How Phenolphthalein Modulates Enzyme Activities Under Variable pH
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein and Enzyme Interaction Background
Phenolphthalein, a widely recognized pH indicator, has long been utilized in various scientific and industrial applications. Its unique property of changing color in response to pH variations has made it an invaluable tool in analytical chemistry and biochemistry. However, recent research has unveiled a more complex role for phenolphthalein in biological systems, particularly in its interactions with enzymes under varying pH conditions.
The relationship between phenolphthalein and enzyme activity is rooted in the fundamental principles of enzyme kinetics and the influence of environmental factors on protein structure and function. Enzymes, as biological catalysts, are highly sensitive to their surrounding conditions, with pH being a critical parameter affecting their performance. Phenolphthalein, traditionally viewed as a passive indicator, has been found to actively modulate enzyme activities in ways that were previously unrecognized.
The molecular structure of phenolphthalein plays a crucial role in its interaction with enzymes. At different pH levels, phenolphthalein undergoes structural changes that alter its chemical properties and potential binding affinities to various enzyme active sites. This pH-dependent structural flexibility allows phenolphthalein to interact with a diverse range of enzymes across different pH environments, potentially influencing their catalytic activities.
Research has shown that phenolphthalein can act as both an activator and inhibitor of enzyme activity, depending on the specific enzyme and the pH of the environment. This dual nature of phenolphthalein's influence on enzyme kinetics has opened up new avenues for understanding enzyme regulation and potential applications in biotechnology and medicine.
The interaction between phenolphthalein and enzymes is not limited to direct binding effects. Studies have revealed that phenolphthalein can also induce conformational changes in enzyme structures, altering their catalytic properties indirectly. These conformational changes can lead to enhanced or diminished enzyme activity, providing a novel mechanism for enzyme regulation that is sensitive to both pH and the presence of phenolphthalein.
Understanding the background of phenolphthalein and enzyme interactions is crucial for interpreting experimental results in biochemistry and developing new methodologies for enzyme studies. The complex interplay between phenolphthalein, pH, and enzyme activity challenges traditional views of pH indicators as passive tools and highlights their potential as active modulators of biological processes.
This evolving understanding of phenolphthalein's role in enzyme modulation has significant implications for various fields, including drug discovery, industrial biotechnology, and environmental science. As researchers continue to unravel the intricacies of these interactions, new opportunities emerge for harnessing the unique properties of phenolphthalein to control and optimize enzyme-catalyzed reactions in diverse applications.
The relationship between phenolphthalein and enzyme activity is rooted in the fundamental principles of enzyme kinetics and the influence of environmental factors on protein structure and function. Enzymes, as biological catalysts, are highly sensitive to their surrounding conditions, with pH being a critical parameter affecting their performance. Phenolphthalein, traditionally viewed as a passive indicator, has been found to actively modulate enzyme activities in ways that were previously unrecognized.
The molecular structure of phenolphthalein plays a crucial role in its interaction with enzymes. At different pH levels, phenolphthalein undergoes structural changes that alter its chemical properties and potential binding affinities to various enzyme active sites. This pH-dependent structural flexibility allows phenolphthalein to interact with a diverse range of enzymes across different pH environments, potentially influencing their catalytic activities.
Research has shown that phenolphthalein can act as both an activator and inhibitor of enzyme activity, depending on the specific enzyme and the pH of the environment. This dual nature of phenolphthalein's influence on enzyme kinetics has opened up new avenues for understanding enzyme regulation and potential applications in biotechnology and medicine.
The interaction between phenolphthalein and enzymes is not limited to direct binding effects. Studies have revealed that phenolphthalein can also induce conformational changes in enzyme structures, altering their catalytic properties indirectly. These conformational changes can lead to enhanced or diminished enzyme activity, providing a novel mechanism for enzyme regulation that is sensitive to both pH and the presence of phenolphthalein.
Understanding the background of phenolphthalein and enzyme interactions is crucial for interpreting experimental results in biochemistry and developing new methodologies for enzyme studies. The complex interplay between phenolphthalein, pH, and enzyme activity challenges traditional views of pH indicators as passive tools and highlights their potential as active modulators of biological processes.
This evolving understanding of phenolphthalein's role in enzyme modulation has significant implications for various fields, including drug discovery, industrial biotechnology, and environmental science. As researchers continue to unravel the intricacies of these interactions, new opportunities emerge for harnessing the unique properties of phenolphthalein to control and optimize enzyme-catalyzed reactions in diverse applications.
Market Applications of pH-Sensitive Enzyme Modulation
The market applications of pH-sensitive enzyme modulation, particularly those involving phenolphthalein, span across various industries and offer significant potential for innovation and growth. In the pharmaceutical sector, this technology has found applications in drug delivery systems, where pH-sensitive enzymes can be used to control the release of active ingredients in specific parts of the digestive tract. This allows for targeted drug delivery, improving efficacy and reducing side effects.
In the food industry, pH-sensitive enzyme modulation has been utilized in the development of smart packaging materials. These materials can change color or properties in response to pH changes, indicating food spoilage or contamination. This technology enhances food safety and reduces waste by providing visual cues to consumers about the freshness of products.
The environmental sector has also benefited from pH-sensitive enzyme modulation. Water treatment plants use this technology to optimize the efficiency of their processes. Enzymes that are sensitive to pH changes can be employed to remove contaminants more effectively, adapting to varying water conditions and improving overall water quality.
In biotechnology, pH-sensitive enzyme modulation has applications in biocatalysis and industrial fermentation processes. By controlling the pH environment, researchers can optimize enzyme activity for the production of valuable compounds, such as biofuels, fine chemicals, and pharmaceuticals. This leads to more efficient and cost-effective manufacturing processes.
The cosmetics industry has incorporated pH-sensitive enzyme technology in skincare products. These formulations can adapt to the skin's natural pH, providing personalized care and improving the efficacy of active ingredients. This technology allows for the development of smart cosmetics that respond to individual skin conditions.
In agriculture, pH-sensitive enzyme modulation has been applied to the development of smart fertilizers and soil amendments. These products can release nutrients or adjust soil pH based on environmental conditions, leading to more efficient use of resources and improved crop yields.
The textile industry has also found applications for this technology in the development of smart fabrics. pH-sensitive dyes and enzymes can be incorporated into textiles to create clothing that changes color in response to environmental conditions or body chemistry, opening up new possibilities for functional and interactive fashion.
In the food industry, pH-sensitive enzyme modulation has been utilized in the development of smart packaging materials. These materials can change color or properties in response to pH changes, indicating food spoilage or contamination. This technology enhances food safety and reduces waste by providing visual cues to consumers about the freshness of products.
The environmental sector has also benefited from pH-sensitive enzyme modulation. Water treatment plants use this technology to optimize the efficiency of their processes. Enzymes that are sensitive to pH changes can be employed to remove contaminants more effectively, adapting to varying water conditions and improving overall water quality.
In biotechnology, pH-sensitive enzyme modulation has applications in biocatalysis and industrial fermentation processes. By controlling the pH environment, researchers can optimize enzyme activity for the production of valuable compounds, such as biofuels, fine chemicals, and pharmaceuticals. This leads to more efficient and cost-effective manufacturing processes.
The cosmetics industry has incorporated pH-sensitive enzyme technology in skincare products. These formulations can adapt to the skin's natural pH, providing personalized care and improving the efficacy of active ingredients. This technology allows for the development of smart cosmetics that respond to individual skin conditions.
In agriculture, pH-sensitive enzyme modulation has been applied to the development of smart fertilizers and soil amendments. These products can release nutrients or adjust soil pH based on environmental conditions, leading to more efficient use of resources and improved crop yields.
The textile industry has also found applications for this technology in the development of smart fabrics. pH-sensitive dyes and enzymes can be incorporated into textiles to create clothing that changes color in response to environmental conditions or body chemistry, opening up new possibilities for functional and interactive fashion.
Current Challenges in pH-Dependent Enzyme Activity Control
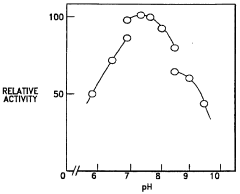
The control of enzyme activity in varying pH environments remains a significant challenge in biochemistry and biotechnology. While phenolphthalein has shown promise in modulating enzyme activities under different pH conditions, several obstacles persist in fully harnessing its potential.
One of the primary challenges is achieving precise and predictable control over enzyme activity across a wide pH range. Enzymes typically exhibit optimal activity within narrow pH windows, and maintaining consistent performance outside these ranges is difficult. Phenolphthalein's pH-dependent color change offers a visual indicator, but translating this into fine-tuned enzyme activity modulation requires further research and development.
The stability of enzyme-phenolphthalein complexes under varying pH conditions poses another hurdle. As pH fluctuates, the interactions between phenolphthalein and enzymes may change, potentially leading to unpredictable enzyme behavior or loss of activity. Developing strategies to stabilize these complexes across a broader pH spectrum is crucial for practical applications.
Furthermore, the reversibility of phenolphthalein-induced enzyme modulation presents both an opportunity and a challenge. While reversibility allows for dynamic control, achieving rapid and complete reversibility without compromising enzyme integrity or activity remains difficult. This is particularly important in industrial processes where quick adjustments to enzyme activity are necessary.
The specificity of phenolphthalein's effects on different enzymes is another area requiring attention. Not all enzymes respond uniformly to phenolphthalein, and understanding the molecular mechanisms behind these variations is essential for developing targeted applications. Researchers must investigate how structural differences among enzymes influence their interactions with phenolphthalein under various pH conditions.
Additionally, the potential for phenolphthalein to interfere with other components in complex biological or industrial systems needs to be addressed. In multi-enzyme systems or in the presence of other pH-sensitive compounds, phenolphthalein's effects may be altered or may inadvertently affect unintended targets.
Scaling up phenolphthalein-based enzyme activity control from laboratory to industrial scales presents its own set of challenges. Factors such as mixing efficiency, pH gradients in large reactors, and the economic feasibility of using phenolphthalein in bulk processes need to be carefully evaluated and optimized.
Lastly, the long-term effects of phenolphthalein exposure on enzyme stability and activity are not fully understood. Continuous or repeated use of phenolphthalein in enzyme systems may lead to cumulative effects that could impact enzyme performance or lifespan. Comprehensive studies on the long-term implications of phenolphthalein-mediated enzyme modulation are necessary to ensure the sustainability and reliability of this approach in various applications.
One of the primary challenges is achieving precise and predictable control over enzyme activity across a wide pH range. Enzymes typically exhibit optimal activity within narrow pH windows, and maintaining consistent performance outside these ranges is difficult. Phenolphthalein's pH-dependent color change offers a visual indicator, but translating this into fine-tuned enzyme activity modulation requires further research and development.
The stability of enzyme-phenolphthalein complexes under varying pH conditions poses another hurdle. As pH fluctuates, the interactions between phenolphthalein and enzymes may change, potentially leading to unpredictable enzyme behavior or loss of activity. Developing strategies to stabilize these complexes across a broader pH spectrum is crucial for practical applications.
Furthermore, the reversibility of phenolphthalein-induced enzyme modulation presents both an opportunity and a challenge. While reversibility allows for dynamic control, achieving rapid and complete reversibility without compromising enzyme integrity or activity remains difficult. This is particularly important in industrial processes where quick adjustments to enzyme activity are necessary.
The specificity of phenolphthalein's effects on different enzymes is another area requiring attention. Not all enzymes respond uniformly to phenolphthalein, and understanding the molecular mechanisms behind these variations is essential for developing targeted applications. Researchers must investigate how structural differences among enzymes influence their interactions with phenolphthalein under various pH conditions.
Additionally, the potential for phenolphthalein to interfere with other components in complex biological or industrial systems needs to be addressed. In multi-enzyme systems or in the presence of other pH-sensitive compounds, phenolphthalein's effects may be altered or may inadvertently affect unintended targets.
Scaling up phenolphthalein-based enzyme activity control from laboratory to industrial scales presents its own set of challenges. Factors such as mixing efficiency, pH gradients in large reactors, and the economic feasibility of using phenolphthalein in bulk processes need to be carefully evaluated and optimized.
Lastly, the long-term effects of phenolphthalein exposure on enzyme stability and activity are not fully understood. Continuous or repeated use of phenolphthalein in enzyme systems may lead to cumulative effects that could impact enzyme performance or lifespan. Comprehensive studies on the long-term implications of phenolphthalein-mediated enzyme modulation are necessary to ensure the sustainability and reliability of this approach in various applications.
Existing Methodologies for Enzyme Activity Modulation
01 Phenolphthalein as enzyme substrate
Phenolphthalein is used as a substrate for various enzymatic assays. It can be modified or conjugated to create specific substrates for detecting enzyme activities. These substrates change color or become fluorescent when cleaved by the target enzyme, allowing for quantitative measurement of enzyme activity.- Phenolphthalein as enzyme substrate: Phenolphthalein is used as a substrate in various enzymatic assays. It can be modified or conjugated to create specific substrates for different enzymes, allowing for the detection and measurement of enzyme activities. The color change of phenolphthalein upon enzymatic action makes it suitable for colorimetric assays.
- Phenolphthalein in β-glucuronidase assays: Phenolphthalein glucuronide is commonly used as a substrate to measure β-glucuronidase activity. The enzyme cleaves the glucuronide moiety, releasing free phenolphthalein, which can be detected colorimetrically. This assay is useful in various fields, including clinical diagnostics and environmental monitoring.
- Phenolphthalein in esterase activity detection: Phenolphthalein esters are used to detect and measure esterase activities. The enzymatic hydrolysis of these esters releases phenolphthalein, which can be quantified spectrophotometrically. This method is applied in various fields, including food science and environmental monitoring.
- Phenolphthalein in enzyme inhibition studies: Phenolphthalein and its derivatives are used in enzyme inhibition studies. They can act as inhibitors for certain enzymes or be used as substrates in assays to evaluate the inhibitory effects of other compounds. This application is particularly relevant in drug discovery and toxicology research.
- Phenolphthalein in enzyme-linked immunosorbent assays (ELISA): Phenolphthalein phosphate is used as a substrate in alkaline phosphatase-based ELISA systems. The enzyme cleaves the phosphate group, releasing phenolphthalein, which can be detected colorimetrically. This application is widely used in immunodiagnostics and protein detection assays.
02 Phenolphthalein in β-glucuronidase assays
Phenolphthalein-β-D-glucuronide is commonly used as a substrate for measuring β-glucuronidase activity. The enzyme cleaves the glucuronide bond, releasing free phenolphthalein, which can be detected colorimetrically. This assay is useful in various fields, including clinical diagnostics and environmental monitoring.Expand Specific Solutions03 Phenolphthalein in esterase activity detection
Phenolphthalein derivatives, such as phenolphthalein diphosphate, are used to detect esterase activities. These substrates are hydrolyzed by esterases, releasing phenolphthalein, which can be measured spectrophotometrically. This method is applied in various fields, including food safety and environmental monitoring.Expand Specific Solutions04 Phenolphthalein in enzyme immunoassays
Phenolphthalein and its derivatives are used in enzyme immunoassays as substrates for enzyme labels. The enzyme-catalyzed reaction produces a colored or fluorescent product, allowing for the detection and quantification of specific analytes. This technique is widely used in clinical diagnostics and research applications.Expand Specific Solutions05 Novel phenolphthalein-based enzyme substrates
Researchers are developing new phenolphthalein-based substrates with improved properties for enzyme activity detection. These novel substrates may offer higher sensitivity, specificity, or stability compared to traditional phenolphthalein derivatives. They are designed to meet the evolving needs of various enzymatic assays in biotechnology and medical diagnostics.Expand Specific Solutions
Key Players in Biochemical Indicator Research
The research into "How Phenolphthalein Modulates Enzyme Activities Under Variable pH" is in a developing stage, with a growing market potential in biochemistry and related industries. The competitive landscape is characterized by a mix of academic institutions and pharmaceutical companies, indicating a balance between fundamental research and practical applications. Key players like Halozyme, Inc., BioMarin Pharmaceutical, Inc., and Ajinomoto Co., Inc. are likely at the forefront of enzyme modulation research, leveraging their expertise in biopharmaceuticals and fine chemicals. The involvement of universities such as Yunnan Normal University and Nanjing Tech University suggests ongoing basic research efforts, potentially leading to future breakthroughs in understanding enzyme behavior under varying pH conditions.
Halozyme, Inc.
Technical Solution: Halozyme has developed a proprietary enzyme technology platform called ENHANZE® that utilizes recombinant human hyaluronidase PH20 (rHuPH20) to enhance the delivery of injected drugs and fluids. While not directly related to phenolphthalein, their expertise in enzyme modulation under varying pH conditions is relevant. They have demonstrated that rHuPH20 remains active across a wide pH range (6.0-8.0) and can temporarily degrade hyaluronan in the extracellular matrix[1]. This technology allows for improved drug absorption and dispersion, potentially applicable to studies involving pH-sensitive enzyme activities[2].
Strengths: Extensive experience in enzyme technology and pH-dependent activity modulation. Weaknesses: Primary focus on hyaluronidase may limit direct applicability to phenolphthalein studies.
Ajinomoto Co., Inc.
Technical Solution: Ajinomoto has developed advanced enzyme engineering techniques to optimize enzyme performance under various pH conditions. Their research includes the use of directed evolution and rational design to create pH-stable variants of industrially important enzymes[3]. While not specifically focused on phenolphthalein, their approach to understanding and manipulating enzyme behavior across pH ranges is highly relevant. They have successfully engineered enzymes with improved activity and stability at both acidic and alkaline pH, which could provide insights into how phenolphthalein might interact with enzymes under variable pH conditions[4].
Strengths: Strong expertise in enzyme engineering and pH-dependent activity optimization. Weaknesses: May lack specific experience with phenolphthalein interactions.
Core Mechanisms of Phenolphthalein-Enzyme Interactions
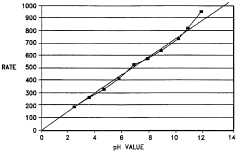
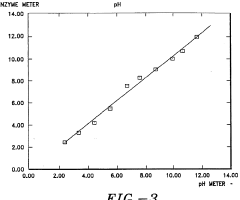
Method and kit for enzymatically determining the pH of a specimen
PatentInactiveUS5595867A
Innovation
- A method involving the use of a buffered solution with an enzyme and substrate to establish a direct proportional relationship between enzyme activity and specimen pH, allowing for accurate and simple pH determination using conventional clinical chemistry analyzers, and a kit containing the necessary components for this method.
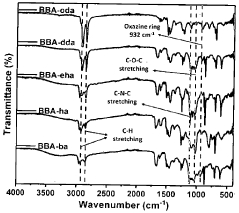
Production of extreme range of PH indicators from benzoxazines
PatentActiveIN202341027342A
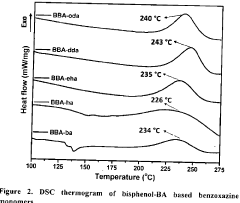
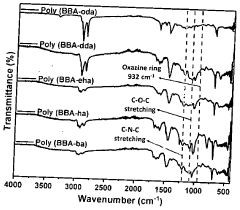
Innovation
- Development of bisphenol-BA/aliphatic amine based hydrophobic polybenzoxazines coated on cellulose paper, synthesized through Mannich condensation, which exhibit distinct color changes across a wide pH range from -1.8 to 14, offering thermal stability and repeated use capability.
Regulatory Considerations for Biochemical Indicators
The regulatory landscape for biochemical indicators, particularly those involving phenolphthalein and enzyme activity modulation under variable pH conditions, is complex and multifaceted. Regulatory bodies across different jurisdictions have established guidelines and standards to ensure the safety, efficacy, and reliability of these indicators in various applications.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating biochemical indicators used in medical diagnostics and research. The FDA's Center for Devices and Radiological Health (CDRH) oversees the approval process for in vitro diagnostic devices, which often incorporate biochemical indicators. For phenolphthalein-based enzyme activity assays, manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and provide substantial evidence of analytical and clinical validity.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation imposes stricter requirements on the performance, risk classification, and post-market surveillance of biochemical indicators. Under the IVDR, indicators like phenolphthalein-based enzyme assays may fall into higher risk classes, necessitating more rigorous conformity assessment procedures and involvement of notified bodies.
Internationally, the International Organization for Standardization (ISO) has developed several standards relevant to biochemical indicators. ISO 13485, which specifies requirements for quality management systems in the medical device industry, is particularly pertinent. Manufacturers of phenolphthalein-based enzyme activity assays must ensure their products meet these standards to gain global market acceptance.
Environmental regulations also come into play, especially when considering the disposal of phenolphthalein and related compounds. The Environmental Protection Agency (EPA) in the US and the European Chemicals Agency (ECHA) have established guidelines for the handling and disposal of these chemicals, given their potential environmental impact.
In the context of research applications, institutional review boards (IRBs) and ethics committees play a crucial role in overseeing the use of biochemical indicators in human subjects research. These bodies ensure that the use of such indicators complies with ethical standards and protects the rights and welfare of research participants.
As the understanding of enzyme modulation under variable pH conditions advances, regulatory bodies are likely to refine their approaches. There is an increasing emphasis on harmonizing regulations across different regions to facilitate global research and development. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working towards this goal, potentially impacting future regulatory considerations for biochemical indicators like phenolphthalein-based enzyme assays.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating biochemical indicators used in medical diagnostics and research. The FDA's Center for Devices and Radiological Health (CDRH) oversees the approval process for in vitro diagnostic devices, which often incorporate biochemical indicators. For phenolphthalein-based enzyme activity assays, manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and provide substantial evidence of analytical and clinical validity.
The European Union has implemented the In Vitro Diagnostic Regulation (IVDR), which came into full effect in May 2022. This regulation imposes stricter requirements on the performance, risk classification, and post-market surveillance of biochemical indicators. Under the IVDR, indicators like phenolphthalein-based enzyme assays may fall into higher risk classes, necessitating more rigorous conformity assessment procedures and involvement of notified bodies.
Internationally, the International Organization for Standardization (ISO) has developed several standards relevant to biochemical indicators. ISO 13485, which specifies requirements for quality management systems in the medical device industry, is particularly pertinent. Manufacturers of phenolphthalein-based enzyme activity assays must ensure their products meet these standards to gain global market acceptance.
Environmental regulations also come into play, especially when considering the disposal of phenolphthalein and related compounds. The Environmental Protection Agency (EPA) in the US and the European Chemicals Agency (ECHA) have established guidelines for the handling and disposal of these chemicals, given their potential environmental impact.
In the context of research applications, institutional review boards (IRBs) and ethics committees play a crucial role in overseeing the use of biochemical indicators in human subjects research. These bodies ensure that the use of such indicators complies with ethical standards and protects the rights and welfare of research participants.
As the understanding of enzyme modulation under variable pH conditions advances, regulatory bodies are likely to refine their approaches. There is an increasing emphasis on harmonizing regulations across different regions to facilitate global research and development. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is working towards this goal, potentially impacting future regulatory considerations for biochemical indicators like phenolphthalein-based enzyme assays.
Environmental Impact of Phenolphthalein Usage
The environmental impact of phenolphthalein usage is a critical consideration in its application as a pH indicator and enzyme activity modulator. Phenolphthalein, while widely used in laboratory settings, can have significant effects on ecosystems when released into the environment.
One of the primary concerns is the potential for phenolphthalein to act as an endocrine disruptor. Studies have shown that it can mimic estrogen in biological systems, potentially affecting the reproductive cycles of various organisms. This estrogenic activity can lead to altered hormone levels in aquatic life, potentially causing reproductive abnormalities and population imbalances in affected ecosystems.
The persistence of phenolphthalein in the environment is another key issue. While it can degrade under certain conditions, its stability in water and soil can lead to accumulation over time. This accumulation may result in long-term exposure for organisms in contaminated areas, potentially leading to chronic health effects and bioaccumulation in food chains.
Water pollution is a significant risk associated with phenolphthalein disposal. Improper handling and disposal of laboratory waste containing phenolphthalein can lead to contamination of water bodies. This contamination can affect water quality, potentially impacting drinking water sources and aquatic ecosystems. The colorimetric properties of phenolphthalein can also alter the visual characteristics of water, potentially affecting light penetration and photosynthetic activity in aquatic environments.
Soil contamination is another environmental concern. Phenolphthalein that enters soil systems can persist and potentially affect soil microorganisms. These microorganisms play crucial roles in nutrient cycling and soil health, and any disruption to their populations could have cascading effects on terrestrial ecosystems.
The impact on plant life is also noteworthy. While phenolphthalein is primarily used in controlled laboratory settings, any release into the environment could potentially affect plant growth and development. Its ability to modulate pH could alter soil chemistry, potentially affecting nutrient availability and root uptake in plants.
Given these environmental concerns, proper handling, use, and disposal of phenolphthalein are crucial. Laboratory protocols should include strict guidelines for its use and disposal to minimize environmental release. Additionally, research into more environmentally friendly alternatives for pH indication and enzyme activity modulation could help reduce the reliance on phenolphthalein and mitigate its potential environmental impacts.
One of the primary concerns is the potential for phenolphthalein to act as an endocrine disruptor. Studies have shown that it can mimic estrogen in biological systems, potentially affecting the reproductive cycles of various organisms. This estrogenic activity can lead to altered hormone levels in aquatic life, potentially causing reproductive abnormalities and population imbalances in affected ecosystems.
The persistence of phenolphthalein in the environment is another key issue. While it can degrade under certain conditions, its stability in water and soil can lead to accumulation over time. This accumulation may result in long-term exposure for organisms in contaminated areas, potentially leading to chronic health effects and bioaccumulation in food chains.
Water pollution is a significant risk associated with phenolphthalein disposal. Improper handling and disposal of laboratory waste containing phenolphthalein can lead to contamination of water bodies. This contamination can affect water quality, potentially impacting drinking water sources and aquatic ecosystems. The colorimetric properties of phenolphthalein can also alter the visual characteristics of water, potentially affecting light penetration and photosynthetic activity in aquatic environments.
Soil contamination is another environmental concern. Phenolphthalein that enters soil systems can persist and potentially affect soil microorganisms. These microorganisms play crucial roles in nutrient cycling and soil health, and any disruption to their populations could have cascading effects on terrestrial ecosystems.
The impact on plant life is also noteworthy. While phenolphthalein is primarily used in controlled laboratory settings, any release into the environment could potentially affect plant growth and development. Its ability to modulate pH could alter soil chemistry, potentially affecting nutrient availability and root uptake in plants.
Given these environmental concerns, proper handling, use, and disposal of phenolphthalein are crucial. Laboratory protocols should include strict guidelines for its use and disposal to minimize environmental release. Additionally, research into more environmentally friendly alternatives for pH indication and enzyme activity modulation could help reduce the reliance on phenolphthalein and mitigate its potential environmental impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!