using carefully balanced electrolyte compositions to maintain cellular integrity during sample processing. Pfizer's isotonic solutions are designed to mimic physiological conditions, ensuring that biomarkers and cellular components remain stable during analysis. The company has also incorporated novel preservatives that extend the shelf-life of their solutions without compromising diagnostic accuracy[1]. Additionally, Pfizer has invested in developing specialized isotonic formulations for specific diagnostic applications, such as flow cytometry and immunoassays, optimizing the performance of these tests[3].
Strengths: Extensive R&D capabilities, global distribution network, and a strong reputation in the pharmaceutical industry. Weaknesses: High development costs and potential regulatory hurdles in bringing new diagnostic solutions to market.
Novartis AG
Technical Solution: Novartis has made significant strides in the field of isotonic solutions for clinical diagnostics, particularly in the area of personalized medicine. Their approach focuses on developing isotonic media that preserve the integrity of circulating tumor cells and cell-free DNA, crucial for liquid biopsy diagnostics. Novartis has patented a novel isotonic solution that incorporates nanoparticles to stabilize rare biomarkers in blood samples, enhancing the sensitivity of cancer detection and monitoring[2]. The company has also developed isotonic solutions with tailored osmolality for different tissue types, improving the accuracy of tissue-based diagnostics. Furthermore, Novartis has integrated machine learning algorithms to optimize the composition of their isotonic solutions based on specific diagnostic test requirements and patient characteristics[4].
Strengths: Strong focus on innovation, extensive experience in personalized medicine, and a robust pipeline of diagnostic technologies. Weaknesses: Complex regulatory landscape for combined diagnostic and therapeutic products, and potential high costs associated with personalized solutions.
Innovative Approaches in Isotonic Solution Research
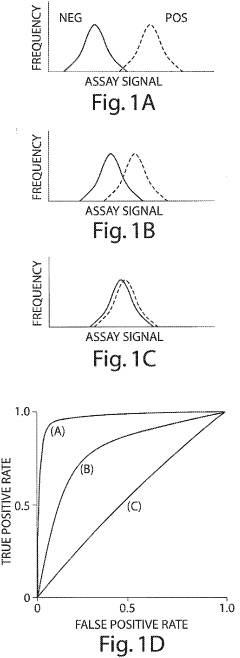
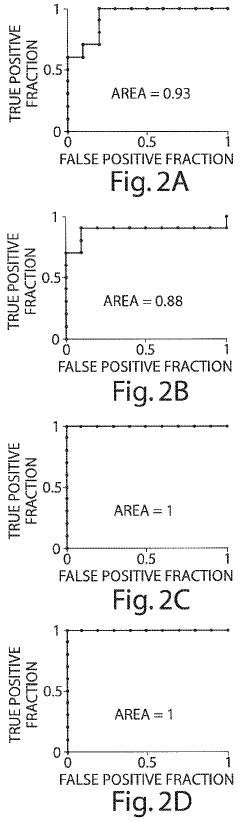
Method of using cytokine assays to diagnose treat, and evaluate inflammatory and autoimmune diseases
PatentInactiveUS20060094056A1
Innovation
- A method involving the analysis of cytokine expression levels in patient samples, comparing them to predefined reference levels to diagnose or predict disease development and treatment efficacy, using specific cytokines associated with various inflammatory and autoimmune diseases.
Methods for evaluation of the effectiveness of a drug or drug candidate in treating an inflammatory bowel disease by use of a multiplexed assay kit comprising various biomarkers
PatentActiveUS20230038393A1
Innovation
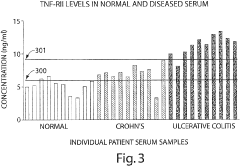
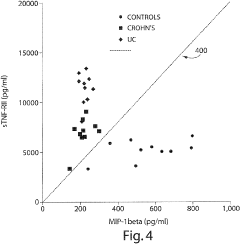
- Measuring levels of specific cytokines such as sTNFRII, RANTES, sIL-6R, IL-1β, IL-13, and IL-6 in blood, serum, or plasma samples using non-invasive methods to diagnose and differentiate between IBDs, with cut-off values and ratios aiding in diagnosis and monitoring disease progression.
Regulatory Framework for Diagnostic Solutions
The regulatory framework for diagnostic solutions plays a crucial role in ensuring the safety, efficacy, and quality of isotonic solutions used in clinical diagnostics. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including diagnostic products. The FDA classifies medical devices into three categories based on their risk level, with most diagnostic solutions falling under Class II or Class III.
For isotonic solutions used in clinical diagnostics, manufacturers must comply with the FDA's Quality System Regulation (QSR) and Good Manufacturing Practices (GMP). These regulations ensure that the production process meets stringent quality standards and that the final product is safe and effective for its intended use. Additionally, manufacturers are required to submit a 510(k) premarket notification or a Premarket Approval (PMA) application, depending on the device classification.
In the European Union, the regulatory landscape for diagnostic solutions is governed by the In Vitro Diagnostic Regulation (IVDR). This regulation, which replaced the previous In Vitro Diagnostic Directive (IVDD), introduces more stringent requirements for manufacturers, including enhanced clinical evidence, post-market surveillance, and traceability of devices throughout the supply chain.
The IVDR also introduces a new risk-based classification system for in vitro diagnostic medical devices, which may impact the regulatory pathway for isotonic solutions used in clinical diagnostics. Manufacturers must ensure compliance with the IVDR's requirements, including obtaining CE marking before placing their products on the EU market.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory approaches across different countries. Their guidelines and recommendations influence the development of regulatory frameworks for diagnostic solutions worldwide, promoting consistency and facilitating international trade.
Regulatory bodies also require manufacturers to implement robust quality management systems and conduct post-market surveillance to monitor the performance and safety of their products. This includes reporting adverse events and implementing corrective actions when necessary.
As the field of clinical diagnostics continues to evolve, regulatory frameworks are adapting to address new technologies and emerging risks. For instance, the increasing use of artificial intelligence and machine learning in diagnostic solutions has prompted regulatory bodies to develop guidelines specific to these technologies.
In conclusion, the regulatory framework for diagnostic solutions, including isotonic solutions used in clinical diagnostics, is complex and continually evolving. Manufacturers must navigate these regulations carefully to ensure compliance and maintain market access for their products.
For isotonic solutions used in clinical diagnostics, manufacturers must comply with the FDA's Quality System Regulation (QSR) and Good Manufacturing Practices (GMP). These regulations ensure that the production process meets stringent quality standards and that the final product is safe and effective for its intended use. Additionally, manufacturers are required to submit a 510(k) premarket notification or a Premarket Approval (PMA) application, depending on the device classification.
In the European Union, the regulatory landscape for diagnostic solutions is governed by the In Vitro Diagnostic Regulation (IVDR). This regulation, which replaced the previous In Vitro Diagnostic Directive (IVDD), introduces more stringent requirements for manufacturers, including enhanced clinical evidence, post-market surveillance, and traceability of devices throughout the supply chain.
The IVDR also introduces a new risk-based classification system for in vitro diagnostic medical devices, which may impact the regulatory pathway for isotonic solutions used in clinical diagnostics. Manufacturers must ensure compliance with the IVDR's requirements, including obtaining CE marking before placing their products on the EU market.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory approaches across different countries. Their guidelines and recommendations influence the development of regulatory frameworks for diagnostic solutions worldwide, promoting consistency and facilitating international trade.
Regulatory bodies also require manufacturers to implement robust quality management systems and conduct post-market surveillance to monitor the performance and safety of their products. This includes reporting adverse events and implementing corrective actions when necessary.
As the field of clinical diagnostics continues to evolve, regulatory frameworks are adapting to address new technologies and emerging risks. For instance, the increasing use of artificial intelligence and machine learning in diagnostic solutions has prompted regulatory bodies to develop guidelines specific to these technologies.
In conclusion, the regulatory framework for diagnostic solutions, including isotonic solutions used in clinical diagnostics, is complex and continually evolving. Manufacturers must navigate these regulations carefully to ensure compliance and maintain market access for their products.
Quality Control in Isotonic Solution Production
Quality control is a critical aspect of isotonic solution production, ensuring the safety and efficacy of these solutions for clinical diagnostic applications. The production process involves stringent measures to maintain consistency, purity, and accuracy in the final product.
One of the primary quality control measures is the precise measurement and control of osmolality. Isotonic solutions must have an osmolality closely matching that of human blood, typically around 290 mOsm/kg. Advanced osmometers are employed to verify the osmolality of each batch, with strict tolerance limits enforced to ensure physiological compatibility.
Chemical composition analysis is another crucial quality control step. High-performance liquid chromatography (HPLC) and mass spectrometry techniques are utilized to confirm the exact concentrations of electrolytes and other solutes in the solution. This analysis ensures that the ionic balance closely mimics that of human extracellular fluid, which is essential for accurate diagnostic results.
Sterility testing is paramount in isotonic solution production. Rigorous microbiological screening is conducted to detect any bacterial, fungal, or viral contamination. This typically involves membrane filtration techniques, followed by incubation and observation of growth media. Additionally, endotoxin testing using the Limulus Amebocyte Lysate (LAL) assay is performed to detect any bacterial endotoxins that could interfere with diagnostic procedures or pose risks to patients.
pH monitoring is another critical quality control measure. The pH of isotonic solutions must be carefully adjusted to match physiological levels, typically between 7.35 and 7.45. Precise pH meters and buffer solutions are used to ensure accuracy, with regular calibration and maintenance of equipment to maintain reliability.
Stability testing forms an integral part of the quality control process. Accelerated stability studies are conducted to assess the shelf life of the isotonic solutions under various environmental conditions. This involves exposing samples to different temperatures, humidity levels, and light conditions to evaluate any potential degradation or changes in composition over time.
Packaging integrity is also rigorously tested to prevent contamination and maintain the solution's sterility throughout its shelf life. This includes leak testing, seal integrity checks, and evaluation of the packaging material's compatibility with the solution.
Finally, comprehensive documentation and traceability systems are implemented to record every step of the production and quality control process. This allows for rapid identification and resolution of any issues that may arise, ensuring consistent quality and regulatory compliance in isotonic solution production for clinical diagnostics.
One of the primary quality control measures is the precise measurement and control of osmolality. Isotonic solutions must have an osmolality closely matching that of human blood, typically around 290 mOsm/kg. Advanced osmometers are employed to verify the osmolality of each batch, with strict tolerance limits enforced to ensure physiological compatibility.
Chemical composition analysis is another crucial quality control step. High-performance liquid chromatography (HPLC) and mass spectrometry techniques are utilized to confirm the exact concentrations of electrolytes and other solutes in the solution. This analysis ensures that the ionic balance closely mimics that of human extracellular fluid, which is essential for accurate diagnostic results.
Sterility testing is paramount in isotonic solution production. Rigorous microbiological screening is conducted to detect any bacterial, fungal, or viral contamination. This typically involves membrane filtration techniques, followed by incubation and observation of growth media. Additionally, endotoxin testing using the Limulus Amebocyte Lysate (LAL) assay is performed to detect any bacterial endotoxins that could interfere with diagnostic procedures or pose risks to patients.
pH monitoring is another critical quality control measure. The pH of isotonic solutions must be carefully adjusted to match physiological levels, typically between 7.35 and 7.45. Precise pH meters and buffer solutions are used to ensure accuracy, with regular calibration and maintenance of equipment to maintain reliability.
Stability testing forms an integral part of the quality control process. Accelerated stability studies are conducted to assess the shelf life of the isotonic solutions under various environmental conditions. This involves exposing samples to different temperatures, humidity levels, and light conditions to evaluate any potential degradation or changes in composition over time.
Packaging integrity is also rigorously tested to prevent contamination and maintain the solution's sterility throughout its shelf life. This includes leak testing, seal integrity checks, and evaluation of the packaging material's compatibility with the solution.
Finally, comprehensive documentation and traceability systems are implemented to record every step of the production and quality control process. This allows for rapid identification and resolution of any issues that may arise, ensuring consistent quality and regulatory compliance in isotonic solution production for clinical diagnostics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!